Abstract
Intensive care unit (ICU) patients receive highly complex care and often require sedation as part of their management. ICU sedation has traditionally been delivered using intravenous (IV) agents due to the impractical use of anaesthetic machines in this setting, which are used to deliver volatile sedation. Sedaconda anaesthetic conserving device (ACD)-S (previously known as AnaConDa-S) is a device which allows for the delivery of volatile sedation via the majority of mechanical ventilators by being inserted in the breathing circuit where the heat and moisture exchanger is normally placed. The National Institute of Health and Care Excellence (NICE), as part of the Medical Technologies Evaluation Programme, considered the potential benefits of using Sedaconda ACD-S compared to standard IV sedation in ICU patients. Here we describe the evidence evaluation undertaken by NICE on this technology, supported by CEDAR. CEDAR considered the evidence present in 21 publications that compared the clinical outcomes of patients receiving Sedaconda ACD-S-delivered sedation and IV sedation, and critiqued the economic model provided by the manufacturer. Clinical expert input during the evaluation process was used extensively to ensure that the relevant clinical evidence was captured and that the economic model was suitable for the UK setting. Due to the uncertainty of the evidence, sensitivity analysis was carried out on the key economic inputs to ensure the reliability of the results. Economic modelling has shown that Sedaconda ACD-S–delivered isoflurane sedation is cost saving on a 30-day horizon compared to IV sedation by £3833.76 per adult patient and by £2837.41 per paediatric patient. Clinical evidence indicated that Sedaconda ACD-S-delivered isoflurane sedation is associated with faster patient wake-up times than standard of care. Consequently, NICE recommended Sedaconda ACD-S as an option for delivering sedation in the ICU setting, but noted that further research should inform whether Sedaconda ACD-S–delivered sedation is of benefit to any particular subgroup of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The National Institute of Health and Care Excellence (NICE) concluded that Sedaconda ACD-S should be considered as an option for people requiring sedation in intensive care units. |
The NICE highlighted that further research is required to identify any patient populations that might particularly benefit from Sedaconda ACD-S–delivered sedation. |
1 Introduction
In the UK, the National Institute for Health and Care Excellence (NICE) Medical Technologies Guidance (MTG) programme produces recommendations on innovative medical devices and diagnostics to facilitate their adoption [1, 2]. This paper forms part of a series of publications commenting on the development of various MTGs, describing the development of the guidance on the Sedaconda anaesthetic conserving device (ACD)-S volatile anaesthetics system.
Here we discuss the manufacturer’s evidence submission, CEDAR’s original assessment report and how it was used by NICE in their development of MTG65 ‘Sedaconda ACD-S for sedation with volatile anaesthetics in intensive care’ [3]. CEDAR is a collaboration between Cardiff and Vale University Health Board and Cardiff University.
1.1 Background to the Technology and Application
Sedaconda ACD-S was originally called AnaConDa-S when the assessment process began, but the technology changed its name during the evaluation process. As such, while the device will be called Sedaconda ACD-S throughout this paper, the referenced literature will mostly use the AnaConDa-S name.
Sedaconda ACD-S is a device that can be attached to the breathing circuit of a mechanical ventilator to allow the delivery of isoflurane or sevoflurane sedation, without the use of a conventional anaesthetic machine in intensive care units (ICUs). The device is relatively compact, having a dead space of 50 mL, and replaces the heat and moisture exchanger typically used on ventilator circuits. The sedative is delivered to the device via an infusion pump connected via a sedative line, which delivers the drug into a porous rod that allows for its vaporisation. The device has a carbon filter, which minimises the loss of the agent during exhalation and allows for its desorption during subsequent inhalations, decreasing the amount of agent that needs to be used to achieve the desired alveolar concentration or end-tidal concentration. It provides a feasible alternative to intravenous (IV) sedation in ICUs, with a hope that it would allow for faster post-sedation patient recovery, resulting in less patient time spent on a ventilator and faster discharge from the ICU and hospital.
2 Decision Problem (Scope)
The National Institute for Health and Care Excellence defined the scope (population, intervention, comparator, outcomes) as:
-
Population: people who are invasively ventilated in intensive care using a mechanical ventilator but not a high frequency ventilator.
-
Intervention: Sedaconda ACD-S, and the original AnaConDa device, which had a 100 mL dead space.
-
Comparator: IV sedatives and standard vaporisers.
-
Outcomes: wake-up time after sedation; cognitive recovery; sedation efficacy (time to extubating, proportion of time within desired sedation level and titration ability using the Richmond Agitation-Sedation Scale); markers of cardiac injury, liver [function], gut [function], kidneys [function] and brain [function] for short-term operative sedation; sedation effectiveness in patients with life-threatening bronchospasm and asthma; oxygenation and inflammatory markers in patients with acute respiratory distress syndrome (ARDS); psychological outcomes (e.g., memories of hallucination, and long-term psychological morbidity, post-traumatic stress disorder [PTSD]); effectiveness of ventilation on people with bronchoconstriction; reduction of additional bronchodilators; duration of mechanical ventilation/increased ventilator-free days; length of stay in the ICU; hospital length of stay/hospital-free days; amount of volatile anaesthetic agent used; staff exposure to volatile anaesthetic agents; staff time in the ICU; amount of opioid drug used; device-related adverse events.
2.1 Equality and Diversity
The NICE equality assessment for Sedaconda ACD-S highlighted that volatile sedation might be of particular benefit to children and the elderly. It also highlighted that in the case of pregnant women, especially in the first trimester, clinical judgement has to be used to balance the risk of potential teratogenic effects of volatile anaesthetics to the foetus and the potential benefit to the pregnant women. Age and pregnancy status are protected characteristics.
3 CEDAR’s Review of the Evidence
The company, Sedana Medical, provided an evidence submission to NICE, presenting the available clinical and cost evidence alongside a de novo cost model. CEDAR’s assessment report aimed to provide the NICE Medical Technologies Advisory Committee (MTAC) with an independent appraisal of the evidence surrounding the use of Sedaconda ACD-S in the sedation of ICU patients.
3.1 Review of Clinical Effectiveness Evidence
The company submitted evidence based on 25 studies from 26 publications; one of these studies was provided in the form of an unpublished report, which has since been published during the MTG process [4]. CEDAR excluded nine of these studies; eight because the volatile sedation was not delivered through Sedaconda ACD-S [5,6,7,8,9,10,11,12], and one because it did not compare the effectiveness of sedation strategies [13]. The company submission also referred to four meta-analyses [14,15,16,17], but as all the relevant studies included in these meta-analyses were included in the evidence identified by the company and CEDAR, and some of the sedation in these studies was not delivered via Sedaconda ACD-S, none of the meta-analysis results were considered further in the evidence review. No evidence was found comparing Sedaconda ACD-S to standard vaporisers delivering volatile sedation.
CEDAR conducted its literature search in 10 bibliographic databases using both free text terms and indexed terms. Additionally, two trial registers, the company’s website and two registers of medical device related adverse events were also searched. Six additional publications relevant to the scope were identified. Two publications compared Sedaconda ACD-S to its 100 mL dead space predecessor [18, 19], one publication included additional outcomes of an already included study [20], and three studies compared Sedaconda ACD-S-delivered volatile sedation to IV sedation and reported relevant outcomes [21,22,23]. This resulted in a total of 21 studies (23 publications) included in the report, two of which only compared the two versions of the device.
Of the 21 publications (19 studies) comparing Sedaconda ACD-S-delivered volatile sedation to IV sedation, 2 compared isoflurane to propofol, 3 compared isoflurane to midazolam, 2 compared isoflurane to both propofol and midazolam, 1 compared both isoflurane and sevoflurane to propofol, 2 studies compared sevoflurane to both propofol and midazolam, 9 compared sevoflurane to propofol, 1 compared sevoflurane to midazolam and 1 compared sevoflurane to dexmedetomidine. At the time of assessment, CEDAR and NICE had access to unpublished data of one study on a confidential basis. While our narrative describes the results as presented in the NICE report, only the published data from that study are presented here, which might result in some discrepancies with the NICE assessment report, which has the original confidential data redacted.
3.2 Critical Appraisal of Studies
Of the included studies, 12 were randomised controlled trials (RCTs), 6 were cohort studies, 2 were case series and there was 1 before-after study. The overall risk of bias of the RCT was of ‘some concern’ in eight studies and ‘high’ in four studies, as evaluated by Cochrane revised risk of bias tool for randomised trials [24]. With respect to the non-randomised studies, two were judged as being of high quality, six were judged to be of medium quality, and one was of low quality, as evaluated by JBI’s checklists for case series and cohort studies, as well as the NHLBI Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group [25,26,27]. No study was conducted in the UK.
3.3 Clinical Results
Based on the available evidence, it was concluded that trials utilising the 100 mL dead space predecessor to Sedaconda ACD-S could be included in the evidence review, as the technologies worked on the same principle and achieved comparable results [18, 19]. A summary of these studies is presented in Table 1.
As the list of considered outcomes in the population, intervention, comparison and outcome (PICO) was lengthy, to help with the decision-making process, CEDAR collected the opinions of clinical experts as to which clinical outcomes they considered most important. From the collated responses, three outcomes were consistently mentioned as important: ventilation duration, wake-up time and sedation efficiency. Supplementary Table 1 provides a summary of these outcomes, as well as ICU and hospital lengths of stay (LoS) reported in the included studies. The included studies also reported on other outcomes mentioned in the scope, but as they were not of primary interest to the assessment of this technology we do not discuss these here and refer the reader to the NICE assessment report [3].
Of these five outcomes (ventilation duration, wake-up time, sedation efficiency, ICU and hospital LoS, it is only patient wake-up time that all the six publications that reported on it found this outcome to be significantly shorter in the Sedaconda ACD-S arm [4, 28,29,30,31,32]. Most studies that reported on the other four outcomes found no significant difference between study arms, although a minority reported results favouring the Sedaconda ACD-S arm, and none reported the Sedaconda ACD-S arm as inferior (Supplementary Table 1). As such, the use of the Sedaconda ACD-S arm is associated with faster patient wake-up times from sedation, which might allow for more flexible patient management.
3.4 Review of Safety Outcomes
CEDAR found no entries pertaining to Sedaconda ACD-S in the US Food and Drug Administration (FDA) MAUDE database. Two entries were found on the UK’s Medicines & Healthcare Products Regulatory Agency (MHRA) database. One pertained to inconsistent instructions for the product, which were rectified in 2005 when Sedana Medical took over the manufacturing of the device. The second entry was dated to January 2020 and concerned three defective batches that could have resulted in loose fitting connections. CEDAR also compiled a list of adverse events reported in the included publications, but as ICU patients are highly complex, often receiving multiple interventions and drugs, it is hard to reliably attribute any of these to the use of the Sedaconda ACD-S system [3]. Moreover, both Sedana Medical and the clinical experts highlighted that adverse events relating to the medication used with Sedaconda ACD-S should be distinguished from those relating to the device itself. Additionally, the experts highlighted that adverse events relating to Sedaconda ACD-S are likely to be similar to those relating to the use of heat and moisture exchangers. Therefore, CEDAR does not believe that there any safety concerns regarding the use of Sedaconda ACD-S that differ from those associated with the use of ventilatory circuits in general.
It is noteworthy that the use of inhaled sedatives in ICUs is an off-label use of these volatile agents. Nevertheless, it is not uncommon (particularly in the paediatric setting) to use medication off-label. Yet, the UK Health and Safety regulations (EH40/2005) has a long-term exposure limit for isoflurane stated as 50 parts per million (ppm) and time weight average of 383 mg/m3, although there is no stated limit for sevoflurane exposure [33]. All studies that reported on Sedaconda ACD-S use-associated isoflurane exposure, used the device with an appropriate scavenging system and reported isoflurane exposure levels as below 2 ppm except in care situations, where the ventilatory circuit might be opened, where the levels would not exceed 10 ppm [34,35,36]. As such the device is likely to be compliant with UK staff exposure regulations.
4 Economic Evidence
Sedana Medical conducted a literature search to identify economic publications relevant to the decision problem. Two records were identified by the company as relevant for inclusion [37, 38]. CEDAR’s own literature search did not identify any other relevant publications. While CEDAR agreed that these two studies were relevant, the evidence they provided was extremely limited. One was a conference abstract reporting a decision model comparing propofol or midazolam sedation to inhaled sedation delivered via Sedaconda ACD-S. The other study assessed the cost of isoflurane sedation delivered using Sedaconda ACD-S, and included a limited cost comparison.
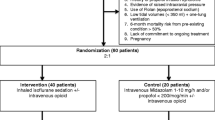
4.1 The Manufacturer’s de novo Economic Model
The manufacturer submitted a cost consequence analysis based on a simple decision tree (Fig. 1). The model assumes that patients are mechanically ventilated in ICU for at least 24 hours and takes a 30-day time horizon, and therefore did not utilise discounting. It utilises a National Health Service (NHS) and personal social services perspective.
4.2 Manufacturer’s Base-Case Results
For the base-case analysis, the company utilised data from patients in the SED001 trial (EudraCT trial number 2016–004551–67; funded by the manufacturer) that did not have their mode of sedation switched throughout the duration of follow-up [39]. These patients received either Sedaconda ACD-S-delivered isoflurane sedation or IV propofol sedation. The model also included several assumptions that were scrutinised by CEDAR:
-
Isoflurane is used as the inhaled sedative.
-
Propofol is the IV sedative that is used most commonly.
-
Sedation efficiency, tolerability and safety do not differ between modes of sedation.
-
Cost differences exist between sedation strategies with regard to the sedatives used, patient monitoring and sedative administration.
-
Intravenous sedation requires more frequent dose renewal (i.e., syringes with the drugs need to be changed more often).
-
Daily sedation interruption protocols are more likely with IV sedation.
CEDAR agreed with these assumptions. Clinical experts noted that while sevoflurane is also used, it’s use is less common than isoflurane. Intravenous midazolam use is more common as a sedative in children, and is included in scenario analysis.
Additional to the previously stated assumptions, CEDAR identified that the following assumptions were incorporated into the manufacturer’s model:
-
Mean adult weight is 70 kg.
-
The cost of a mixed gas analyser is included as a Sedaconda ACD-S sedation-associated cost.
-
The mixed gas analyser cost assumes that it is used for 180 days per year, and replaced every 5 years.
-
Training is required to move from an IV sedation strategy to one using Sedaconda ACD-S.
The result of the company’s base-case analysis was that Sedaconda ACD-S-delivered isoflurane sedation was cost saving by £3649 per patient when compared to IV-delivered propofol sedation.
4.3 Appraisal of Model Structure, Model Inputs and Changes Made by the EAC
CEDAR agreed with the submitted model structure, and made only minor changes to the inputs. The main change was to include a training cost of £621.60 per patient, based on training ICU team members who then deliver sedation to 100 patients. This was not included in the company model, and its inclusion is seen as a conservative estimate, as the price will decrease if more patients are sedated. As no sources were provided for a mean patient weight assumption of 70 kg, which determines the quantity of sedative required, CEDAR utilised the mean weight from the SED001 trial instead. Furthermore, while not all units required the purchase of a mixed gas analyser, this cost was kept by CEDAR as a conservative assumption. No source was provided to justify the assumption of 180 days per year use of a mixed gas analyser; however, this was accepted as having a minimal impact on the model.
When considering changes to be made to the model, CEDAR also made three additional assumptions:
-
Training costs involve all key ICU staff and are primarily incurred at the outset of the move to the use of inhaled sedation, as once it becomes standard practice it will become a normal staff training process.
-
As Sedaconda ACD-S training is provided free of charge, the training cost is associated with staff time.
-
The training cost assumes that 100 patients will be sedated using Sedaconda ACD-S, and as such, the per-patient cost will decrease if more patients are sedated using this device.
CEDAR made minor changes to the dosage and price of sedatives. While the company’s model utilised eMIT and Pharmex data for sedative prices alongside a dose of 3 mg/kg/h, CEDAR utilised prices from the British National Formulary (BNF) and a midpoint of the BNF recommended range for dose (2.15 mg/kg/h). For nursing costs associated with sedation, CEDAR agreed with the use of a band 6 nurse cost from Personal Social Services Research Unit, but included the overhead costs that had been removed in the company submission. Similarly, while both the company and CEDAR utilised the 2018/2019 NHS Reference Costs, the manufacturer’s model used national average costs for critical care, while CEDAR utilised adult critical care in standard location weighted mean, excluding the condition where ‘0 organs supported’.
4.4 Effects of Base-Case Changes Made by the EAC
Despite the addition of training costs, CEDAR’s base-case changes resulted in a potential increase of cost savings to £3833.76. This increase in cost saving is primarily associated with the higher cost and dosage of propofol and higher cost per ICU bed day in CEDAR’s model.
4.5 Sensitivity Analysis
In the company’s submission, duration of mechanical ventilation was identified as the main driver of cost savings. This was confirmed by CEDAR using one-way sensitivity analysis, and is linked primarily to the cost of increased ICU bed days. The company’s threshold analysis indicated that if duration of mechanical ventilation is the same between both sedation strategies, the duration of non-ventilated ICU days must be 0.33 days lower for Sedaconda ACD-S to not be cost incurring. Using CEDAR’s preferred inputs, this would be reduced to 0.2 days. Additionally, CEDAR carried out sensitivity analysis for a range of doses and costs for propofol and isoflurane, based on the different references for costs and isoflurane usage indicated by the clinical experts. Sedaconda ACD-S-delivered sedation proved to be cost saving across all the scenarios in this analysis.
4.5.1 Additional Scenario Analysis
Four additional scenarios were considered. In the first scenario, rather than using the mean duration of ventilation of all patients, the company used the mean duration of each study arm. This resulted in a potential cost saving of £5395.98 per patient when Sedaconda ACD-S was used, based on CEDAR’s preferred inputs. The second scenario calculated the duration of mechanical ventilation and ICU stay for the whole study population, including those patients who had their mode of sedation switched. In this scenario the Sedaconda ACD-S-associated cost saving was £1574.30 per patient when using CEDAR’s input values. The third scenario considered sevoflurane-delivered inhaled sedation compared to propofol IV sedation. This resulted in a Sedaconda ACD-S-associated cost saving of £2657.08 per patient. Lastly, costs of delivering inhaled isoflurane sedation in the paediatric population were considered, where the IV comparator is midazolam rather than propofol. This analysis was based on data from a different published study, and required the use of different critical-care costs and assumptions about body weight [40]. The company’s analysis indicated a Sedaconda ACD-S-associated cost saving of £5758 per patient, while CEDAR’s preferred inputs resulted in a potential cost saving of £2837.41 per sedated patient.
5 National Institute of Health and Care Excellence Guidance
5.1 Development of Guidance
NICE Medical Technologies Advisory Committee (MTAC) met in July 2021 and considered evidence from a range of sources, including the company’s submission, CEDAR’s report and testimony from clinical experts. The committee made provisional recommendations that went to public consultation.
During the consultation process, NICE received 16 comments from two consultees (the company and CEDAR). Comments covered issues of the technology name itself, licensing of isoflurane, discrepancies with CEDAR’s assessment report and wording changes. As these were only minor comments a second formal meeting between MTAC and other relevant parties did not happen.
5.2 Recommendations
Following a period of public consultation and a second committee meeting to discuss responses to the consultation, MTAC produced the following recommendations [41]:
-
Sedaconda anaesthetic-conserving device‑S (Sedaconda ACD‑S) is recommended as a cost‑saving option for delivering inhaled sedation in an intensive care setting when the volatile anaesthetics isoflurane or sevoflurane are being considered.
-
Further research is recommended to identify any health conditions or groups of patients that would benefit more from inhaled sedation with Sedaconda ACD‑S than from standard care.
6 Key Challenges and Learning Points
ICU patients are clinically a very complex and diverse population, therefore ascertaining the impact of any one intervention on their overall outcomes is very difficult. Moreover, evaluation of Sedaconda ACD-S posed a particular challenge as it was unclear whether any potential benefits of this intervention should be attributed to device or the volatile sedatives used with it. To overcome this challenge, CEDAR made extensive use of clinical expert input to decide which outcome measures were the most relevant for the assessment of this device.
Through discussions with clinical experts, CEDAR found that there is variation across the UK on how sedation in general and Sedaconda ACD-S in particular are being utilised. Guidance from the UK’s Intensive Care society on patient sedation is very generic, and practice differences are particularly wide between the adult and paediatric settings [42]. Some centres might only utilise the Sedaconda ACD-S system on specific patient populations, although there was a lack of evidence on pre-specified subgroups, and none of the of the studies included where carried out in the UK. As such, while CEDAR made extensive use of clinical expert input to ensure the relevance of the clinical evidence and appropriateness of the economic model to the UK as a whole, variations in local practice might have resulted in these assumptions being less reflective of how the device is utilised in some centres.
There was some uncertainty regarding the clinical evidence used in the economic modelling. The duration of ventilation data was taken from a subset of patients from the SED001 trial, meaning that the data were not powered appropriately for this outcome. Moreover, midazolam is the primary IV agent used in the paediatric setting, but the Krannich et al study, which was used as the basis for the economic model comparing Sedaconda ACD-S-delivered isoflurane sedation to midazolam IV sedation, was conducted in the adult population, and as such there is uncertainty about the applicability of its data to the paediatric population [40]. SED001 and Krannich et al were the largest trials included in this evidence review. CEDAR made extensive use of sensitivity analysis to ensure that the conclusion —that Sedaconda ACD-S delivered isoflurane sedation is cost saving—is robust with respect to the uncertainty in the data.
7 Conclusions
The evidence reviewed suggests that Sedaconda ACD-S-delivered isoflurane sedation in the ICU can improve patient outcomes relating to extubation and post-sedation wake-up times, and can be cost saving to the NHS. There is some uncertainty regarding some of the inputs driving the economic analysis, but CEDAR tried to minimise the uncertainty regarding these inputs through the use of sensitivity analysis. Moreover, analysis of ICU-patient outcomes is always difficult due to the complexity of the patients and the interventions they receive. In this case, it was particularly difficult to decide whether any clinical benefits arising from the use of Sedaconda ACD-S should be associated with the device or the volatile sedatives used with it.
The NICE recommended the use of Sedaconda ACD-S as an option for ICU patient care. Nevertheless, further research was recommended to identify whether any groups of patients would especially benefit from Sedaconda ACD-S-delivered sedation.
References
Campbell B, Campbell M. NICE medical technologies guidance: a novel and rigorous methodology to address a new health technology assessment challenge. Appl Health Econ Health Policy. 2012;10:295–7.
National Institute for Health and Care Excellence. Medical technologies guidance [Internet]. NICE. NICE. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-medical-technologies-guidance. Cited 4 Apr 2024.
National Institute for Health and Care Excellence. Sedaconda ACD-S for sedation with volatile anaesthetics in intensive care: Supporting documentation [Internet]. 2021: https://www.nice.org.uk/guidance/mtg65/documents/supporting-documentation. Cited 4 Apr 2024.
Meiser A, Volk T, Wallenborn J, Guenther U, Becher T, Bracht H, et al. Inhaled isoflurane via the anaesthetic conserving device versus propofol for sedation of invasively ventilated patients in intensive care units in Germany and Slovenia: an open-label, phase 3, randomised controlled, non-inferiority trial. Lancet Respir Med. 2021;9:1231–40.
Bellgardt M, Georgevici AI, Klutzny M, Drees D, Meiser A, Gude P, et al. Use of MIRUSTM for MAC-driven application of isoflurane, sevoflurane, and desflurane in postoperative ICU patients: a randomized controlled trial. Ann Intensive Care. 2019;9:118.
Daume P, Weis J, Bomberg H, Bellgardt M, Volk T, Groesdonk HV, et al. Washout and awakening times after inhaled sedation of critically ill patients: desflurane versus isoflurane. J Clin Med. 2021;10:665.
Gómez JI, Tamayo E, Cortejoso J, Cóbreces MJ. Isoflurano frente a propofol en la sedación tras cirugía cardíaca [Isoflurane versus propofol for sedation after heart surgery]. Rev Esp Anestesiol Reanim. 1995;42:261–8.
Guinot P-G, Ellouze O, Grosjean S, Berthoud V, Constandache T, Radhouani M, et al. Anaesthesia and ICU sedation with sevoflurane do not reduce myocardial injury in patients undergoing cardiac surgery. Medicine (Baltimore). 2020;99: e23253.
Kong KL, Willatts SM, Prys-Roberts C. Isoflurane compared with midazolam for sedation in the intensive care unit. BMJ. 1989;298:1277–80.
Meiser A, Sirtl C, Bellgardt M, Lohmann S, Garthoff A, Kaiser J, et al. Desflurane compared with propofol for postoperative sedation in the intensive care unit. BJA Br J Anaesth. 2003;90:273–80.
Millane TA, Bennett ED, Grounds RM. Isoflurane and propofol for long-term sedation in the intensive care unit. A crossover study. Anaesthesia. 1992;47:768–74.
Spencer EM, Willatts SM. Isoflurane for prolonged sedation in the intensive care unit; efficacy and safety. Intensive Care Med. 1992;18:415–21.
Sackey PV, Radell PJ, Granath F, Martling CR. Bispectral index as a predictor of sedation depth during isoflurane or midazolam sedation in ICU patients. Anaesth Intensive Care. 2007;35:348–56.
Landoni G, Pasin L, Cabrini L, Scandroglio AM, Baiardo Redaelli M, Votta CD, et al. Volatile agents in medical and surgical intensive care units: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2016;30:1005–14.
Jerath A, Panckhurst J, Parotto M, Lightfoot N, Wasowicz M, Ferguson ND, et al. Safety and efficacy of volatile anesthetic agents compared with standard intravenous midazolam/propofol sedation in ventilated critical care patients: a meta-analysis and systematic review of prospective trials. Anesth Analg. 2017;124:1190–9.
Kim HY, Lee JE, Kim HY, Kim J. Volatile sedation in the intensive care unit: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96: e8976.
Spence J, Belley-Côté E, Ma HK, Donald S, Centofanti J, Hussain S, et al. Efficacy and safety of inhaled anaesthetic for postoperative sedation during mechanical ventilation in adult cardiac surgery patients: a systematic review and meta-analysis. Br J Anaesth. 2017;118:658–69.
Marcos-Vidal JM, Merino M, González R, García C, Rey S, Pérez I. Comparison of the use of AnaConDa® versus AnaConDa-S® during the post-operative period of cardiac surgery under standard conditions of practice. J Clin Monit Comput. 2020;34:89–95.
Bomberg H, Meiser F, Zimmer S, Bellgardt M, Volk T, Sessler DI, et al. Halving the volume of AnaConDa: initial clinical experience with a new small-volume anaesthetic reflector in critically ill patients—a quality improvement project. J Clin Monit Comput. 2018;32:639–46.
Hellström J, Öwall A, Bergström J, Sackey PV. Cardiac outcome after sevoflurane versus propofol sedation following coronary bypass surgery: a pilot study. Acta Anaesthesiol Scand. 2011;55:460–7.
Foudraine NA, Algargoush A, van Osch FH, Bos AT. A multimodal sevoflurane-based sedation regimen in combination with targeted temperature management in post-cardiac arrest patients reduces the incidence of delirium: an observational propensity score-matched study. Resuscitation. 2021;159:158–64.
Jung S, Na S, Kim HB, Joo HJ, Kim J. Inhalation sedation for postoperative patients in the intensive care unit: initial sevoflurane concentration and comparison of opioid use with propofol sedation. Acute Crit Care. 2020;35:197–204.
Meiser A, Groesdonk HV, Bonnekessel S, Volk T, Bomberg H. Inhalation sedation in subjects with ARDS undergoing continuous lateral rotational therapy. Respir Care. 2018;63:441–7.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127.
Moola S, Munn Z, Tufanaru C, Aromataris EC, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris EC, Munn Z, editors., et al., JBI Man Evid Synth. JBI; 2020.
National Heart, Lung, and Blood Institute. NHLBI Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Sackey PV, Martling C-R, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the anesthetic conserving device. Crit Care Med. 2004;32:2241–6.
Hanafy MA. Clinical evaluation of inhalational sedation following coronary artery bypass grafting. Egypt J Anaesth. 2005;21:237–42.
Jerath A, Beattie SW, Chandy T, Karski J, Djaiani G, Rao V, et al. Volatile-based short-term sedation in cardiac surgical patients: a prospective randomized controlled trial. Crit Care Med. 2015;43:1062–9.
Mesnil M, Capdevila X, Bringuier S, Trine P-O, Falquet Y, Charbit J, et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med. 2011;37:933–41.
Hellström J, Öwall A, Sackey PV. Wake-up times following sedation with sevoflurane versus propofol after cardiac surgery. Scand Cardiovasc J SCJ. 2012;46:262–8.
Health and Safety Executive. EH40/2005 (Fourth Edition) Workplace exposure limits [Internet]. The Stationery Office; 2020. https://www.hse.gov.uk/pubns/priced/eh40.pdf.
Pickworth T, Jerath A, DeVine R, Kherani N, Wąsowicz M. The scavenging of volatile anesthetic agents in the cardiovascular intensive care unit environment: a technical report. Can J Anaesth. 2013;60:38–43.
Sackey PV, Martling C-R, Nise G, Radell PJ. Ambient isoflurane pollution and isoflurane consumption during intensive care unit sedation with the Anesthetic Conserving Device*. Crit Care Med. 2005;33:585.
Herzog-Niescery J, Vogelsang H, Gude P, Seipp H-M, Bartz H, Uhl W, et al. The impact of the anesthetic conserving device on occupational exposure to isoflurane among intensive care healthcare professionals. Minerva Anestesiol. 2018;84:25–32.
Sackey P, Mumby-Croft J, Last V, Hickey DA. A cost-consequence analysis of anaconda versus propofol and midazolam in the long-term sedation of critically-ill surgical patients from the UK NHS perspective. Value Health. 2018;21:S254.
L’her E, Dy L, Pili R, Prat G, Tonnelier J-M, Lefevre M, et al. Feasibility and potential cost/benefit of routine isoflurane sedation using an anesthetic-conserving device: a prospective observational study. Respir Care. 2008;53:1295–303.
EU Clinical Trials Register. EudraCT Number 2016-004551-67 [Internet]. 2021. https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-004551-67/results. cited 26 Jun 2024.
Krannich A, Leithner C, Engels M, Nee J, Petzinka V, Schröder T, et al. Isoflurane sedation on the ICU in cardiac arrest patients treated with targeted temperature management: an observational propensity-matched study. Crit Care Med. 2017;45:e384–90.
National Institute for Health and Care Excellence. Sedaconda ACD-S for sedation with volatile anaesthetics in intensive care: Guidance [Internet]. NICE; 2022: https://www.nice.org.uk/guidance/mtg65/chapter/1-Recommendations. cited 4 Apr 2024.
Grounds M, Snelson C, Whitehouse T, Willson J, Tulloch L, Linhartova L, et al. Intensive Care Society Review of Best Practice for Analgesia and Sedation in the Critical Care [Internet]. Sedation Committee of the Intensive Care Society United Kingdom; 2014. https://www.wyccn.org/uploads/6/5/1/9/65199375/sedation_for_patients_in_icu_2014.pdf.
Acknowledgements
We wish to acknowledge Helen Morgan (SURE, Cardiff University) and Sarah Kotecha (SURE, Cardiff University) for conducting the literature searches for this project. We thank Federica Ciamponi (NICE) and Kimberley Carter (NICE) for their support in managing this project and helpful advice. We thank Megan Dale (CEDAR) for proof-reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
MP, SO, LK and RM contributed to the preparation of this manuscript. RM reviewed the article, and can act as a guarantor for the overall content.
Funding
CEDAR was funded by the NICE Medical Technologies Evaluation Programme for its work.
Conflict of interest
Michal Pruski, Laura Knight and Rhys Morris are employees of Cardiff and Vale University Health Board. Susan O’Connell was an employee of Cardiff and Vale University Health Board at the time this research was conducted. Cardiff and Vale University Health Board is part of NHS Wales, which has a financial interest in the guidance on which this project is based.
Compliance with Ethical Standards
This summary of the Medical Technology Guidance was produced following the publication of the final guidance report, and NICE had an opportunity to comment on a draft of this manuscript. The article has not been externally peer reviewed by Applied Health Economics and Health Policy, but has been reviewed externally by NICE.
Additional information
Susan O’Connell has now moved institutions.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pruski, M., O’Connell, S., Knight, L. et al. Sedaconda ACD-S for Sedation with Volatile Anaesthetics in Intensive Care: A NICE Medical Technologies Guidance. Appl Health Econ Health Policy (2024). https://doi.org/10.1007/s40258-024-00903-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40258-024-00903-2