Abstract
Rationale
The increased risk of asthma mortality in association with long-acting β2-agonist (LABA) monotherapy is well documented but the risk associated with LABA plus inhaled corticosteroid (ICS) therapy remains unclear.
Objective
We assessed the feasibility of a large pharmacoepidemiological study to compare the effect of combined LABA + ICS therapy with non-LABA maintenance therapy on the risk of asthma mortality.
Methods
This observational retrospective study used electronic data from ten US data partners to construct a cohort of patients with persistent asthma (defined as: four or more asthma maintenance medication dispensings in 12 months and a code diagnosis of asthma). Asthma deaths were determined by linking patient data with the National Death Index.
Results
From 5,881,438 asthma patients, a cohort of 994,627 met the criteria for persistent asthma and provided 2.4 million person-years of follow-up. The total number of deaths was 278 with only three of these occurring after incident exposure to an asthma maintenance medication. The overall pooled asthma mortality rate, standardized by age and data partner, was 1.16 [95 % confidence interval (CI) 0.98–1.34] per 10,000 person-years; crude mortality rates (per 10,000 person-years) increased with age and were higher in female individuals (1.34; 95 % CI 1.15–1.55) than in male individuals (0.92; 95 % CI 0.74–1.12).
Conclusions
Despite a cohort size of almost 1 million asthma patients, the asthma mortality risk associated with combined LABA + ICS therapy could not be determined. This study showed that very few patients with persistent asthma have asthma-related deaths, and confirmed that those who die are more likely to be older and female.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In a retrospective cohort of almost 1 million patients with persistent asthma, with 2.4 million years of follow-up, the asthma mortality risk associated with combined long-acting β-agonist/inhaled corticosteroid therapy could not be determined. |
Very few patients with persistent asthma have asthma-related deaths; those who die are more likely to be older and female. |
1 Introduction
Asthma is a heterogeneous chronic disease afflicting approximately 300 million individuals worldwide [1], and is responsible for the loss of an estimated 15 million disability-adjusted life-years annually [2]. Uncontrolled asthma is associated with an increased healthcare and social burden [3–5]. Despite this, asthma mortality rates have progressively declined over the past 20 years [6], and this is largely attributed to the improved management and treatment of asthma, including the effective use of inhaled corticosteroids (ICS) [7].
Although deaths due to asthma are rare, it remains important to learn more about the causes and characteristics of these potentially preventable fatalities. In the last decade, much attention has focused on the reported safety risks of long-acting β2-agonist (LABA) monotherapy following the results from the Salmeterol Multicenter Asthma Research Trial (SMART) [8] and a review of clinical data for formoterol [9], which raised concerns about increased severe asthma-related events in relation to LABA therapy. As a result, the US Food and Drug Administration issued a requirement for LABA medication package inserts and a ‘boxed’ warning of the risks, and mandated the manufacturers of LABAs to perform five, large post-marketing safety trials, all comparing the treatment of LABA with concurrent ICS therapy with ICS alone [10]. While the majority of these trials are ongoing, the results of the first completed study showed no increased risk of serious asthma events with combined LABA + ICS (salmeterol + fluticasone propionate) compared with ICS (fluticasone propionate) alone [11]. These results are consistent with those of observational health database studies that have reported treatment with combined LABA + ICS to be at least as effective as ICS alone in reducing severe asthma exacerbations [12–14].
This article describes the clinical outcomes from the Asthma Safety Observational Study (ASSESS), a large cohort feasibility study that aimed to use multiple electronic data sources to compare the risk of asthma mortality associated with combined LABA + ICS therapy with non-LABA maintenance therapy.
2 Methods
A detailed description of the methodology and feasibility of ASSESS has been reported previously [15], and is summarized here. ASSESS was a retrospective cohort study that used electronic data from ten collaborating institutions (data partners) in USA from January 1, 2000 to December 31, 2010. The data partners conducted analyses using electronic healthcare claims or electronic medical record data. In qualifying as a collaborative partner, each institution could access all required data during the relevant enrollment period, could identify patients and therefore retrieve information on deaths from the US National Death Index (NDI) database, and work under a commonly applied protocol. Each data partner constructed a general asthma cohort (patients with an International Classification of Diseases code diagnosis of asthma and at least one recorded pharmacy dispensing of an asthma maintenance medication). Central programming and a common data model based on a validated algorithm [16] were then used to provide a cohort of patients aged 4–100 years with persistent asthma defined as: (1) four or more dispensings for any asthma maintenance medication recorded during any 12-month interval of continuous health plan enrollment, and (2) an International Classification of Diseases diagnosis of asthma between January 1, 2000 and the date of the fourth qualifying medication dispensing (cohort entry date).
The study protocol was granted an institutional review board exemption by each local institution’s institutional review board owing to the anonymization of data before being provided to the coordinating center.
2.1 Asthma Medication Exposures
Asthma medication categories of interest were: (1) LABA + ICS (of primary interest), in a single inhaler or in separate inhalers dispensed concomitantly; (2) ICS monotherapy, ICS dispensed without concomitant non-ICS controller medication and with no dispensing of any non-ICS controller medication in the 12 months before the ICS; and (3) ICS + non-LABA controller therapy, ICS dispensing(s) plus concomitant dispensing(s) of theophylline, leukotriene receptor antagonist, or other non-LABA controller. The asthma index date was defined as the first new use of a medication in any of the above-mentioned exposure groups on or after the cohort entry date.
Once patients entered the persistent asthma cohort, they were followed up until the earliest of (1) start of a health insurance enrollment gap greater than 35 days, (2) end of enrollment, (3) date of death, (4) date of dispensing of a prescription for omalizumab, or (5) end of the study period.
2.2 Asthma Mortality
The primary outcome was death from asthma. Deaths and causes of death were ascertained by individual data partners by linking patient data with the US NDI, and using a match-rating computer algorithm adapted by the coordinating center. The coding for assignment of which deaths were ‘from asthma’ was accepted without manual review of the NDI output.
2.3 Statistical Analysis
Descriptive statistics were used to characterize the asthma cohort. The overall crude mortality rate was the sum of the number of asthma deaths across all data partners divided by the sum of the total number of person-years across data partners. Rates, overall and stratified by age and sex, were expressed per 10,000 person-years, with 95 % confidence intervals (CIs) estimated using the Poisson distribution in Episheet [17]. The overall asthma mortality rate was standardized to the age distribution of person-years for the index LABA + ICS exposure category across research partners.
3 Results
3.1 Patient Characteristics of the Persistent Asthma Cohort
A total of 5,881,438 patients from the ten participating data partners met the criteria of having asthma. From this group, study criteria were applied and yielded a persistent asthma cohort of 994,627 patients (Table 1). During the 10-year follow-up period, 14.5 % of the persistent asthma cohort initiated one of the asthma treatments of interest, with the most common treatments being ICS monotherapy, ICS + LABA (predominantly Advair®), and ICS + leukotriene receptor antagonist. From the total 2,399,564 person-years of follow-up, the most common age group was 40–64 years, representing 959,165 person-years (40 %), and female individuals represented 1,373,534 person-years (57 %).
3.2 Asthma Mortality
The total number of asthma deaths identified in the persistent asthma cohort, across all data partners, was 278, and of these, only one occurred during a period of index exposure (i.e., first medication taken from exposure categories of interest during observation period), and two occurred during a period of subsequent new exposure (i.e., subsequent medication taken from exposure categories of interest during the observation period) (Table 1). The overall crude asthma mortality rate was 1.16 (95 % CI 1.03–1.30) per 10,000 person-years. The pooled overall rate, standardized by age and data partner, was 1.16 (95 % CI 0.98–1.34) per 10,000 person-years (Table 1).
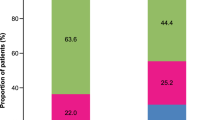
Of the 278 patients who died of asthma during the follow-up period, the number of deaths reported per age group was: 8 for age 4–11 years (number of person-years, n = 507,380); 4 for age 12–17 years (n = 247,595); 36 for age 18–39 years (n = 480,427); 120 for age 40–64 years (n = 959,165); 66 for age 65–79 years (n = 171,151); and 44 for age ≥80 years (n = 33,846). The pooled mortality rate per 10,000 person-years increased with age (Fig. 1). A higher number of the deaths were among female individuals (n = 184), and the pooled mortality rate per 10,000 person-years was higher in female individuals (1.34; 95 % CI 1.15–1.55) than in male individuals (0.92; 95 % CI 0.74–1.12).
4 Discussion
The initial aim of this observational study was to assess the feasibility of a comparative safety pharmacoepidemiological study on the association between combined LABA + ICS therapy and asthma mortality in patients with persistent asthma. Then, if it was possible to generate a meaningful result, we planned to compare the effect of combined LABA + ICS therapy with non-LABA maintenance therapy on the risk of asthma mortality in this very large cohort of patients. As previously reported, we demonstrated a successful collaboration between multiple institutions to produce the current largest cohort of patients that has been used to study the association between combined LABA + ICS therapy and asthma mortality [15]. Despite a huge dataset, the number of deaths reported to be due to asthma was so infrequent that the study did not have the statistical power to definitively answer the question of risk of mortality in association with combined LABA + ICS therapy. The feasibility precision analyses resulted in a very low probability (less than 0.10) of meeting the pre-defined thresholds with respect to the mortality rate ratio (MRR) (upper 95 % confidence limit (CL) of the MRR would be less than 1.4 if the true MRR was 1.0) and rate difference (RD) (upper 95 % CL of the RD would be (1) less than 0.4 and (2) less than 0.8 per 10,000 person-years, if the true RD was 0) for combined LABA + ICS therapy compared with non-LABA medications [15]. Despite this, the low number of asthma deaths per se does suggest that if there is any such attributable risk, it is extremely small.
The reported benefit:risk profile of LABA treatment in asthma is an ongoing debate, and this study highlights the challenges faced in designing studies to measure the effects of treatment on the endpoint of asthma mortality. The study results indicate that neither an observational nor a randomized controlled trial (RCT) study design can be of sufficient magnitude to answer the key question of whether adding a LABA to ICS, when the use of the ICS is assured, increases the risk of asthma death and suggests that the results of the ongoing Food and Drug Administration-mandated RCTs will also be underpowered for this endpoint. Recognizing that the rate of asthma-related deaths and asthma-related intubations are infrequent, these RCTs will most likely be assessing the risk of asthma-related hospitalization and not death. The results of the first completed RCT which enrolled 11,679 patients, reported small numbers of serious asthma-related events overall and no difference in the composite primary endpoint between the LABA + ICS (salmeterol + fluticasone propionate)- and ICS (fluticasone propionate)-only groups [11]. There were no asthma-related deaths reported in the trial, supporting the findings of the current study that asthma deaths per se are rare.
Our findings in this persistent asthma cohort, of a trend of increasing asthma mortality rate with age, and a greater mortality rate in female individuals compared with male individuals, are consistent with data reported in the US National Surveillance of Asthma summary [18]. The overall mortality rate of 1.16 per 10,000 persons is also consistent with a rate of 1.4 reported in 2009 in the US summary, and provides reassurance about the message that asthma mortality rates have declined to their lowest level over the last 20 years [6]. Our data provide a clear message for clinicians that very few managed care patients with asthma have asthma-related deaths. While this may be largely attributed to improved treatment, this may also reflect the use of self-management plans and a greater public awareness of asthma and its proper management [10, 19]. The limitations of our dataset did not allow us to evaluate any temporal relationship between asthma mortality and usage of ICS- and LABA-containing products, but temporal trends using other data sources clearly show a decrease in asthma mortality concurrent with an increase in the use of ICS and ICS/LABA over the period of 1991–2014 (Fig. 2).

Data sources: 1Vector One: National (VONA) from Verispan (1991–2004); IMS Health (2005–2014); prescriptions are not disease specific, 2DiSantostefano et al. [22], 3American Lung Association Epidemiology and Statistics Unit Research and Scientific Affairs [23], 4Deaths: Final data for 2010–2014; CDC/NCHS, National Vital Statistics System
US prescriptions for LABA- and ICS-containing products1,¥ and asthma death rate2,3,4,*. ¥Change in data vendor for prescription data from Verispan to IMS Health, *1979–1998 rates reflect the International Classification of Disease (ICD) 9th Revision Code; 1999–2013 rates reflect the ICD 10th Revision Code. ICS inhaled corticosteroid, LABA long-acting β2-agonist
The current study relied on using the US NDI to capture asthma deaths and with no gold standard as a comparator, we cannot determine the validity of this method. Previous studies have shown that asthma mortality may be underestimated when using asthma as the primary cause of death on death certificates, and in older people (in whom the rates are higher) there is a higher likelihood of potential misclassification (e.g., chronic obstructive pulmonary disorder, pneumonia) [20, 21]. The inclusion of asthma in any position on the death certificate (not just primary) would have increased the sensitivity of our classification and, as reported by To et al., the even wider broadening of groups of conditions (asthma-contributing classification) would enable the further exploration of the contribution of asthma to mortality risk estimates. ASSESS was designed with the objective of assessing the feasibility of evaluating a risk associated with a class of medications that emerged in a RCT, we therefore erred on the side of matching our methods to what was used in the RCT for the sake of consistency (a conservative approach). A new study would likely be more successful in identifying asthma mortality by using the approach taken by To et al., i.e., by analyzing sub-populations that include both asthma-specific and asthma-contributing mortality [20].
This type of study does have other limitations. The diagnosis of persistent asthma relied on administrative claims data and the assessment of exposure was based on the medication dispensed and not the drug taken. In addition, the large attrition rate between the general asthma cohort and the persistent asthma cohort may suggest a mis-identification of some patients as not having persistent asthma. The large attrition may also have been related to the broad criteria for defining the general asthma cohort, which aimed to maximize sensitivity in identifying asthma patients, vs. the persistent cohort, which was strictly defined based on a validated algorithm; the latter arguably a strength of this study. Nevertheless, this type of study design did allow for the evaluation of almost 1 million asthma patients, a sample size that would be infeasible using a RCT design.
5 Conclusion
In summary, despite a very large cohort of patients, identified over 10 years of clinical practice, from ten healthcare systems across USA, the small number of asthma deaths meant that this study did not yield a sufficient sample size to evaluate the putative excess risk of asthma mortality associated with combined LABA + ICS therapy relative to non-LABA therapy. Importantly, this study showed that very few patients originally meeting the criteria for persistent asthma have asthma-related deaths, and confirmed that those who die are more likely to be older and female. Although the increasing availability of very large real-world datasets allows us to evaluate possible adverse drug events that could not previously be studied, some questions remain unstudiable because of their very low event rates. Until linked real-world health data infrastructure for research takes another leap forward, to enable studies of very rare events, the low frequency of asthma deaths should be factored into the development of future research priorities.
References
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2016. Available from: http://www.ginasthma.com. Accessed 31 Aug 2016.
Masoli M, Fabian D, Holt S, Beasley R, for the Global Initiative for Asthma (GINA) program. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59:469–78.
Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24.
Guilbert TW, Garris C, Jhingran P, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. 2011;48:126–32.
Accordini S, Corsico A, Cerveri I, et al. Therapy and Health Economics Working Group of the European Community Respiratory Health Survey II. The socio-economic burden of asthma is substantial in Europe. Allergy. 2008;63:116–24.
Wijesinghe M, Weatherall M, Perrin K, et al. International trends in asthma mortality rates in the 5- to 34-year age group: a call for closer surveillance. Chest. 2009;135:1045–9.
Suissa S, Ernst P. Inhaled corticosteroids: impact on asthma morbidity and mortality. J Allergy Clin Immunol. 2001;107:937–44.
Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26.
Mann M, Chowdhury B, Sullivan E, et al. Serious asthma exacerbations in asthmatics treated with high-dose formoterol. Chest. 2003;124:70–4.
Camargo CA Jr. ICS-LABA safety studies in asthma: an update. Respir Drug Del. 2014;1:49–60.
Stempel DA, Raphiou IH, Kral KM, For the AUSTRI Investigators, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374:1822–30.
Wells KE, Peterson EL, Ahmedani BK, et al. The relationship between combination inhaled corticosteroid and long-acting beta-agonist use and severe asthma exacerbations in a diverse population. J Allergy Clin Immunol. 2012;129:1274–9.
Stanford RH, Blanchette CM, Roberts MH, et al. Effect of combination fluticasone propionate and salmeterol or inhaled corticosteroids on asthma-related outcomes in a Medicare-eligible population. Am J Geriatr Pharmacother. 2012;10:343–51.
Sadatsafavi M, Lynd LD, Marra CA, Fitzgerald JM. Dispensation of long-acting beta agonists with or without inhaled corticosteroids, and risk of asthma-related hospitalisation: a population-based study. Thorax. 2013;69:328–34.
Tennis P, Johannes C, Camargo C, et al. Feasibility of ruling out small treatment-associated increase in asthma mortality risk. Pharmacoepidemiol Drug Saf. 2013;22(Suppl 1):34–5.
Schatz M, Zeiger RS, Yang SJ, et al. Relationship of asthma control to asthma exacerbations using surrogate markers within a managed care database. Am J Manag Care. 2010;16:327–33.
Rothman KJ. Episheet: spreadsheets for the analysis of epidemiologic data. October 2012. Available from: http://www.krothman.org/episheet.xls. Accessed 31 Aug 2016.
Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat. 2012;3:1–67.
Beasley R, Crane J. Reducing asthma mortality with the asthma self-management plan system of care. Am J Respir Crit Care Med. 2001;163:3–4.
To T, Simatovic J, Zhu J, et al. Asthma deaths in a large provincial health system: a 10-year population-based study. Ann Am Thorac Soc. 2014;11:1210–7.
McCoy L, Redelings M, Sorvillo F, Simon P. A multiple cause-of-death analysis of asthma mortality in the United States, 1990–2001. J Asthma. 2005;42:757–63.
DiSantostefano RL, Davis KJ, Yancey S, et al. Ecologic analysis of asthma-related events and dispensing of inhaled corticosteroid- and salmeterol-containing products. Ann Allergy Asthma Immunol. 2008;100(6):558–65.
American Lung Association Epidemiology and Statistics Unit Research and Scientific Affairs. Trends in asthma morbidity and mortality. 2005, 2012. Available at: http://www.lung.org. Accessed 25 July 2016.
Acknowledgments
The authors thank RTI Health Solutions as the coordinating center responsible for conducting the study and all the institutions who collaborated in providing the data. We also thank David Hinds and Jean Strelitz for producing and formatting the ‘temporal trends between asthma mortality and usage of ICS- and LABA-containing products’ figure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was conducted by RTI Health Solutions with financial support from GlaxoSmithKline (GSK) (Protocol WEUSRTP4942). Editorial support was received in the form of draft manuscript development, collating of author comments, and copyediting, which was provided by Kate Hollingworth of Continuous Improvement Ltd. This support was funded by GSK.
Conflict of interest
EA is an employee of RTI Health Solutions. KJD and DAS are GSK employees and hold GSK shares. Dr. Camargo has performed asthma-related consulting for GSK, Novartis, and Teva; he has received research grants from Novartis. MS receives research funding from GSK, AstraZeneca/Medimmune, and Merck and is a consultant to Amgen and Boston Scientific. There are no other conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Camargo, C.A., Davis, K.J., Andrews, E.B. et al. Pharmacoepidemiological Study of Long-Acting β-agonist/Inhaled Corticosteroid Therapy and Asthma Mortality: Clinical Implications. Clin Drug Investig 36, 993–999 (2016). https://doi.org/10.1007/s40261-016-0448-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0448-1