Abstract
Background and Objective
Blood pressure (BP) control in hypertensive patients remains poor worldwide, particularly in high-risk patients with hypertension and diabetes. Guidelines recommend that such patients receive prompt pharmacological therapy at maximal doses to rapidly control BP. We aimed to evaluate efficacy and safety of single-pill combination (SPC) perindopril/indapamide (PER/IND) at full dose (10/2.5 mg) in hypertensive patients, including diabetics, with BP uncontrolled by previous medication.
Methods
Twelve-week prospective, observational study in patients with uncontrolled hypertension (≥160–200 mmHg systolic BP [SBP] and <110 mmHg diastolic BP [DBP]) on a previous SPC or free-dose combination of renin-angiotensin system blocker plus thiazide diuretic, substituted with PER/IND 10/2.5 mg. Office BP, quality of life, and blood parameters were evaluated in the whole cohort and patients with type 2 diabetes mellitus.
Results
2120 ambulatory hypertensive patients were enrolled, including 307 with type 2 diabetes. Two weeks after substitution, SBP significantly decreased from 171.0 ± 13.3 to 148.6 ± 13.4 mmHg, and DBP from 98.6 ± 8.3 to 88.8 ± 7.9 mmHg (both p < 0.00001). A similar rapid decrease was noted in the diabetes subgroup. After 12 weeks, BP had reduced by 42/19 mmHg in the whole cohort (diabetes subgroup: 41/18 mmHg). Most (84%; diabetes subgroup: 77%) patients reached BP target (<140/90 mmHg). Laboratory tests and quality of life improved in the whole cohort and the diabetic subgroup.
Conclusions
Switching to PER/IND at full dose (10/2.5 mg) was well tolerated, leading to fast BP reduction and control in the majority of patients with uncontrolled hypertension, including difficult-to-treat patients with diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Switching to the single-pill combination perindopril/indapamide at full dose (10/2.5 mg) provided rapid blood pressure (BP) control in patients uncontrolled by a previous combination of renin-angiotensin-aldosterone inhibitor plus diuretic. |
A similar rapid decrease in BP and achievement of BP targets were observed in the whole cohort of the study, and in patients with type 2 diabetes. |
The treatment was well tolerated, and had a favorable impact on metabolic profile. |
1 Introduction
Arterial hypertension is one of the basic independent risk factors for stroke and coronary artery disease (CAD), and effective antihypertensive treatment contributes considerably to decreased risk of cardiovascular mortality [2, 3]. One of the main ways of measuring the efficacy of antihypertensive therapy is blood pressure (BP) control—the achievement of BP <140/90 mmHg. Combination antihypertensive therapy allows BP to be controlled in most patients, and usually includes a renin-angiotensin-aldosterone system (RAAS) blocker in combination with a diuretic or calcium channel blocker (CCB) [4]. The single-pill combination (SPC) perindopril/indapamide (PER/IND) is a widely prescribed antihypertensive treatment in Russia [5]. The efficacy of this combination has been confirmed in international and Russian clinical trials [6–11].
In Russia, an SPC of perindopril arginine/indapamide is available as Noliprel A (2.5/0.625 mg) or Noliprel A Forte (5/1.25 mg). Recently a new 10/2.5 mg full dose (Noliprel A Bi-Forte) was introduced. This combination therapy may not only improve BP control in patients with difficult-to-control hypertension (patients with uncontrolled hypertension on two antihypertensive drugs [including a diuretic]), but may also improve treatment adherence, protect target organs, and reduce mortality [12–14]. Patients with diabetes and hypertension, a population at high risk of microvascular and macrovascular complications showing especially low rates of BP control, are also difficult to control [2]. The American Diabetes Association (ADA) has recommended that hypertensive patients with diabetes should receive prompt pharmacological therapy in order to rapidly control BP [15]. In particular they endorse treatment with a combination therapy of either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB) accompanied by a thiazide diuretic, both at maximal doses.
Under the aegis of the Russian Medical Society on Arterial Hypertension (RMSAH), the FORTISSIMO study was performed to investigate the efficacy and safety of the new dosage of SPC PER/IND (10/2.5 mg) in patients with poorly controlled BP on a fixed- or free-dose RAAS inhibitor plus diuretic (hydrochlorothiazide [HCTZ] or indapamide). The effect of treatment was evaluated in the overall cohort of the FORTISSIMO study, and in the subgroup of patients with type 2 diabetes.
2 Patients and Methods
Russian general practitioners and cardiologists followed up suitable outpatients in the FORTISSIMO study, a 12-week open-label, prospective, multicenter, observational, non-comparative study in men or women aged >18 years with essential hypertension poorly controlled by a RAAS inhibitor—ACE inhibitors or ARBs—plus diuretic (HCTZ or indapamide), given separately or as an SPC. These stable patients (stable hypertension and stable antihypertensive therapy over the last 3 months) had office systolic BP (SBP) ≥160 to 200 mmHg and diastolic BP (DBP) <110 mmHg. All patients gave their written informed consent.
Exclusion criteria included age <18 years, secondary hypertension, type 1 or decompensated type 2 diabetes mellitus, and contraindications or allergies to perindopril or indapamide. Patients with severe concomitant diseases (Canadian Cardiovascular Society class III-IV effort angina; unstable angina; New York Heart Association grade III-IV heart failure; acute myocardial infarction or cerebrovascular accident in the last 6 months; renal insufficiency (creatinine clearance <30 mL/h); or impaired liver function) were also excluded.
Office BP, quality of life, and blood composition were measured in patients switched to SPC PER/IND at full dose (10/2.5 mg once daily, Noliprel A Bi-Forte, Servier, Serdix Co. Ltd, Sof’ino, Moscow, Russia) from an ACE inhibitor or ARB plus diuretic. Other antihypertensive therapy was unchanged. Dietary or exercise interventions were not part of the study. Visits took place 2, 4, 8, and 12 weeks after baseline. If required, physicians were able to modify the dose of SPC PER/IND after 2 or 8 weeks, and to add additional amlodipine 5 mg once daily with BP >140/90 mmHg at 8 weeks. Arterial pressure was measured using Korotkoff’s auscultatory method at all visits (in the physician’s office in the morning after a 5-min rest and 24 h after the last intake of SPC PER/IND 10/2.5 mg). The mean value was calculated from three separate measurements taken with the patient in a sitting position. Potassium, glucose, total cholesterol, triglycerides, low- and high-density lipoprotein cholesterol, creatinine, uric acid, hemoglobin, and erythrocyte sedimentation rate were recorded at baseline (sample taken within 3 months before inclusion) and at 12 weeks. Obesity was assessed at baseline and was defined as a waist circumference >102 cm in men and >88 cm in women. Quality of life was assessed at baseline and at 12 weeks using the universal health status SF-36 questionnaire (physical function, activities, pain, general health, vitality, social function, emotional state, and mental health), and two summary scores were derived (the physical component summary and the mental component summary). General health state was assessed by both patient and physician at the end of the study. Safety was assessed by recording adverse events at each visit during the study. Adverse events were defined as any untoward medical occurrence in a patient administered a medicinal product and which does not necessarily have a causal relationship with this treatment. Serious adverse events were defined as an adverse reaction which results in death, is life-threatening, requires in-patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a congenital anomaly/birth defect, or might jeopardize the patient or might require intervention to prevent one of the other outcomes listed above.
Efficacy criteria included BP change from baseline to 12 weeks, and BP control (defined as the percentage of patients who reached the target BP level of <140/90 mmHg). Other criteria were change in quality of life and change in blood parameters. Change in BP is presented in the overall population (i.e., all patients who completed the study), in patients with type 2 diabetes, in patients switching from an ACE inhibitor plus diuretic, in patients switching from an ARB plus diuretic, and according to the grade of SBP at baseline. BP control is presented in the overall population, in patients with type 2 diabetes, and in subgroups who took an ACE inhibitor plus a diuretic or an ARB plus a diuretic. Response to treatment was calculated in patients with type 2 diabetes, and was defined as the percentage of patients fulfilling at least one of the three following criteria: SBP reduction ≥20 mmHg, DBP reduction ≥10 mmHg, or achievement of target BP. Quality of life and blood parameters are presented in the overall population, and in the subgroup of patients with type 2 diabetes.
2.1 Statistical Methods
Data are presented as means ± standard deviations for continuous variables or numbers and percentages for categorical variables. Comparisons at baseline and at 12 weeks were assessed using nonparametric tests (Wilcoxon and signed-rank tests) in addition to Student’s t test for paired samples. A p value <0.05 indicated a significant difference. SPSS software (version 16.0) was used for statistical analysis.
3 Results
3.1 Baseline Characteristics
In the FORTISSIMO study, 2120 patients were enrolled by 700 general practitioners and cardiologists from 51 regions in the Russian Federation (see Acknowledgements section). A total of 1628 (77%) patients fulfilled the inclusion criterion of a previous antihypertensive treatment with a RAAS inhibitor plus diuretic (HCTZ or indapamide).
The characteristics at baseline for the overall study population (n = 2120) and for patients with type 2 diabetes (n = 307 [14%]) are shown in Table 1. When compared with the overall study population, diabetic patients were slightly older (62.1 vs. 59.0 years), more likely to be female (74 vs. 66%), to be obese (79 vs. 63%), and to have raised cholesterol (54 vs. 49%). Approximately a third of the overall population (30%) and of patients with diabetes (31%) had a family history of early onset of cardiovascular disease. Hypertensive retinal angiopathy (84%) or left ventricular hypertrophy (87%) was present in most patients in the overall population, and nearly half (48%) had chronic heart failure (class I–II). Patients with diabetes had a greater proportion of microvascular or hypertension-related disease as compared with the overall population: 92% of them had hypertensive retinal angiopathy, 93% had left ventricular hypertrophy, and 61% had chronic heart failure (class I–II). Microalbuminuria was present in 27% of patients with diabetes and in 13% of the overall population.
When compared with the overall study population, patients with diabetes were more likely to be prescribed a diuretic (92 vs. 84%) or a CCB (39 vs. 30%) at baseline. The proportion of patients taking a beta-blocker (50 and 53% for the overall population and for patients with diabetes, respectively), an ACE inhibitor (78 and 75%), and an ARB (18 and 21%) was similar in both groups. Of the overall population, 81% were taking a fixed- or free-dose ACE inhibitor or ARB plus diuretic, including 37% taking an ACE inhibitor or ARB plus HCTZ at baseline.
The mean duration of hypertension was 11.0 years in the overall population, and 13.0 years in patients with diabetes. Mean heart rate, SBP, and DBP were similar in both patient groups (heart rate: 76.4 beats/minute (bpm) in both groups; SBP/DBP: 171.0/98.6 mmHg overall, vs. 172.0/98.4 mmHg in patients with diabetes). The distribution of grades of hypertension was similar in both groups (85 and 87% of patients in Grade 2 or 3, for the overall population and patients with diabetes, respectively).
3.2 Study Participants
Overall, there were 151 (7%) premature withdrawals, mainly lost to follow-up (n = 41 [27%]), adverse events (n = 40 [27%]), and patient refusal (n = 29 [19%]). Other causes included unknown reasons (n = 25 [17%]), no effect (n = 8 [5%]), treatment regimen deviation (n = 5 [3%]), physician decision (n = 2 [1%]), and supply problems (n = 1 [<1%]). The remaining 1969 patients (93%) completed the study (including 280 patients with type 2 diabetes). Of the 1969 patients who completed the study, 1873 (95%) were still taking SPC PER/IND 10/2.5 mg after 12 weeks; 87 (4%) were on a lower dose of 5/1.25 mg and nine (<1%) on 2.5/0.625 mg.
3.3 Effect of Treatment with Single-Pill Combination (SPC) Perindopril/Indapamide (PER/IND) 10/2.5 mg on Blood Pressure
3.3.1 Overall Population
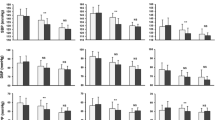
In the overall population, SBP decreased by 41.5 ± 13.9 mmHg from 171.0 ± 13.3, and DBP by 18.5 ± 8.7 mmHg from 98.6 ± 8.3 mmHg after 12 weeks of treatment (Fig. 1a). SBP decreased by 22.4 ± 13.5 mmHg (54%), 31.9 ± 14.1 mmHg (77%), and 38.2 ± 14.0 mmHg (92%) at 2, 4, and 8 weeks, respectively (all p < 0.00001 vs. previous visit). Equivalent decreases in DBP were 9.8 mm ± 8.0 Hg (53%), 14.0 ± 8.4 mmHg (76%), and 16.8 ± 8.7 mmHg (91%) (all p < 0.00001 vs. previous visit). Similar trends in SBP and DBP reduction were observed in patients previously taking an ACE inhibitor/diuretic or ARB/diuretic (Table 2). BP reduction was rapid, falling by 22/10 mmHg after 2 weeks in the main study. The greater the severity of hypertension at baseline, the greater the reduction in BP with SPC PER/IND 10/2.5 mg: between baseline and 12 weeks, SBP/DBP decreased by 26.5 ± 8.8/13.8 ± 7.9 mmHg in patients with grade 1 hypertension, 39.3 ± 9.3/18.2 ± 8.2 mmHg in patients with grade 2 hypertension, and 56.1 ± 11.7/22.2 ± 8.3 mmHg in patients with grade 3 hypertension (all p < 0.00001 vs. previous visit) (Fig. 2). The degree of SBP and DBP reduction was similar in men and women, and was dependent on the duration of treatment with SPC PER/IND. SPC PER/IND 10/2.5 mg was effective regardless of previous treatment, age, gender, risk factors, or diseases associated with hypertension.
Systolic and diastolic blood pressure reduction with single-pill combination perindopril/indapamide 10/2.5 mg in patients with uncontrolled hypertension despite previous antihypertensive therapy. Results are presented for the overall population (a) and diabetic patients (b). p < 0.00001 vs. previous visit
Systolic and diastolic blood pressure reduction with single-pill combination perindopril/indapamide 10/2.5 mg according to grade of hypertension in patients with uncontrolled hypertension despite previous antihypertensive therapy. Values are presented as means ± standard deviations. Hypertension was classified according to systolic blood pressure (SBP) at baseline: grade 1, 140–159 mmHg; grade 2, 160–179 mmHg; and grade 3, ≥180 mmHg. *p < 0.00001 vs. previous visit for all grades
In the overall population, the majority of patients reached the standard BP target of <140/90 mmHg (n = 1646 [84%]). Rates of BP control were comparable in patients who previously took an ACE inhibitor/diuretic (78%) or ARB/diuretic (76%). BP control was rapid: after 4 and 8 weeks, 43 and 65%, respectively, of patients previously on an ACE inhibitor/diuretic achieved BP control. The equivalent percentages at 4 and 8 weeks in patients previously on an ARB/diuretic were 49 and 67%, respectively.
3.3.2 Patients with Diabetes
With regard to patients with diabetes, SBP decreased by 40.5 ± 13.6 mmHg from 171.7 ± 13.7, and DBP by 18.0 ± 9.5 mmHg from 98.6 ± 9.0 mmHg after 12 weeks of treatment (Fig. 1b). SBP decreased by 21.2 ± 12.6 mmHg (52%), 30.8 ± 13.2 mmHg (76%), and 37.2 ± 13.7 mmHg (92%) at 2, 4, and 8 weeks, respectively (all p < 0.00001 vs. previous visit). Equivalent decreases in DBP were 9.4 ± 8.1 mmHg (52%), 13.7 ± 8.8 mmHg (76%), and 16.2 ± 9.5 mmHg (90%) (all p < 0.00001 vs. previous visit). Similar trends in SBP and DBP reduction were observed in patients previously taking an ACE inhibitor/diuretic or ARB/diuretic (Table 2). As in the overall population, BP reduction was rapid (21/9 mmHg after 2 weeks), and decreases in office BP with SPC PER/IND 10/2.5 mg were greater in patients with more severe hypertension at baseline; between baseline and 12 weeks, BP decreased by 26.3 ± 7.8/13.7 ± 8.0 mmHg in patients with grade 1 hypertension, 37.6 ± 9.1/17.7 ± 8.8 mmHg in patients with grade 2 hypertension, and 54.6 ± 10.7/21.5 ± 8.9 mmHg in patients with grade 3 hypertension.
BP target was reached in 215 diabetic patients (77%). Nearly all (98%) diabetic patients were responders to treatment with SPC PER/IND. Rates of BP control were comparable in patients who previously took an ACE inhibitor/diuretic (77%) or ARB/diuretic (81%). BP control was rapid: After 4 and 8 weeks, 42 and 66%, respectively, of patients previously on an ACE inhibitor/diuretic achieved BP control. The equivalent percentages at 4 and 8 weeks in patients previously on ARB/diuretic were 46 and 71%, respectively.
3.4 Effect of Treatment with SPC PER/IND 10/2.5 mg on Quality of Life
3.4.1 Overall Population
A total of 1512 patients completed the SF-36 questionnaire at baseline and study end. All eight scores rose after 12 weeks’ therapy with SPC PER/IND 10/2.5 mg compared with baseline scores. Physical and mental summary scales significantly increased from 38.9 ± 7.2 and 39.8 ± 9.6% at baseline to 44.5 ± 6.1 and 50.7 ± 6.5% after 12 weeks of treatment, respectively (p < 0.001 for both, Fig. 3).
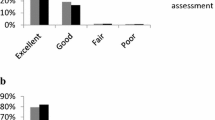
At the end of the study, patients were asked to rate their general condition: 31% rated it excellent, 55% good, 11% satisfactory, and <1% described no change. No patient reported worsening. Physicians rated the results of treatment excellent, good, and satisfactory in 37, 49, and 4% of cases, respectively. No change with treatment was reported for one patient, and no physicians described worsening of the patient’s condition.
3.4.2 Patients with Diabetes
In patients with diabetes, all eight scores in the SF-36 questionnaire increased between baseline and study end. Physical and mental summary scales significantly increased from 36.4 ± 7.3 and 39.4 ± 9.7% at baseline to 41.8 ± 6.2 and 50.1 ± 6.4% after 12 weeks of treatment, respectively (p < 0.00001 for both, Fig. 3).
With regard to self-assessment of their general condition by diabetic patients, 24% rated it excellent, 58% good, 14% satisfactory, and <1% described no change. Physicians rated the general condition of patients with diabetes excellent or good in 79% of cases.
3.5 Tolerability and Safety of SPC PER/IND 10/2.5 mg
After 12 weeks, in the overall population slight reductions were observed in hemoglobin, erythrocyte sedimentation rate, blood glucose, creatinine, total cholesterol, low-density lipoprotein cholesterol, triglycerides, and uric acid (all p ≤ 0.00001). High-density lipoprotein cholesterol increased marginally (p = 0.00039). Blood potassium remained unchanged.
Blood parameters of patients with diabetes are presented in Table 3. After 12 weeks, slight but significant improvements in several metabolic and clinical blood parameters were observed, including a reduction in triglycerides, total cholesterol, low-density lipoprotein cholesterol, creatinine, and uric acid. Blood glucose was significantly reduced from 6.88 ± 1.55 at baseline to 6.33 ± 1.41 after 12 weeks (p < 0.00001). Blood potassium was not changed.
Evaluation of adverse events was performed in all patients who started treatment with SPC PER/IND (2120 patients). Overall, adverse events were registered in 85 patients (4%), of which two were fatal cases (both unrelated to the treatment: stroke of unknown type and death in automobile accident [the patient was a passenger]). Adverse events that were related to the treatment (adverse drug reactions) were registered in 57 patients. Of these, six were considered as serious: three cases of excessive BP reduction, one case of Quince‘s edema, one case of urticaria, and one case of dysuria; the outcomes were favorable. The most commonly registered other adverse events were cough (<1%), dizziness (<1%), asthenia (<1%), and BP decrease (<1%).
4 Discussion
Administration of the SPC PER/IND at full dose (10/2.5 mg) in hypertensive outpatients poorly controlled by a previous combination of fixed- or free-dose ACE inhibitor or ARB plus diuretic resulted in a rapid and significant reduction in BP over 12 weeks in the overall cohort and in the subgroup of patients with type 2 diabetes, with a high rate of responders to treatment. The SPC PER/IND at full dose (10/2.5 mg) controlled BP effectively, regardless of the type of previous antihypertensive combination (ACE inhibitor or ARB plus diuretic). Quality of life improved significantly, and safety was good, including a favorable effect on metabolic profile.
The antihypertensive efficacy—in terms of BP reduction and BP control—and safety of SPC PER/IND at low and high dosages has been well established in hypertensive patients [6–9]. This combination has also been shown to have nephroprotective benefits in patients with type 2 diabetes mellitus [10, 11]. Positive long-term outcomes—antihypertensive efficacy, target organ protection, and cardiovascular benefits—of treatment with perindopril and indapamide have been observed in other studies [11, 13, 14, 16]. The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation-Observational study (ADVANCE-ON) recently showed that positive survival benefits of treatment with PER/IND lasted for up to 10 years after treatment initiation, which included a 6-year post-trial period [16]. SPC PER/IND is currently the only RAAS blocker-based SPC to contain indapamide, a diuretic preferred to thiazides in British hypertension guidelines [17]. HCTZ is not a suitable alternative, owing to its short (<24 h) duration of action, and its lesser efficacy compared to thiazide-like diuretics and other classes of antihypertensive drugs [18]. Indapamide, which acts via both a diuretic and a direct vasorelaxant effect, is more potent in reducing SBP than HCTZ (by −5 mmHg), as demonstrated in a recent meta-analysis [19, 20].
In our study, a higher proportion of patients were obese in the diabetes subgroup than the overall group (79 vs. 63%). These difficult-to-control patients could benefit from a combination treatment of an ACE inhibitor and a diuretic, as advocated by Wenzel et al. [21]. This combination may help counterbalance the salt-retaining effects of diabetes or hyperinsulinemia in obesity. Moreover, it has been demonstrated that adding a diuretic may increase the antiproteinuric effect of RAAS blockade [22]. This suggests that patients with both hypertension and type 2 diabetes should benefit from initiating treatment with either an ACE inhibitor or an ARB in combination with a thiazide-type diuretic [23]. Recently, the American Diabetes Association (ADA) has endorsed the need for a maximal dose of such a combination to achieve BP target in hypertensive patients with diabetes [15]. Our results support these recommendations, and further show that among the RAAS inhibitor/thiazide diuretic combinations strategy, the SPC PER/IND used at the full dose of 10/2.5 mg is an attractive alternative in hypertensive patients with diabetes, by offering rapid BP control in patients previously uncontrolled by other RAAS inhibitor/thiazide combinations, in parallel with a favorable impact on the metabolic profile.
Our findings confirm those observed in other observational studies with a full dose of SPC PER/IND 10/2.5 mg in hypertensive patients in real life [24, 25]. BP in 9257 hypertensive patients in the Perindopril Plus Indapamide Combination Blood Pressure Reduction (PICASSO) study decreased from 159/93 mmHg to 132/80 mmHg (p < 0.001), and target BP (<140/90 mmHg) was reached by 73% [24]. In Stratification of Cardiovascular Risk and Evidence Based Medicine Hypertension Treatment FORTE (FALCO FORTE) [25], SBP was reduced in 2327 hypertensive patients (69% with BP uncontrolled by other antihypertensives, 27% newly diagnosed) from 156.9 to 132.3 mmHg, and DBP from 94.9 to 81.3 mmHg (both p < 0.0001). Target BP was reached by 87%. Quality of life improved significantly in FALCO FORTE, as seen in our study.
A full dose of PER/IND 10/2.5 mg showed good antihypertensive efficacy in patients with type 2 diabetes in our study. These findings are similar to those observed in previous observational studies in diabetic patients [26, 27]. Noliprel Forte A as the Key Therapy for Diabetic Patients with Hypertension (NIKA) study included 397 patients with type 2 diabetes, and newly diagnosed hypertension or BP uncontrolled with previous therapy. BP decreased from 160/95 to 130/81 mmHg in patients after 6 months treatment with PER/IND 5/1.25 mg or 10/2.5 mg, and BP control was achieved in 90% of patients treated with the highest dose [26]. In a subgroup analysis of the PICASSO trial in 2762 patients with type 2 diabetes or pre-diabetes, BP was reduced from 159/93 mmHg to 132/80 mmHg after 3 months of treatment with PER/IND 10/2.5 mg [27]. Treatment also significantly decreased ambulatory BP. Similar to our findings, both the PICASSO subgroup analysis and the NIKA study showed an improvement of laboratory parameters after treatment with PER/IND in diabetic patients. These results indicate that, unlike thiazide-type diuretics such as HCTZ, indapamide does not negatively impact the metabolic status of patients with type 2 diabetes mellitus.
BP control in hypertensive patients has been shown to be greater when treated with SPCs than with free-dose combinations or monotherapy [28], and antihypertensive SPCs have been shown to improve treatment adherence [29, 30]. This could be useful, as rates of BP control in clinical trials are not always reproduced in routine clinical practice [31]. Aside from poor treatment adherence, physicians’ reluctance to insist on more active and aggressive treatment of hypertension may be a reason [32].
Further possible advantages of antihypertensive SPCs versus monotherapy include a quicker BP response in a greater percentage of patients, and greater BP reduction compared with increasing the dosage of one antihypertensive agent [2]. There may also be potential advantages in terms of fewer drops-out and a reduced incidence of side effects. Treatment initiation with SPCs is currently recommended by European guidelines in hypertensive patients at high risk or with high initial BP values [2]. In current Russian hypertension guidelines [3], the main indication for changing treatment or first administering combination therapy is failure of monotherapy or other combinations to control BP. ACE inhibitor/diuretic SPCs remain a preferred choice of antihypertensive strategy [2, 4].
4.1 Limitations
Our study has the typical benefits and limitations of an observational study. A major limitation of this study is the lack of a control arm, which makes it difficult to determine the extent of a true drug effect versus the result of intense BP monitoring. For some patients, inclusion criteria (mainly baseline BP values and previous antihypertensive treatments) deviated from the protocol, which might reduce the validity of these findings. Time since diagnosis of diabetes was not collected for diabetic patients. Despite this, our study provides a real-life assessment of BP reduction and BP control with the SPC PER/IND at full dose (10/2.5 mg) in a large range of patients, including patients with type 2 diabetes who are more difficult to treat.
5 Conclusion
Switching to SPC PER/IND at full dose (10/2.5 mg) effectively reduced BP over the short term in patients with poorly controlled BP despite previous treatment with an ACE inhibitor or ARB plus diuretic. This resulted in rapid and effective BP control (<140/90 mmHg) in most patients (84%), good tolerability including a favorable impact on metabolic profile, and a significant improvement in quality of life. This efficacy was maintained in patients with type 2 diabetes—who are more difficult to treat—and 77% of them achieved BP control after failure of previous combination therapy. Simplification of treatment and reduction of noncompliance with SPC PER/IND at full dose may have contributed to the efficacy observed.
References
Karpov I. The FORTISSIMO program: advantages of fixed full dose combination of perindopril arginine and indapamide in the treatment of poorly controlled arterial hypertension. Kardiologiia. 2013;53:37–43.
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219.
Russian Medical Society on Arterial Hypertension (RMSAH) and Russian Society of Cardiology (RSC). Russian guidelines on the diagnosis and treatment of arterial hypertension. 4th ed [in Russian]. Syst Hypertens. 2010;3:5–26.
Sever PS, Messerli FH. Hypertension management 2011: optimal combination therapy. Eur Heart J. 2011;32:2499–506.
Leonova MV, Belousov DJu, Shteinberg LL, et al. Analysis of physicians’ practice of antihypertensive therapy conduction in Russia (according to the data of the PIFAGOR III study) [in Russian]. Pharmateca. 2009;12:114–9.
Martynyuk TV, Kolos IP, Chazova IE. The efficacy and safety of a fixed perindopril/indapamide combination in small doses in patients with arterial hypertension within the scope of the real clinical practice (multicenter, open-label, prospective study STRATEGY) [in Russian]. Cardiovasc Ther Prev. 2007;6:21–7.
Chazova IE, Ratova LG, Martynyuk TV. First results of the Russian STRATEGY A study (Russian multicenter program to evaluate the efficacy of Noliprel A Forte in high-risk arterial hypertension patients with inadequate blood pressure control) on the way to optimize antihypertensive therapy in patients with high-risk arterial hypertension [in Russian]. Syst Hypertens. 2010;4:42–8.
Mourad JJ, Lameira D, Guillausseau PJ. Blood pressure normalization by fixed perindopril/indapamide combination in hypertensive patients with or without associate metabolic syndrome: results of the OPTIMAX 2 study. Vasc Health Risk Manag. 2008;4:443–51.
Dahlof B, Gosse P, Gueret P, et al. Perindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL study. J Hypertens. 2005;23:2063–70.
Mogensen CE, Viberti G, Halimi S, et al. Effect of low-dose perindopril/indapamide on albuminuria in diabetes: preterax in albuminuria regression: PREMIER. Hypertension. 2003;41:1063–71.
Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40.
Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407.
Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98.
van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension - a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone-system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088–97.
American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2016;39:S60–71.
Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–406.
National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available from: http://nice.org.uk/guidance/cg127. Accessed 15 Apr 2016.
Roush GC, Ernst ME, Kostis JB, et al. Not just chlorthalidone: evidence-based, single tablet, diuretic alternatives to hydrochlorothiazide for hypertension. Curr Hypertens Rep. 2015;17:540.
Waeber B, Rotaru C, Feihl F. Position of indapamide, a diuretic with vasorelaxant activities, in antihypertensive therapy. Expert Opin Pharmacother. 2012;13:1515–26.
Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with indapamide and chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041–6.
Wenzel UO, Benndorf R, Lange S. Treatment of arterial hypertension in obese patients. Semin Nephrol. 2013;33:66–74.
Esnault VL, Ekhlas A, Delcroix C, et al. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol. 2005;16:474–81.
Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601–10.
Farsang C. Blood pressure and metabolic efficacy of fixed-dose combination of perindopril and indapamide in everyday practice. Blood Press. 2013;22(Suppl 1):3–10.
Pella D. Efficacy and safety of treatment of hypertensive patients with fixed combination perindopril/indapamide up to 10/2.5 mg: results of the FALCO FORTE programme. High Blood Press Cardiovasc Prev. 2011;18:107–113.
Netchessova TA, Shepelkevich AP, Gorbat TV. Efficacy of single-pill perindopril/indapamide in patients with hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev. 2014;21:63–9.
Farsang C. Efficacy and tolerability of fixed-dose combination of perindopril/indapamide in type 2 diabetes mellitus: PICASSO trial. Adv Ther. 2014;31:333–44.
Egan BM, Bandyopadhyay D, Shaftman SR, et al. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012;59:1124–31.
Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–9.
Chrysant SG. Using fixed-dose combination therapies to achieve blood pressure goals. Clin Drug Invest. 2008;28:713–34.
Oganov RG, Timofeeva TN, Koltunov IE. Arterial hypertension epidemiology in Russia: the results of 2003–2010 federal monitoring [in Russian]. Cardiovasc Ther Prev. 2011;10:9–13.
Germino FW. Therapeutic inertia and measurement inertia in hypertension: a call to action. J Clin Hypertens (Greenwich). 2013;15:365–6.
Acknowledgements
Part of the data presented here was previously published in the Russian journal Kardiologiia [1].
Physicians participating in the FORTISSIMO study: Abbasova EV, Abdualimova MH, Abdullina AR, Abramova VV, Avdonina NM, Avilova OV, Agapova VV, Agoeva MB, Adamova HA, Adamian MM, Adelshina LR, Aznaurova NR, Aydashova NA, Akimochkina AG, Aksenova NA, Akulova NA, Aleynik ON, Alexandrova VN, Alpaeva YU, Amerdzhanyan YS, Amichba GN, Ananicheva GV, Ananyeva AA, Andreev AN, Andreeva GV, Andreeva OV, Andriyasheva TA, Androsova IA, Anisimova II, Anisimova NV, Anpalova EY, Antonova TG, Anoufriev DY, Aryukova AV, Aslambekova FN, Astapenko TS, Astakhova MA, Afanasieva TN, Akhmetzyanova RR, Akhmetshina OA, Babaeva AV, Baboshina NS, Baburenkova TD, Baburina NI, Bagdulina EN, Badlina AN, Bakal OP, Balaeva ZV, Balakina VI, Balobina OI, Barannikova IA, Baranova SP, Bargueva SG, Bardash GA, Barinova TN, Batmanova LN, Batsoeva AS, Baccina TV, Belkina NM, Belkorei OS, Belokopytova IS, Bichunskaya VA, Blinova GA, Blinova MN, Bobkova LV, Boboshko TN, Bogatova TK, Bogdanova VA, Bogdanova TI, Bogdashkina YV, Bodrihin NV, Bolshakova TV, Bondareva EI, Bondar MN, Borodina EV, Botasheva TT, Bochkova YV, Boyarshinova GA, Bredneva AI, Bronnikova VV, Budanova OV, Buldakov IM, Burlaka AP, Burljaeva TS, Burma VI, Burmina EL, Booth-Gusaim VI, Bydanova SS, Bykov AN, Bykova LV, Bylinaa OL, Bystrova EA, Vavilova VA, Vavilova NV, Vagaytseva EA, Valieva GT, Valiullova GR, Varezhnikova OV, Varnakova LN, Vasilyeva EV, Vasilyeva LB, Vasilyeva MV, Vasilyeva NV, Vasilyeva SS, Vasilchenko VN, Vasilchenko II, Vedeneva TP, Velykanova EE, Verkhovskaya VA, Vetronogova GI, Vikulova SL, Vinogradova NS, Vishnevskaya MS, Bishnyakov AA, Vojvodina MS, Volkova EI, Vologzhanina TF, Volodina EI, Volodova SI, Volynkina AD, Vorobyova SG, Vorontsova IV, Vorontsova IE, Gahan NG, Gabova NE, Gavrilova AA, Galiahmetova OV, Galieva LF, Garifullina BR, Garcia VP, Gevorgyan AB, Geybatova IA, Geyisova EV, Gimadieva RI, Glazunova OA, Gluschenko NP, Gnezdilova YG, Golotvin MV, Golubeva AG, Golubeva LV, Golubeva MG, Golubeva OB, Golubickaya NV, Gorbunova AS, Gordeeva SV, Gordina VI, Gorin AV, Gorlanova LV, Gornaeva LI, Gorpinyak BB, Gosteva EP, Grebenets LV, Grebneva TV, Grigorenko II, Grigorieva VS, Grigorieva TA, Grigoryan CA, Grishina EV, Grudtsyna IV, Gusakova TV, Guseva AS, Davydova IV, Davidovich EB, Danilova EA, Dvornikova IV, Degtyareva TY, Degtyarenko TV, Demchuk TI Denyak IV, Deryabina ES, Deryabina NG, Dehont LJ, Jha OA, Dmitrieva LV, Dobina AS, Dobkes AV, Dolmatova KO, Donets TV, Donskih GP, Doroshkova NG, Dubrovina NG, Duviryak LN, Dudchenko EV, Dulatova FF, Dyukova VV, Evdokimova SR, Egorenko EA, Yekimova NV, Eremeeva OA, Eremenko OA, Yermolayeva NA, Yermolayeva CV, Erokhina IS, Erokhina OV, Efimova OA, Zharikova LV, Zheltova VL, Zeltuha NA, Zherebker EM, Zhizhko NV, Zhukova OV, Zhuravleva AA, Zhuravleva EA, Zaglyadova IA, Zaitseva IV, Zakirova VA, Zapolskikh LG, Zaripova RA, Zarubina MN, Zakharova EG, Zakharova EL, Zakharova IV, Zakharova YV, Zatsarina EP, Zelentsova AB, Zorina NS, Zotova OP, Zubova OV, Zuzak LT, Ivankova SV, Ivanova AP, Ivanova AF, Ivanova AS, Ivanova MV, Ivanova MM, Ivanova NV, Ivanova OA, Ivanova SS, Ivanova TA, Ivanova TV, Ivankova II, Ivashchenko TN, Ivochkina MI, Idiyatullina VR, Ilicheva GA, Imhovik MV, Isaeva MV, Isakova MV, Iskhakova AZ, Itkulova NZ, Ishmurzin GP, Kadnikova AN, Kazakovtseva MV, Kazantseva MO, Kayrapetyan LB, Kalinina EG, Kalinina SN, Kalinichenko GA, Kamyshenkova EA, Kaneva NL, Kantseva LV, Kaperiz YS, Kaplun TV, Karamova LM, Karandasova TM, Karukes NA, Katarzhnova MA, Kafanova MS, Kashipova NN, Kelareva EN, Kechina EP, Kindzersky ON, Kirillov GI, Kitaeva VM, Kichigina TM, Klementeva EA, Klimashevich EI, Knyazeva OV, Koblyakova II, Kovalenko AA, Kowalskaya MV, Kogut NN, Kozlov SS, Kolganova EB, Kolevatova GA, Koltakova JF, Kolchanova OV, Kolchina EV, Komarov AA, Komarov OA, Komissarov LE, Komkova NA, Konischeva EA, Konovalov VA, Konovalov GV, Konovalov LP, Konohova IV, Koposova OY, Korepanova VE, Korneva VA, Kornilova NV, Koroleva NM, Koryakina TL, Koryakova EP, Kostin LP, Kochetkov EB, Kochkina TG, Kochuganova IA, Koshelev IP, Koshrokova RA, Kraynov AN, Kramnik GE, Krapivnaya NV, Krasnova TS, Krepetula IF, Krechunova TN, Krivaya AA, Krochmal OV, Kruglov OA, Kudoyarova MK, Kuznetsov DP, Kuznetsova EA, Kuznetsova NV, Kuzmina IP, Kulalaeva II, Kulahmetova RG, Kulik NA, Kulygina VE, Kumaneeva OF, Kumpan IV, Kurakova EN, Kurgan NI, Kurguzova OV, Kurzyakova NA, Kukhalashvili DA, Kuharets YV, Kucheryavaya TY, Kuchina NV, Lazarenko EV, Lapin EA, Laptev MA, Laputenko TA, Lapshin SA, Larina OV, Latypova VN, Lebedev EV, Lebedev EG, Lebedev IN, Levchenko GI, Levchenko NA, Lezhnina NV, Lezginova TO, Lepeshkina TV, Lee LA, Lee LF, League MB, Linz NI, Lisin SV, Lisovska OS, Litvinov II, Lobanov LM, Loy MG, Lonkina LA, Lubinetskaya TA, Lukanina GS, Lukashou IN, Lukmanova NN, Lukyanchikova VV, Lytaeva TN, Lyubimov OV, Makarov EA, Makarov IN, Makarova MV, Makodzeba TD, Malachieva MC, Malevich SM, Malin AL, Malikova IR, Malic SI, Mal’tseva J N, Mamonova AV, Maneeva ID, Mark AM, Maslikova TN, Masyukova MR, Matveeva LV, Matvienko AY, Matkovskiy AS, Machova YA, Melent’ev AV, Melnikova EN, Melnikova EJ, Melnichenko IN, Menzhevitskaya TI, Menshikov VA, Meteleva MV, Mehedko LN, Meshcheriakova MA, Milovatskaya NB, Minibaeva AF, Mitrofanov VN, Mitrofanov C V, Mikhailova EA, Mikhailov LV, Mikhailova NN, Mikhailova RY, Mikhalkov DF, Mikheeva MN, Moiseeva OS, Molodtseva EY, Molodtsov AN, Morgun NK, Morozova LN, Moscaleva RA, Motsnyi OA, Muzalevskii II, Muradova OV, Murasova RI, Murzagulov AF, Musaeva FK, Nabokov NB, Nazarov LI, Nalbandian SN, Naumova EN, Naumchuk AY, Nafikova RS, Nesterenko SV, Nesterov AA, Nefedov LV, Nechayev EV, Nechitailo OV, Nikiforova TY, Nikolaev OA, Nikolaev LY, Nikolina TT, Nikulin EG, Nikulin IV, Novik LM, Novikova GA, Novikova MV, Novoselov GI, Nomokonova OA, Nosyreva OV, Nurgaliyeva SY, Obukhov NS, Gadfly VG, Ovchinnikov OV, Oleinikova ID, Onishchenko EG, Orehova GA, Orlina OV, Osinchuk YB, Osipova EN, Osmonalieva ME, Osokina GL, Osminkin LA, Pavlova TN, Panina VA, Pantyukhov VV, Panfilov NL, Panchenko NN, Parshin IA, Pascal EV, Patrikeeva LV, Pashkovskaya TN, Pentegova VA, Peredernin SA, Perepelitsa EI, Permitin EA, Permjakova OV, Petukhov DA, Piven ON, Brewers AM, Pivovarova NG, Pimenov LA, Pichugina IM, Plenkova IM, Pletnikova TP, Plotnikova IN, Pogostina AL, Podgorbunskikh AP, Podugolnikova NV, Pozdnjakova OA, Poletaeva LK, Polishchuk NG, Polikarpov LF, Polinsky DG, Politova LM, Polyakov IM, Ponomareva NV, Ponomareva OV, Popkov EV, Popov RV, Popova ZV, Popova IL, Popova MP, Popovic SV, Pospelov NV, Potekhina JA, Privalov OJ, Prishchenko EG, Prokop’ev AV, Prokofiev DA, Prokudin IA, Prosyannikova NY, Purgina GY, Pushkin OR, Razvedchenko NV, Rasstrygina LM, Ratnikova IY, Revina NA, Reznichenko MI, Resetnicova NG, Resetnicova SI, Rogach ME, Romashkina LA, Rosin DE, Rossal LL, Rossolovich SV, Rothenberger VR, Rudnev GA, Rudomanova VV, Rusanov TS, Rusovich NG, Rybakova TY, Rybinskaya VS, Ryzhkova SI, Ryzhova LA, Ryskina YS, Ryazanov EV, Ryazantseva LG, Sabirov RA, Sabitova OV, Savina LG, Savkina AI, Savushkina GV, Sazonov TV, Salishcheva TL, Samojlenko TV, Sanatina LI, Sannikov MI, Sapronova OP, Saprykina EE, Safonov EV, Safronova VV, Svetchikov IV, Svistova EV, Svistunova LN, Sevastianova OP, Sedov IN, Sekretareva NI, Selezneva NI, Selezneva SM, Sam AS, Semenova NN, Semenova TV, Semenycheva AA, Semycheva SG, Senkova LV, Sergeev AS, Sergeeva TS, Sergius GV, Serikov ED, Skachkova NA, Skvortsov IY, Skudarnova TV, Skuratova NN, Slepchenkova NG, Smirnova EN, Sozonik EP, Sokolov VA, Solovyov LV, Solohova TI, Sonin SV, Sorokin OA, Sosnowski OI, Sohonchuk AN, Starikov AV, Starichkova EM, Startseva EI, Stepanenko EO, Stepanova NA, Stepura LI, Studennikova SP, Stupkin EH, Stupnikova NK, Subach EV, Subbotina TY, Suvorova SG, Sunchalieva FD, Suryaninova MA, Sousse VA, Suslikova AV, Sukhocheva EI, Suchkova NV, Sychevskii VP, Tabolina SV, Talanov GB, Tepleeva FB, Tepliakova YN, Ternavschi SP, Tipsina EM, Tito NA, Tikhomirov YI, Tkachenko SN, Tolmachev EA, Tomilova VI, Tomkina TM, Tribun OF, Trubitsyna IV, Tuktarova FS, Turenko EV, Tynyankina EE, Urinsky ME, Usankina SA, Usachenko NN, Ustinov IV, Ushakov NI, Fanina ER, Fateev NV, Fateeva OV, Fahretdinova LZ, Fedotova NG, Fedotova YA, Fedrunova OV, Fedko NP, Filatkina EA, Filene LA, Filippenko OA, Filonov YV, Firsh EP, Fomenko AV, Fominih EV, Frolov OM, Fuchs NV, Khaziyeva AS, Hayrtdinov ShF, Hairulina OM, Hamedova MSh, Harrasova GZ, Hahaleva NV, Hisamutdinova TA, Hismatulin AK, Hovanskaya MN, Hojaeva DV, Holov TA, Holueva NE, Hokhlova EP, Hrabrova SN, Huzina AA, Tsyrulnik GN, Chaadaeva MI, Chashina OI, Cheypesh IS, Chelyadenkova LR, Cherenkov OP, Cherkashina ML, Cherneckaya IA, Chernikova IV, Chernikova TM, Chernova VN, Chernish VN, Chernysheva VA, Chernysheva GN, Chernysheva IN, Chernysheva NG, Chernysheva TV, Chernyaeva EV, Chernjakova LY, Chernjakova NS, Chikunov VD, Chirkova SA, Chistyakov AG, Chumakova OV, Shabalova VA, Sharifyanova GN, Shelankova MV, Shelegeda EJ, Shelekova AV, Shelipova AA, Shershneva LV, Shib TL, Shigina AV, Shilov LI, Shipilova TG, Shirochkina LV, Shirshakova OV, Shmelev EA, Shmelev KI, Shokurov NA, Shoshina OY, Shtembik VG, Shtets AV, Shtyrov TV, Shugaeva EN, Shulenina GV, Shulikova IE, Shumnik SG, Sherbak MF, Shcherbakova NI, Schinova ON, Shchukina HV, Yumaev NG, Yreva LS, Yusova IA, Yakovenko EI, Yakubenko AN, Yakupova RR, Yakupova SP, Yanina YA, Yarchenko SV.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This study was funded by Servier, Russia. Medical writing assistance was provided by Julie Salzmann, PhD, on behalf of Servier, France.
Conflict of interest
YAK would like to declare the following: research grants from AstraZeneca; consultancy for Novartis, MSD, Sanofi-Aventis, Servier, and AstraZeneca; and lectures for Novartis, MSD, Sanofi-Aventis, Pfizer, Servier, Boehringer Ingelheim, Bayer, and AstraZeneca.
Ethical approval
At the time the study was performed, ethics committee approval was not required in the Russian Federation if the investigated drug was used in strict accordance with its registered indication, which was the case for the FORTISSIMO study.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Members of FORTISSIMO physicians are listed in Acknowledgements.
An erratum to this article is available at http://dx.doi.org/10.1007/s40261-017-0551-y.
Rights and permissions
About this article
Cite this article
Karpov, Y.A., On behalf of the FORTISSIMO physicians. Full-dose Perindopril/Indapamide in the Treatment of Difficult-to-Control Hypertension: The FORTISSIMO Study. Clin Drug Investig 37, 207–217 (2017). https://doi.org/10.1007/s40261-016-0479-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0479-7