Abstract
Aim
This study aimed to describe the prescription pattern of selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) in an Italian setting, focusing on therapy duration, switching and adherence.
Method
Historic cohort study, based on administrative databases of three Italian local health-units, was conducted. Patients with a prescription of antidepressants (ADs) in 2009 were enrolled and grouped into: (1) naïve, (2) already in treatment with the same drug and (3) already in treatment with a different drug. Therapy duration, switching and adherence [as medication possession ratio-(MPR)] were evaluated. A logistic regression model was performed to identify predictors of adherence.
Results
There were 88,755 subjects recruited: 37 % naïve, 58 % already in treatment with the same drug and 4 % with different drug. A treatment duration of ≤3 months was found in 41 % of naïve patients, whereas the majority of patients already in treatment had a duration of ≥6 months. Switches occurred in 0.7 % of the whole cohort and mostly occurred between two different SSRIs. The 63 % of naïve patients had a low adherence (MPR < 60 %), whereas a good adherence (MPR ≥ 80 %) was found in 58 % of patients already in treatment with the same drug and in 39 % of those already in treatment with different drug. Predictors of adherence were: female gender, increasing comorbidity and pain absence. All ADs, except for fluoxetine and venlafaxine, showed a better adherence than paroxetine.
Conclusion
Notwithstanding the difficulty to associate the AD prescription to the specific diagnosis of depression, this study highlighted a short duration and a low adherence of AD therapies, especially at the initiation of treatment. Physicians should carefully balance the need to prescribe these drugs, considering the great likelihood of a short duration of treatment and a very low level of adherence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite the fact that diagnosis was not retrieved from prescription data, the use of antidepressants resulted in a short duration and a low adherence, especially among naïve patients: 41 % of naïve subjects were treated with SSRIs or SNRIs for ≤3 months and 60 % of these subjects showed a low level of adherence. |
Physicians should carefully consider the choice to prescribe antidepressants as initial treatment of depression, taking into account the high likelihood of a short duration of treatment and a very low level of adherence to continuous treatment, especially in naïve subjects. |
The knowledge of antidepressant use patterns in real practice is crucial to establish what measures are needed to increase the appropriateness of antidepressant prescription, thus improving the standard of care for patients, and better allocating resources of the national health systems. |
1 Introduction
The consumption of antidepressants (ADs) has largely increased over the past decade, with an average increment of around 20 % per year in different European countries between 1980 and 2009 [1]. In Italy, as in various EU countries, the consumption of ADs, expressed as defined daily dose (DDD) per 1000 inhabitants/day, rose from 9 to 36, from 1995 to 2009, respectively [1].
In addition to economic crisis, the main reasons for this increase could be related to a better awareness of clinicians (especially general practitioners [2]) on depression disorders, or to the availability of active substances perceived as safer (i.e. selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) [3]. Apart from major depressive disorders, these new ADs are labelled for different therapeutic indications (e.g. anxiety disorders, panic disorders, obsessive–compulsive disorders, eating disorders, diabetic neuropathy), and they are prescribed also for off-label uses (e.g. sleeping disorders, migraine, neuropathic pain and nocturnal enuresis, functional dyspepsia) [4].
A cross-national comparison showed differences in prescribing patterns of ADs among countries [5], thus making the analysis of prescription attitudes in various geographical settings a crucial tool.
In Italy, the prevalence of depression is growing [6], and consequently relevant prescriptions of ADs are increasing (from 27.5 DDD/1000 inhabitants/day in 2005 to 39.1 DDD/1000 inhabitants/day in 2013), especially for SSRIs and SNRIs [7].
Although the increase in the use of AD seems to be related to the decrease in suicide rates in different European countries [1], concerns on the correct use of these drugs still remain.
Apart from unlabelled indications, the goal of the AD treatment is the virtual removal of depression symptoms and, to reach this objective, a continuous treatment is essential, as showed by various studies on this issue documenting that AD discontinuation may lead to a therapeutic failure [8]. For this reason, the National Institute for Health and Clinical Excellence (NICE) guidelines on depression advise a minimum of 6 months’ treatment [9]; whereas the World Health Organization (WHO) recommends at least 9–12 months’ of treatment [10].
The modification of AD therapy can occur in different ways, such as switching among active substances, discontinuing treatment or early stopping of therapy. Moreover, different factors could be related to non-adherence: side effects, severity of the disease, co-morbidity, personal characteristics and health provider support [11, 12].
2 Aim of the Study
The study aimed to describe the pattern of use of SSRIs and SNRIs in an Italian general practice setting of patients, by analysing duration of therapy, switching and adherence of AD therapy. Furthermore, this analysis investigated possible predictors of adherence, by identifying differences among patients naïve for treatment and those already in treatment.
3 Method
3.1 Data Source
Administrative databases of three Italian local health units (two located in Tuscany and one in Sardinia), covering approximately 1.5 million health-assisted individuals, were used. These data sources are complete and accurate, and they were previously used for several epidemiological research studies [13, 14].
In this study, the following databases were searched: the Health-Assisted Subjects’ Database, containing patients’ demographic data; the Medications Prescription Database, providing information on reimbursed prescriptions [e.g. prescription date, Anatomical Therapeutic Chemical (ATC) code [15] of the dispensed drug, number of packages]; Hospital Discharge Database, including all hospitalisation data [discharge diagnosis codes, classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [16] and diagnosis-related groups (DRG) [17]] Ambulatory Care Specialist, recording outpatient specialist services (visits, laboratory tests, diagnostic tests).
3.2 Study Design and Population
This study was an observational historic cohort analysis, with a whole observation period of 3 years (2008–2010).
Patients aged ≥18 years with at least one prescription of SSRI or SNRI (ATC codes: N06AB*, N06AX16, and N06AX21) from January 1st 2009 to December 31st 2009 were included.
The first AD prescription date, observed in the enrolment period, was considered as the index date.
Based on AD prescriptions in the 12 months preceding the index date, patients were grouped into three classes of exposure: (1) naïve for any AD treatment (patients without any AD prescriptions in the 12 months preceding the index date), (2) already in treatment with the same AD (patients with a prescription of the same active substance in the 12 months preceding the index date, including the index date) and (3) already in treatment with a different AD (patients with a prescription of AD in the 12 months preceding the index date but with a different active substance at the index date). In order to define subjects already in treatment with the same or different AD, the last prescription occurred in 12 months prior to the index date was compared with the AD prescription at the index date.
Each patient was followed-up for 1 year starting from the index date; subjects who died or moved to another Local Health Unit during the follow-up period were excluded from the analysis.
In order to characterise clinical status of each patients, the Charlson comorbidity index [18], which comprises different diseases (i.e. AIDS, solid tumour, liver disease, malignant lymphoma, leukaemia, diabetes, hemiplegia, ulcer disease, connective tissue disease, chronic pulmonary disease, dementia, cerebrovascular disease, cardiovascular disease), was computed through information derived from hospital discharges in the 12 months preceding the index date. Pain was an additional clinical parameter considered in the analysis, defined as one of the following criteria evaluated in the year preceding the index date: dispensation of at least four packs of non-steroidal anti-inflammatory drugs (NSAIDs) (ATC: M01A*); previous rheumatologic and/or orthopaedic visits; previous laboratory tests for rheumatoid factor, previous hospital admissions for cancer (ICD9: 140–239), previous hospitalisations related to diabetes (DRG: 213, 218, 219, 225) in combination with medications for diabetes (ATC: A10*) or neurological examination/angiological/peripheral vascular surgery.
3.3 Measures of AD Patterns of Use
AD pattern of use was described through three measures: therapy duration, switching and adherence. All these measures were evaluated during a 1-year follow-up period of each selected subject.
The duration of treatment was calculated as the days between the index date (first AD prescription) and the last prescription date, plus the numbers of days covered by the last prescription in terms of number of DDDs) [15].
Switching was evaluated by considering the first therapeutic change of each patient.
Treatment adherence was calculated by the medication possession ratio (MPR) defined as the total days of supply of medication divided by number of days between the first prescription and the last refill, including the duration of the last refill. A patient was defined adherent when the MPR was ≥80 %.
3.4 Statistical Analysis
Continuous variables were reported as mean and standard deviation, whereas categorical variables were expressed as numbers and percentages.
To investigate predictors of adherence, a multiple logistic regression model was performed by considering as possible predictors of adherence all collected variables (age, gender, Charlson comorbidity index, pain status; AD active substance). The model estimated the adjusted odds ratios (adjORs) for each variable by computing all other variables included in the analysis. For categorical variables, the most represented category was considered as reference, whereas for continuous variables the first value was considered as reference for the analysis.
A variable was defined a predictor of adherence when the adjOR was statistically significant [i.e. its 95 % confidence interval (CI) did not include 1], with a p value <0.05. This approach was conducted on the overall cohort and separately for the three exposure groups. All statistical analyses were carried put using SPSS statistical software for Windows, version 18.0.
4 Results
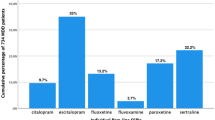
The study cohort included 88,755 subjects (5.9 % of residents) with at least one prescription of SSRI or SNRI during the observational period: 33,054 (37 %) were categorised as naïve patients, those already in treatment with the same AD 51,867 (58 %) as already in treatment with the same AD, 3814 (4 %) were classified as already in treatment with different AD. Males represented 29 % of the cohort and the average age was 61.3 ± 17.7 years. The percentage of male patients was slightly higher in the naïve group (32 %) and lower among patients already in treatment group (27 % in both groups of subjects who were already in treatment with AD). As expected, naïve patients were younger (average age 59.0 years) as compared to those already in treatment (62.7 and 62.3 years, respectively). Out of the overall cohort, 14.5 % of subjects experienced at least a hospitalisation in the 12 months prior to the index date: the majority had low (49 %) or medium (45 %) Charlson comorbidity index and this distribution was similar in the three exposure classes. A diagnosis of pain was reported in 20 % of the cohort and was higher in patients already in treatment with a different AD (25 %). The most prescribed AD was paroxetine (26 % of the whole cohort), followed by citalopram (21 %), sertraline (19 %) and escitalopram (17 %). This ranking was similar in all groups (Table 1).
The median of AD therapy duration during 1-year follow-up was 5.5 months for the naïve group, 12.4 for patients already in treatment with the same AD, and 11.8 for those already in treatment with different AD. The therapy duration (Fig. 1 panel a) was less than 3 months especially in naïve patients (41 %), whereas patients already in treatment had a duration of therapy of 6–12 months (32 % of subjects in treatment with the same AD and 32 % of those treated with different AD) or more than 12 months (56 % of those in treatment with the same AD and 41 % of those with different AD).
Measures of antidepressant (SSRI or SNRI) patterns of use: therapy duration (a), switching (b) and adherence (c), grouped for the three exposure classes (naïve, already in treatment with the same drug and already in treatment with different drug). In a denominators were represented by the overall subjects in each group: naïve 33,054; already in treatment with the same AD 51,867; already in treatment with different AD 3814. In b denominators were represented by patients with at least one switch: naïve 288; already in treatment with the same AD 266; already in treatment with different AD 90. In c denominators were represented by patients without switches in the study period: naïve 28,557; already in treatment with the same AD 45,482; already in treatment with different AD 2302. The error bars represent 95 % confidence interval. AD antidepressants, SNRI serotonin and norepinephrine reuptake inhibitors, SSRI selective serotonin reuptake inhibitors, treat. treatment
Switches occurred in 0.7 % of the entire cohort. These modifications of therapy (Fig. 1 panel b) mostly consisted in shifting between two different SSRIs, in all three groups (76 % in naïve patients, 69 % in patients already in treatment with the same AD and 71 % in those with different AD). Only a small proportion of patients, in all three groups, experienced switching from SSRI to SNRI and vice versa, whereas no switch from SNRI to another SNRI was found.
The level of adherence (Fig. 1 panel c) was evaluated by excluding subjects without switch during the study period. A low adherence with a MPR <60 % was recorded for 63 % of naïve patients, for 27 % of patients already in treatment with the same AD and for 51 % of those with different AD. An adequate adherence (MPR ≥ 80 %) was found in 29, 58 and 39 % of categories (naïve group, already in treatment with the same AD group and already in treatment with different AD group), respectively.
The analysis of predictors of adherent therapy (Table 2) showed that female gender was related to a good adherence in the overall cohort (adjOR 1.15; 95 % CI 1.11–1.19) in all three exposure classes. In comparison to an absence of comorbidity, a rise in comorbidity degree was related to a better therapy adherence (adjOR 1.24 for a medium degree of comorbidity, and adjOR 1.22 for a high degree of comorbidity). However, this correlation did not emerged in patients with very high comorbidity. These results were confirmed for naïve subjects and for patients already in treatment with the same AD. In patients with a diagnosis of pain, the adherence level to AD therapy was low, especially in the naïve group; indeed, in the whole cohort the adOR was 0.89 with 95 % CI 0.86–0.93 and in naïve class adjOR was 0.83 with 95 % CI 0.78–0.90. The analysis of adherence for different active substances found that all ADs, with the exception of fluoxetine and venlafaxine, were associated with a better adherence than paroxetine. In particular, duloxetine and escitalopram showed a two-fold increase in the likelihood of adherence in comparison with paroxetine (adjOR 2.29 95 % CI 2.09–2.50 for duloxetine and adjOR 2.08 95 % CI 1.99–2.17 for escitalopram). These findings were consistent across all the three different classes of exposure.
5 Discussion
This study describes the prescription pattern of SSRIs and SNRIs in an Italian setting, highlighting all clinically important therapy modifications. The SSRI-SNRI prescription rate found in our analysis is in line with similar epidemiological studies conducted in different Italian areas [19, 20].
In addition to previous studies in Italy [19–22], our study showed that the inappropriate use of AD, in terms of inadequate duration and low adherence level, concerns especially naïve patients rather than those already in treatment with the same or with different AD. The duration of AD therapy represents an important issue to achieve clinical response in the treatment of depression, as reported by the most authoritative guidelines underlining the importance of continued medication for at least 6 months after a remission of an episode of depression [9]. However, ADs are prescribed for other disorders apart from depression (e.g. nocturnal enuresis, anxiety disorders, eating disorders, sleeping disorders, migraine and neuropathic pain), where standard recommendations did not exist regarding the correct length of treatment, which often lasted until symptom resolution.
Our data showed that 41 % of naïve subjects were treated with an AD for ≤3 months; therefore it should be reasonable to assume this as an indicator (rate) of incorrect use of ADs. The short duration could be related to adverse effects of SSRIs/SNRIs (e.g. gastrointestinal disturbances, sexual dysfunctions, weight gain, effect on sleep [23]), which could scarcely be tolerated by some patients.
As regards switches between different ADs, our analysis showed that, during 1-year follow-up, a small percentage of subjects shifted between different SSRIs and, to a lesser extent, from a SSRI to a SNRI. Although switches from a SNRI to a SSRI were also observed, these occurred in a negligible number of subjects, in accordance with the guidelines that prefer SSRI as first-line treatment and to change drug class only when the patient response is inadequate or SSRIs are not well tolerated [9].
Another important clinical aspect related to AD therapy, and other chronic therapy, was represented by the level of adherence. Our results showed inadequate adherence >60 % of naïve subjects. Also, this finding could be interpreted as inappropriate treatment. Among predictors of adherence, our analysis identified the patients’ clinical status in terms of comorbidity complexity: subjects with medium or high levels of comorbidity were more compliant in comparison with those with a low level of comorbidity. By contrast, the very high level of comorbidity was not associated to a good level of adherence. This last finding could be related to the complexity of drug therapy administered to these patients that, in some cases, might compromise the continuous intake of AD. Our study also highlighted that diagnosis of pain is quite common among subjects receiving ADs and it is inversely related to adherence. This result may be due to the lack of a need for pharmacological treatment of depression secondary to chronic pain. Consequently, the presence of pain should be interpreted by the physician as an important factor to select the appropriate treatment of the depression status of the subject. However, the relationship between depression and pain is bidirectional (i.e. pain and depression could influence each other) [24] and this should be taken into account in the evaluation of poor adherence to AD therapy in the presence of pain.
Our analysis also showed differences in terms of adherence levels among active substances. In particular, both duloxetine and escitalopram seem to achieve an adequate level of adherence in comparison with paroxetine. These findings are in line with previous studies that reported escitalopram or both drugs as associated with a higher adherence [19, 21, 25, 26]. This could be justified by the different safety profiles of ADs (paroxetine resulted in less tolerance than other SSRIs in specific adverse events [27]) or by a channelling bias (when treating more severe disorders, the physician is likely to resorts to newest ADs) as argued by Poluzzi et al. [19].
5.1 Strengths and Limitation
The major strength of this study is represented by the large population followed throughout a long time period. This has been possible by using complete administrative databases, including validated data used in previous epidemiological studies [28–30]. However, the high number of subjects included in our analysis could have influenced the statistical significance of some results, but not the clinical implications of our findings.
Moreover, the identification of different exposure groups should be mentioned, since it allows identifying the actual inappropriate use of ADs. An additional strength is the analysis of pain as key clinical variables among confounders; indeed pain is often related to depression as reported by various authors [31, 32]. Finally, while various pharmacoepidemiological investigations were performed through administrative databases from local health authorities located in the Tuscany region of Italy (e.g. [13, 14]), to our knowledge this is the first drug utilisation study involving administrative data from a local health authority located in the Sardinia region.
Conversely, our approach has various limitations and weaknesses. The main limitation is the missing information on the reason for therapy modification: the administrative database did not collect these kinds of data. Moreover, Italian administrative databases did not have information on diagnosis, clinical course or health status of subject. Therefore, prescription data used in our study did not report the indication of AD use and this could have generated a misclassification of patients and could affect the interpretation of our findings, as stated by other authors [33]. However, this is supposed to be a minor issue: results from a study performed on databases recording the indication of use showed that SSRIs and SNRIs were prescribed to treat depression or feeling depressed in more than 50 % of cases [33], and SSRI prescriptions emerged as main predictors of depression when databases without indication were used to conduct analysis on this illness [34].
Another limitation of our study derives from the use of the Charlson index to evaluate the health status of each subject. Because this index is based on diagnosis recorded in hospital discharges, patients who did not require any hospitalisation during the follow-up period could be misclassified for this condition. However, this approach is widely used in epidemiology and it is considered a validated method to estimate the level of comorbidity of a cohort of patients. Moreover, as proxy for pain status of patients, we analysed only reimbursed prescriptions for NSAIDs (although this approach could have generated an incomplete identification of subjects suffering from pain, the number of patients using opioids is negligible, considering that in Italy their consumption is 10-fold lower than NSAIDs (2 vs. 23 DDD/1000 inhabitants/day [7]), whereas the over-the-counter number of NSAIDs cannot be captured using the reimbursed prescription databases. Also, the strategy to detect pain status through cancer ICD-9-CM codes could have generated a misclassification of subjects affected by pain; nevertheless this approach is more representative than using specific pain-related codes, often underused by clinicians.
Finally, the strategies used to measure the treatment duration and adherence did not take into account the possible gaps between prescriptions and this could have generated an overestimation of subjects with inadequate duration of therapy, or a misclassification of discontinuing patients as not adherent.
6 Conclusion
The present analysis suggests that the short duration and low adherence of ADs occur especially at the initiation of treatment. Therefore, physicians should balance the need to prescribe these drugs, taking into account the great likelihood of a short duration of treatment and a very low level of adherence to continuous treatment. This analysis provides physicians with several socio-demographic and clinical predictors, as useful tools to improve their prescription of ADs.
Improvement in the appropriateness of AD prescriptions will ultimately succeed in advancing the standard of care for patients who were exposed to fewer adverse reactions and better allocating the resources of the national health systems.
References
Gusmao R, Quintao S, McDaid D, et al. Antidepressant utilization and suicide in Europe: an ecological multi-national study. PLoS One. 2013;8:e66455. doi:10.1371/journal.pone.0066455.
Fernandez A, Pinto-Meza A, Bellon JA, et al. Is major depression adequately diagnosed and treated by general practitioners? Results from an epidemiological study. Gen Hosp Psychiatry. 2010;32:201–9.
MacGillivray S, Arroll B, Hatcher S, et al. Efficacy and tolerability of selective serotonin reuptake inhibitors compared with tricyclic antidepressants in depression treated in primary care: systematic review and meta-analysis. BMJ. 2003;326:1014.
Mercier A, Auger-Aubin I, Lebeau JP, et al. Evidence of prescription of antidepressants for non-psychiatric conditions in primary care: an analysis of guidelines and systematic reviews. BMC Fam Pract. 2013;14:55. doi:10.1186/1471-2296-14-55.:55-14.
Alonso J, Angermeyer MC, Bernert S, et al. Psychotropic drug utilization in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420:55–64.
Sorveglianza Passi. Sintomi depressivi nella popolazione adulta: prevalenze e caratteristiche socio-demografiche (Dati nazionali 2012). 2014. http://www.epicentro.iss.it/passi/rapporto2012/depressione.asp. Accessed 7 July 2014.
Gruppo di lavoro Osmed. L’uso dei farmaci in Italia—Rapporto OsMed 2013. 2014. http://www.agenziafarmaco.gov.it/sites/default/files/Rapporto_OsMed_2013.pdf. Accessed 24 June 2015.
Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9:41–6.
NICE—National Institute for health and Clinical Excellence. NICE guidelines [CG90]: depression in adults: the treatment and management of depression in adults. 2009. http://www.nice.org.uk/Guidance/CG90. Accessed 7 July 2014.
WHO—World Health Organization. Duration of antidepressant treatment. 2012. http://www.who.int/mental_health/mhgap/evidence/depression/q2/en/. Accessed 7 July 2014.
Lee MS, Lee HY, Kang SG, et al. Variables influencing antidepressant medication adherence for treating outpatients with depressive disorders. J Affect Disord. 2010;123:216–21.
Goethe JW, Woolley SB, Cardoni AA, et al. Selective serotonin reuptake inhibitor discontinuation: side effects and other factors that influence medication adherence. J Clin Psychopharmacol. 2007;27:451–8.
Cianferotti L, Parri S, Gronchi G, et al. Changing patterns of prescription in vitamin D supplementation in adults: analysis of a regional dataset. Osteoporos Int. 2015. doi:10.1007/s00198-015-3187-x.
Charlton RA, Jordan S, Pierini A, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG. 2015;122:1010–20.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2014. 2014. http://www.whocc.no/atc_ddd_index/. Accessed 19 Dec 2014.
WHO—World Healh Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2013. http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed 19 Dec 2014.
Italian Ministry of Health. Classification of Diagnosis Related Groups version 24. 2009. http://www.salute.gov.it/imgs/C_17_pubblicazioni_1094_allegato.pdf. Accessed 19 Dec 2014.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Poluzzi E, Piccinni C, Sangiorgi E, et al. Trend in SSRI-SNRI antidepressants prescription over a 6-year period and predictors of poor adherence. Eur J Clin Pharmacol. 2013;69:2095–101.
Parabiaghi A, Franchi C, Tettamanti M, et al. Antidepressants utilization among elderly in Lombardy from 2000 to 2007: dispensing trends and appropriateness. Eur J Clin Pharmacol. 2011;67:1077–83.
Trifiro G, Barbui C, Spina E, et al. Antidepressant drugs: prevalence, incidence and indication of use in general practice of Southern Italy during the years 2003–2004. Pharmacoepidemiol Drug Saf. 2007;16:552–9.
Aguglia E, Ravasio R, Simonetti M, et al. Use and treatment modalities for SSRI and SNRI antidepressants in Italy during the period 2003–2009. Curr Med Res Opin. 2012;28:1475–84.
Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3:22–7.
Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12:964–73.
Esposito D, Wahl P, Daniel G, et al. Results of a retrospective claims database analysis of differences in antidepressant treatment persistence associated with escitalopram and other selective serotonin reuptake inhibitors in the United States. Clin Ther. 2009;31:644–56.
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–58.
Purgato M, Papola D, Gastaldon C, et al. Paroxetine versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2014;4:CD006531. doi:10.1002/14651858.CD006531.pub2.:CD006531.
Carnovale C, Conti V, Perrone V, et al. Paediatric drug use with focus on off-label prescriptions in Lombardy and implications for therapeutic approaches. Eur J Pediatr. 2013;172:1679–85.
Kirchmayer U, Di MM, Agabiti N, et al. Effect of evidence-based drug therapy on long-term outcomes in patients discharged after myocardial infarction: a nested case-control study in Italy. Pharmacoepidemiol Drug Saf. 2013;22:649–57.
De BG, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–94.
Cheatle MD. Assessing suicide risk in patients with chronic pain and depression. J Fam Pract. 2014;63:S6–11.
Salama-Hanna J, Chen G. Patients with chronic pain. Med Clin N Am. 2013;97:1201–15.
Gardarsdottir H, Heerdink ER, van Dijk L, et al. Indications for antidepressant drug prescribing in general practice in the Netherlands. J Affect Disord. 2007;98:109–15.
Gardarsdottir H, Egberts AC, van Dijk L, et al. An algorithm to identify antidepressant users with a diagnosis of depression from prescription data. Pharmacoepidemiol Drug Saf. 2009;18:7–15.
Acknowledgments
The authors thank Dr. Emanuel Raschi (researcher at Department of Medical and Surgical Sciences, University of Bologna) for his comments and editing assistance in the revision of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
In order to guarantee privacy, patients’ personal information was encrypted and each subject was assigned an anonymous univocal code that allowed to link the searched databases. No patient identifiers were provided to the researchers. For this type of study formal consent is not required. The Ethics Committees of the involved local health units approved the study.
Conflict of interest
LDE, CP, DS and SB declare that they have no conflict of interest. AF provided consultancies and/or speaker and/or participation in advisory boards and/or research grants for Angelini, Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Lundbeck, Novartis, Otsuka, Pfizer, Roche, Takeda.
Rights and permissions
About this article
Cite this article
Degli Esposti, L., Piccinni, C., Sangiorgi, D. et al. Patterns of Antidepressant Use in Italy: Therapy Duration, Adherence and Switching. Clin Drug Investig 35, 735–742 (2015). https://doi.org/10.1007/s40261-015-0332-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0332-4