Abstract
Background and Objective
Deep brain stimulation (DBS) is an established treatment for Parkinson’s disease (PD) in patients with advanced motor symptoms with an inadequate response to pharmacotherapies. Despite its effectiveness, the cost effectiveness of DBS remains a subject of debate. This systematic review aims to update and synthesize evidence on the cost effectiveness of DBS for PD.
Methods
To identify full economic evaluations that compared the cost effectiveness of DBS with other best medical treatments, a comprehensive search was conducted of the PubMed, Embase, Scopus, and Tufts Cost-Effective Analysis registry databases. The selected papers were systematically reviewed, and the results were summarized. For the quality appraisal, we used the modified economic evaluations bias checklist. The review protocol was a priori registered with PROSPERO, CRD42022345508.
Results
Sixteen identified cost-utility analyses that reported 19 comparisons on the use of DBS for PD were systematically reviewed. The studies were primarily conducted in high-income countries and employed Markov models. The costs considered were direct costs: surgical expenses, calibration, pulse generator replacement, and annual drug expenses. The majority of studies used country-specific thresholds. Fourteen comparisons from 12 studies reported on the cost effectiveness of DBS compared to best medical treatments. Eleven comparisons reported DBS as cost effective based on incremental cost-utility ratio results.
Conclusions
The cost effectiveness of DBS for PD varies by time horizon, costs considered, threshold utilized, and stage of PD progression. Standardizing approaches and comparing DBS with other treatments are needed for future research on effective PD management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We observed that the cost effectiveness of deep brain stimulation for Parkinson’s disease varies by time horizon, costs considered, threshold utilized, and stage of Parkinson’s disease progression. |
Cost-utility analysis models should be interpreted with consideration of data limitations, such as the absence of long-term effectiveness data and the challenges in modeling disease complications. |
1 Background
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disorder that afflicts aging people globally [1]. The death of dopaminergic neurons in the substantia nigra pars compacta and the presence of alpha-synuclein protein in the brain are the hallmarks of PD [1]. Patients with PD exhibit a range of motor and non-motor symptoms: tremors, rigidity, and difficulty with movement, balance, and coordination [1]. Parkinson’s disease significantly impacts a patient’s quality of life, and managing the disease could be challenging for patients and caregivers [2]. Parkinson’s disease lacks a definitive cure; the treatments primarily involve the use of pharmacological agents to substitute for the loss of dopamine in the striatum, along with non-dopaminergic approaches to manage both motor and non-motor symptoms [1]. The progressive nature of PD poses challenges as patients become less responsive to pharmacotherapies over time, necessitating the need for alternative treatments to manage its symptoms effectively [3, 4].
Deep brain stimulation (DBS) is an effective option for patients with Parkinson’s disease who develop motor complications that do not respond to L-DOPA treatment [1]. Deep brain stimulation is a neurosurgical treatment that involves the implantation of electrodes into the subthalamic nucleus or globus pallidus internus regions of the brain [5, 6]. An implantable pulse generator connected with the electrodes generates electrical impulses for stimulation [5]. Studies have shown that DBS effectively reduces motor symptoms and improves the quality of life of patients with PD [2, 5, 7, 8]. Bilateral subthalamic nucleus DBS is suggested to reduce dopaminergic medications and bilateral globus pallidus internus DBS is suggested to reduce the severity of “on” medication dyskinesias [9]. Meta-analyses of randomized controlled trials have reported that DBS significantly improves the Unified Parkinson’s Disease Rating Scale II (activities of daily living) and the Unified Parkinson’s Disease Rating Scale III (motor scores) in advanced PD [10,11,12,13,14,15]. The potential long-term cost savings associated with DBS may justify its upfront expense, particularly if it reduces the need for costly medications or hospitalizations [16]. However, despite DBS’s demonstrated effectiveness, its higher cost relative to other treatments has raised concerns about its value in terms of overall health outcomes and resource allocation [17].
Several cost-utility analyses (CUAs) have evaluated the cost effectiveness of DBS for PD, with mixed results [18]. Some studies have suggested that DBS is a cost-effective treatment option compared with medical therapy alone [19,20,21,22]. In contrast, others have reported that DBS is not cost effective when considering the high initial cost of the procedure and the need for long-term maintenance [23].
The previous systematic reviews reveal the evolving landscape of cost-effectiveness studies in DBS for PD over time. Becerra et al. reported DBS cost effective for advanced PD despite a higher initial cost than best medical treatments (BMTs) [18]. Dang et al. observed variability in the incremental cost-utility ratios (ICURs) from model-based evaluations for DBS compared with BMTs [24]. Afentou et al. suggest tailored interventions because of varying PD progression, to enhance the quality of life for patients and caregivers [25]. Marsili et al. reported that Levodopa-Carbidopa Intestinal Gel and DBS are more efficacious than BMT but with higher costs for healthcare systems [26].
Because of the evolving landscape of newer publications on the cost effectiveness of DBS for PD, we conducted a systematic review to provide an updated synthesis of the available evidence. Further, we provided an incremental net benefit (INB) of the currency year of each study and we converted all ICURs in 2023 USD to determine the economic value of DBS for PD. Our review will help inform healthcare policy decisions with insights into the economic considerations of DBS as a treatment option for PD.
2 Materials and Methods
The study protocol was pre-registered with PROSPERO, CRD42022345508, and the study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27].
2.1 Data Sources, Eligibility Criteria, Screening, and Search Strategy
PubMed, Embase, Scopus, and the Tufts Medical Centers’ cost-effective analysis registry were searched [28] for cost-utility studies published from inception to 25 July, 2022 (Appendix I of the Electronic Supplementary Material [ESM]). The Population, Intervention, Comparator, Outcome (PICO) approach was used to construct the search terms. We included published CUAs that met the following eligibility criteria: patients with established PD requiring treatment and treated with DBS, studies reporting economic outcomes in ICURs per quality-adjusted life-year (QALY) or INB. We excluded studies with effectiveness measured other than in QALYs, reviews, letters, editorials, abstracts, books, reports, gray literature, and methodological articles. Appendix I of the ESM provides detailed search terms and search strategies.
2.2 Selection of Studies
English language studies that met the eligibility criteria, listed from the electronic database search, were screened independently by two reviewers (BSB and SK) for titles and abstracts using the Rayyan-web application [29]. After title and abstract screening and deduplication, the full text of the finalized studies was independently reviewed in detail by three reviewers (BSB, SK, and AS) using the Rayyan-web application. The reference list of recovered studies was examined for additional suitable papers. The independent assessors’ mutual agreement with the arbitrator (BSB) produced the final list of studies that met the inclusion and exclusion criteria, and data were extracted from the selected studies.
2.3 Data Extraction
Using a data extraction template adapted for the outcomes of interest, AS and SK extracted the following data from the eligible studies: author, year, country of setting, study/patient characteristics, intervention, comparator, and the general characterization of the model, which included model type, perspective, time horizons, discount rate, and currency year. Economic parameters included costs I, incremental costs (ΔC), clinical effectiveness (E), its incremental effectiveness (ΔE), ICURs, INB values, and their measures of dispersion (i.e., standard deviation, standard error, or 95% confidence interval). Willingness to pay and threshold were also extracted. From the cost-effective plane graph, we extracted ΔC and ΔE values using the Web-Plot-Digitaliser [30].
2.4 Data Preparation and Statistical Analysis
Incremental net benefit was calculated for the currency year of each study. Incremental net benefit is defined as INB = K × ΔE − ΔC, where K represents the willingness-to-pay threshold, ΔC denotes incremental cost (i.e., the difference in costs between intervention and comparator), and ΔE denotes incremental effectiveness (i.e., the difference in effectiveness between intervention and comparator). A positive INB favors intervention, i.e., intervention is cost effective, whereas a negative INB favors the comparator, i.e., intervention is not cost effective. We chose to present INBs because of the ambiguity in interpreting ICURs and their inherent limitations [31].
As the included studies reported results in different currencies and at different timepoints (years), ICURs were converted to a common currency (US dollar [USD] 2023), adjusted for inflation using the Consumer Price Index (CPI), and converted to purchasing power parity (PPP)-adjusted USD for the year 2023 [32]. This conversion was done using the formula:
All data were prepared using Microsoft Excel version 2019 [33] and using Stata software version 17 [34].
2.5 Risk of Bias Assessment and Quality Assessment
We assessed quality independently using the modified economic evaluation bias (ECOBIAS) checklist [35]. It considers both overall biases (11 items) and model-specific biases, including structure (four items), data (six items), and internal consistency (one item) [Fig. S1 of the ESM].
3 Results
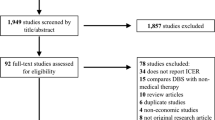
A systematic search of multiple peer-reviewed repositories yielded 2023 studies, from which 57 articles underwent full-text screening. Of these, 16 articles focused on CUAs were included in the final analysis. The screening process is provided in the PRISMA flow chart (Fig. 1). In this current synthesis, apomorphine subcutaneous infusion (ASBI) and continuous subcutaneous apomorphine infusion (CSAI) were not considered as part of the traditional BMT, which encompasses oral dopamine replacement therapies using levodopa as monotherapy or levodopa combined with other antiparkinsonian drugs. [36] This distinction arises because ASBI and CSAI involve a different route of administration and are employed as advanced or adjunctive interventions for PD. These 16 studies reported 19 comparisons [19,20,21,22,23, 37,38,39,40,41,42,43,44,45,46,47] (Fig. 1). Among these, DBS was compared with BMT in 12 studies with 14 comparisons [19,20,21,22,23, 38, 40,41,42,43, 45, 46], while single studies compared DBS with bilateral radiofrequency ablation [44], magnetic resonance-guided focused ultrasound thalamotomy (MRgFUS) [47], ASBI [39], and CSAI [37]. The majority of studies involved individuals with advanced PD, though one study focused on early-stage PD [19] and another on tremor-dominant PD [47] (Table 1).
Eleven studies with 13 comparisons adopted a payer’s perspective [19,20,21,22,23, 37,38,39,40, 45, 46], while the remaining five studies with six comparisons were conducted from a societal perspective [41,42,43,44, 47]. All studies included in our review were conducted in high-income countries, with four comparisons from the UK [21,22,23, 37], three comparisons from the USA [40, 43, 44] and Germany [19, 20, 37], two comparisons from Spain [38, 39], Taiwan [41], and Hong Kong [45], and one study each from Sweden [42], Japan [46], and Canada [47]. Walter and Odin provided data from two countries, Germany and the UK [37]. Ten studies utilized a Markov model [19,20,21,22, 37, 39,40,41,42, 46], while two studies employed a decision tree [44, 47] and prospective studies [38, 45], respectively. Furthermore, McIntosh et al. conducted an alongside trial [23], and Tomaszewski and Holloway used a semi-Markov process [43]. Some studies used a 6-month cycle length [21, 22, 37, 40, 46], while some studies used a 1-year cycle length [19, 20, 22, 43], and Fann et al. used a 3-month cycle length [41].
While disease progression is often determined according to Hoehn and Yahr stages, Fann et al. utilized a regression analysis to obtain a Hoehn and Yahr stage proxy based on the Unified Parkinson’s Disease Rating Scale motor score [41]. Dams et al. used the algorithm by Young et al. for early PD [48]. Among the 16 CUAs, nine comparisons utilized a 3% discount rate for both costs and effects [19, 20, 37, 40,41,42,43, 45], while four comparisons employed a 3.5% discount rate for both costs and effects [21, 22, 37, 39]. One study from Canada reported only a discount rate of 1.5% for effects [47]. Kawamoto et al. did not report the discount rate for costs and effects [46].
Health resource costs were derived from various sources, including national guidelines, national health insurance databases, clinical trials, hospital cost departments, and published studies. These sources included Medicare in the USA [43], the National Health Insurance of Spain [38], clinical databases in Sweden [42], the hospital financial department in China [45], the PD SURG clinical trial [21, 23], guidelines from the Japanese Society of Neurology [46] and German PD guidelines [37], among others. Most of the studies utilized input parameters sourced from the EARLYSTIM Trial [49], the Deuschl randomized controlled trial [50], or the PDSURG trial [51] for the model effectiveness measures. Time horizons for the studies ranged from 1 year to a lifetime, with Fann et al. and Zhu et al. providing two horizons, a shorter horizon and a longer horizon [41, 45]. Four studies with five comparisons reported a lifetime horizon [19, 20, 37, 43].
All CUAs included direct costs specific to DBS costs, such as surgery, calibration, pulse generator replacement, and temporary and permanent DBS complications. Additionally, annual drug costs included expenses related to follow-up visits, annual home or nursing home care, and hospital admissions. Only one recent CUA used Diagnosis Related Groups for battery exchange, [19] while older studies used Diagnosis Related Groups for a cardiac pacemaker exchange [20]. However, equipment costs such as DBS implants were not included in some studies, and Mahajan et al. used Medicare reimbursement as a proxy for the societal cost [44], and Zhu et al. collected baseline costs retrospectively [45]. Some studies excluded the costs of adverse events for DBS [46], and some studies assumed the costs to be constant over time, even when cost data were collected over a more extended period of time [22].
All other studies reported country-specific thresholds except two studies. Zhu et al. did not provide a willingness-to-pay threshold and reported that the treatment cost exceeded the recommended cost-effective range in Europe but was in the upper end of the cost-effective range in the USA. Fann et al. used a threshold of three times the gross domestic product per capita in Taiwan. The year of reference for the studies ranged from 2001 to 2020. Only four studies reported results of a probabilistic sensitivity analysis [22, 23, 37, 41, 46].
3.1 Quality Appraisal
The risk of bias in the selected studies was assessed using the ECOBIAS checklist (31). Nearly 80% of the studies used the BMT as a comparator and all comparators were adequately described. Data transparency was reported to be adequate across all studies. Sufficient information was provided on costs, effectiveness, discount rates, and funding sources. Selection bias related to model choice was negligible. However, the studies were found to have a high risk of bias related to the time horizon, as most studies did not employ a lifetime horizon. Further, the chance of limited scope bias was higher, and the internal consistency related to mathematical logic was not evident in nearly all studies (Fig. 1 of the ESM).
3.2 Cost Effectiveness of DBS vs BMT
A total of 14 comparisons from 12 studies reported on the cost effectiveness of DBS compared to the BMT [19,20,21,22,23, 38, 40,41,42,43, 45, 46]. Eleven comparisons reported DBS as cost effective based on ICUR results [19,20,21,22, 38, 40,41,42,43, 45, 46] (Table 1). Fann et al. provided cost-effective estimates for short-term and long-term evaluations (3-year and 10-year time horizons) from a societal perspective [41], while Zhu et al. 2014 reported on 1-year and 2-year time horizons from a healthcare provider perspective [45]. Although Fann et al. indicated that DBS was not cost effective over a 3-year horizon, it became cost effective over a 10-year horizon. Zhu et al. reported that DBS was cost effective over a 2-year horizon but not over a 1-year horizon.
Studies with a longer time horizon (> 5 years) reported DBS as cost effective compared with BMT [19, 20, 43]. Valldeoriola et al. considered DBS cost effective, even though the ICUR exceeded the generally acceptable threshold used in Spain (< 30,000 Euros) [38]. Figure 2 illustrates the incremental cost, adjusted for PPP and CPI for 2023, plotted against incremental QALYs, with the size of each data point representing the ICUR in USD (PPP 2023)/QALY.
3.3 Cost Effectiveness of DBS Versus Other Device-Aided Therapies
Four studies with five comparisons reported on the cost effectiveness of DBS compared to other treatments [37, 39, 44, 47]. Deep brain stimulation was reported to be cost effective when compared with bilateral radiofrequency ablation [44] and ASBI [39]. However, DBS was not cost effective compared with MRgFUS thalamotomy for tremor-dominant PD from a societal perspective [47]. A study by Walter and Odin concluded that CSAI could be a viable alternative treatment for patients with advanced PD, as it was found to dominate DBS in terms of cost effectiveness [37]. However, the study reported that the utilities and costs were similar for both treatment options in the UK and Germany from a healthcare provider’s perspective [37].
4 Discussion
This systematic review synthesized cost-effectiveness evidence for DBS in PD from published CUAs. The majority of the included studies compared DBS with BMT among patients with advanced PD, while a few other comparisons included bilateral radiofrequency ablation, MRgFUS, ASBI, and CSAI. The included studies in the review are all from high-income countries, with the UK, USA, and Germany being the most represented. Most studies adopted a payer’s perspective, with a few considering a societal perspective. Health resource costs and analytical time horizons varied among studies, and ranged from 1 year to a lifetime. Most studies indicate that DBS is cost effective for PD compared with BMT. However, the cost effectiveness of DBS varies according to the country, time horizon, perspective adopted, and threshold used. Regarding other device-aided therapies, the effectiveness evidence for Levodopa-Carbidopa Intestinal Gel, and apomorphine pumps is limited [42]. Walter et al. concluded CSAI dominated DBS, even though costs and utilities were nearly the same for both treatment options. Although MRgFUS remains a viable option to DBS, the cost-effectiveness advantage is less substantial [47]. Furthermore, even though Mahajan et al. had reported lower treatment costs for focused ultrasound [44], the focused ultrasound equipment is quite costly; hence, even if the procedure is cost effective, its immediate adoption and scalability may be limited. More studies are needed to compare the cost effectiveness of DBS with levodopa-carbidopa intestinal gel subcutaneous apomorphine infusion in order to inform decision making regarding the most effective and efficient treatment approach. Additionally, future studies should investigate the cost effectiveness of DBS for different subgroups of patients with PD, such as those with early-stage or tremor-dominant PD, as well as for different DBS targets.
Reported cost effectiveness of DBS varied based on the source of effectiveness and cost data being considered. Eggington et al. reported favorable ICURs using clinical data from the Deuschl randomized controlled trial [50]. In contrast, McIntosh et al., conducted alongside the PD SURG study, reported less favorable results for DBS [23]. The PD SURG study had used a micro-costing methodology to ascertain costs associated with DBS and BMT, as well as their long-term implications [23]. Nevertheless, considering the elapsed time since the clinical investigation, the possibility of including outdated practices cannot be disregarded.
Quality assessment of the selected studies observed several methodological strengths, including the frequent use of the BMT as a comparator, ensuring a well-defined comparison. Additionally, most included studies exhibited data transparency. Adequate reporting of costs, effectiveness, discount rates, and funding sources further enhanced the transparency of cost-effectiveness analyses. However, we identified notable methodological weaknesses, primarily the relatively short time horizons employed in some studies [23, 38, 41, 45, 47]. Furthermore, some studies did not comprehensively capture the broader economic impact of DBS. Internal consistency was unclear in most studies, raising concerns about the reliability of presented cost-effectiveness estimates.
4.1 Implications and Variability in the Cost Effectiveness of DBS
Determinants of the cost effectiveness of DBS encompass a range of factors, including broader cost considerations from a societal perspective [41, 42], the duration of the DBS effect [22, 43], the age at the time of the DBS intervention (e.g., early intervention at 60 years) [20, 46], the relative cost differences in comparator options [38], and cost reductions in DBS-related expenses occurring after the first year of surgery [19, 45]. In terms of outcomes, these determinants encompass variations in QALY gains [23, 37, 40, 43], utility weights associated with Hoehn and Yahr ‘off’ states, and the extent of improvement in motor complications [20, 50]. On the cost-related front, critical factors include hardware and procedural costs [19,20,21,22, 40, 43, 46], battery life [19, 20, 22, 40, 43], medication costs [19, 21, 40], and the reduction in the length of hospital stays [23]. Model-related factors come into play, including the choice of discount rates [20, 37] and time horizons [22, 23, 40, 43]. Notably, studies with shorter time frames consistently indicate that DBS may not be cost effective [23, 41, 45]. Surprisingly, despite a lifetime time horizon, Tomaszewski and Holloway, which employed a semi-Markov process, found DBS to be cost ineffective [43], underscoring the intricate nature of evaluating DBS cost effectiveness over extended periods.
Owing to a myriad of pragmatic factors, prior cost-effectiveness analyses pertaining to DBS have been hindered by several limitations. First, the absence of long-term effectiveness data, especially for early PD in some model-based evaluations [19,20,21,22, 37, 40, 43, 44, 46], constrains our ability to comprehensively assess DBS outcomes over extended periods. Additionally, the lack of robust quality-of-life evidence associated with DBS effectiveness and its variability in treatment choices, both in model-based [19,20,21,22, 37, 40, 43, 44, 46] and trial-based evaluations [45], pose challenges in fully capturing the impact of DBS on patients’ well-being. Furthermore, difficulties in modeling motor and non-motor complications because of limited data in some model-based studies [20, 21, 37], and issues stemming from limited sample sizes or non-randomized designs in both trial-based [38, 45] and model-based evaluations [20, 21, 37, 43], introduce uncertainties into our analyses. Exclusions of non-medical and indirect costs [19,20,21,22, 37, 40, 43, 44, 46], concerns regarding cost data accuracy [19,20,21, 38, 43, 45], non-standardized timeframes for battery replacement [19, 40], statistical issues, such as double counting or missing data imputation [19, 20, 37, 43], the heterogeneity of data sources in model-based evaluations [22], insufficient data concerning adverse events and associated costs [20, 46], and the exclusion of health and social service follow-up costs [20, 38], collectively underscore the methodological complexities inherent in our assessment.
4.2 Methodological Strengths and Weaknesses
In conducting a cost-effectiveness analysis for DBS in PD differences in methodology, estimation duration and input cohorts, such as disease progression across nationalities or races, may impact the results. Further, implementing DBS treatment for PD presents various complexities. The lack of well-established eligibility criteria and frequent contraindications hinder the appropriate assignment of this treatment modality [52]. Additionally, a scarcity of neurologists with expertise in DBS and other device-aided therapies poses organizational challenges for its broader application [42]. Long waiting times for initiating device-aided therapies, particularly DBS, are common and further exacerbated by the ongoing pressures on healthcare delivery due to the COVID-19 pandemic [53]. The underutilization of DBS may be attributed to suboptimal economic incentives within distinct cost-bearing entities, necessitating further investigation into financial frameworks for treatment allocation [20]. Further, there is a conspicuous paucity of data from lower- to middle-income countries and low-income countries regarding the cost effectiveness of DBS for PD.
In the context of decision making for PD treatments, it is important to acknowledge the key sources of uncertainty in economic modeling, namely, the Markovian assumption that current health rather than previous health history determines the unit costs for health states. It is often not clear in the context of PD whether patients who receive less supportive care, those who moved to less expensive home care, or those who returned to work without delay when PD symptoms improved are reassigned from worse to better health states. Consideration should be given to potential cost offsets associated with reductions in productivity loss and home care, as these depend on the stage of disease at the time of treatment initiation. Additionally, it is important to acknowledge that if DBS is initiated too late in the PD disease progression, the cost offsets would be considerably less. Therefore, careful evaluation of the potential benefits and costs of DBS therapy, including the potential for cost offsets, should be considered when making decisions regarding PD treatment.
There is no clear trend suggesting that DBS is cost effective for patients with PD who do not respond well to medical therapy. The heterogeneity of study methodologies, perspectives, and outcomes in the CUAs makes it challenging to draw definitive conclusions. As such, it is crucial to consider the individual patient’s clinical characteristics, disease stage, and response to medical therapy when making treatment decisions.
4.3 Limitations and Strengths
We chose to limit our approach to a systematic review and refrained from conducting a meta-analysis because of the inherent challenges in meta-analyzing CUAs. Comparing cost-effectiveness results over time and across countries is a complex endeavor, and pooling individual ICURs often lacks meaningful interpretation. Some proposed approaches for a meta-analysis involve CPI conversion to the current year and PPP conversion to USD to achieve a unified analysis with INB and subsequent pooling of INB [54, 55]. However, relying solely on the CPI adjustment may be insufficient for comprehensively assessing the dynamic changes in healthcare costs and is often subject to debate. Furthermore, adjusting for inflation alone may be inadequate, particularly when cost-effectiveness thresholds remain fixed over an extended period. In the context of meta-analyses, calculating INB variance may present a challenge, given that many CUAs provide only point estimates, with dispersion measures for incremental costs, QALYs, and ICURs frequently absent.
Our emphasis on CUAs offers a unique perspective, addressing existing research gaps by providing a more interpretable and comparable measure of cost effectiveness. Unlike previous systematic reviews, our approach facilitates a holistic understanding of the cost-effectiveness landscape and enables direct comparisons of the economic impact of DBS across diverse studies and healthcare contexts. We recommend future research to utilize lifetime horizons, for a more comprehensive assessment, broaden the scope of analysis to consider a wider range of economic and societal factors, ensure internal consistency in modeling and analysis methods, and maintain a high level of data transparency to facilitate transparency and reproducibility in DBS cost-effectiveness analyses.
5 Conclusions
While DBS has demonstrated promising results for the management of PD, there is a need for further research to fully understand whether it is cost effective. The cost-effectiveness evidence for DBS in PD is context specific and varies depending on the study perspective, costs considered, threshold utilized, and the stage of PD progression. Moreover, there is a dearth of data from lower-income countries and lower-income to middle-income countries on the cost effectiveness of DBS for PD. Future research should focus on evaluating the cost effectiveness of DBS in distinct subgroups of patients with PD, including those with early-stage or tremor-dominant PD, and those undergoing DBS at different targets. Furthermore, it is crucial to standardize approaches in CUAs, comparing DBS with other regular or current practice treatment options for any relevant policy translation for the clinical management of PD. It is essential to note that the generalizability of the findings should be considered with caution because of the inherent variability in methodologies and healthcare contexts within health technology assessments. Consensus on the most appropriate methodology, perspective, and reporting guidelines would greatly improve the comparability of study results and facilitate decision making for healthcare providers, policymakers, and patients.
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease Nat Rev Dis Primers. 2017;3(1):17013.
Smith ER, Perrin PB, Tyler CM, Lageman SK, Villasenor T. Parkinson’s symptoms and caregiver burden and mental health: a cross-cultural mediational model. Behav Neurol. 2019;2019:1396572.
Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson’s disease. Trends Neurosci. 2000;23(10 Suppl.):S2-7.
National Collaborating Centre for Chronic Conditions (UK). Symptomatic pharmacological therapy in Parkinson’s disease. London: Royal College of Physicians; 2006.
Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson's disease: surgical technique and perioperative management. Mov Disord. 2006;21 Suppl. 14(S14):S247–58.
Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25(5):578–86.
Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013;123(11):4546–56.
National Institute for Health and Care Excellence. Parkinson's disease in adults. NICE guideline [NG71]. 2017. National Institute for Health and Care Excellence (NICE), London.
Rughani A, Schwalb JM, Sidiropoulos C, Pilitsis J, Ramirez-Zamora A, Sweet JA, et al. Congress of Neurological Surgeons systematic review and evidence-based guideline on subthalamic nucleus and globus pallidus internus deep brain stimulation for the treatment of patients with Parkinson’s disease: executive summary. Neurosurgery. 2018;82(6):753–6.
Nijhuis FAP, Esselink R, de Bie RMA, Groenewoud H, Bloem BR, Post B, et al. Translating evidence to advanced Parkinson’s disease patients: a systematic review and meta-analysis. Mov Disord. 2021;36(6):1293–307.
Perestelo-Perez L, Rivero-Santana A, Perez-Ramos J, Serrano-Perez P, Panetta J, Hilarion P. Deep brain stimulation in Parkinson’s disease: meta-analysis of randomized controlled trials. J Neurol. 2014;261(11):2051–60.
Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21 Suppl. 14(S14):S290–304.
Mansouri A, Taslimi S, Badhiwala JH, Witiw CD, Nassiri F, Odekerken VJJ, et al. Deep brain stimulation for Parkinson’s disease: meta-analysis of results of randomized trials at varying lengths of follow-up. J Neurosurg. 2018;128(4):1199–213.
Peng L, Fu J, Ming Y, Zeng S, He H, Chen L. The long-term efficacy of STN vs GPi deep brain stimulation for Parkinson disease: a meta-analysis. Medicine (Baltimore). 2018;97(35): e12153.
Zhang J, Li J, Chen F, Liu X, Jiang C, Hu X, et al. STN versus GPi deep brain stimulation for dyskinesia improvement in advanced Parkinson’s disease: a meta-analysis of randomized controlled trials. Clin Neurol Neurosurg. 2021;201: 106450.
Hacker ML, Currie AD, Molinari AL, Turchan M, Millan SM, Heusinkveld LE, et al. Subthalamic nucleus deep brain stimulation may reduce medication costs in early stage Parkinson’s disease. J Parkinsons Dis. 2016;6(1):125–31.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–60.
Becerra JE, Zorro O, Ruiz-Gaviria R, Castaneda-Cardona C, Otalora-Esteban M, Henao S, et al. Economic analysis of deep brain stimulation in Parkinson disease: systematic review of the literature. World Neurosurg. 2016;93:44–9.
Dams J, Balzer-Geldsetzer M, Siebert U, Deuschl G, Schuepbach WM, Krack P, et al. Cost-effectiveness of neurostimulation in Parkinson’s disease with early motor complications. Mov Disord. 2016;31(8):1183–91.
Dams J, Siebert U, Bornschein B, Volkmann J, Deuschl G, Oertel WH, et al. Cost-effectiveness of deep brain stimulation in patients with Parkinson’s disease. Mov Disord. 2013;28(6):763–71.
Eggington S, Valldeoriola F, Chaudhuri KR, Ashkan K, Annoni E, Deuschl G. The cost-effectiveness of deep brain stimulation in combination with best medical therapy, versus best medical therapy alone, in advanced Parkinson’s disease. J Neurol. 2014;261(1):106–16.
Fundament T, Eldridge PR, Green AL, Whone AL, Taylor RS, Williams AC, et al. Deep brain stimulation for Parkinson’s disease with early motor complications: a UK cost-effectiveness analysis. PLoS ONE. 2016;11(7): e0159340.
McIntosh E, Gray A, Daniels J, Gill S, Ives N, Jenkinson C, et al. Cost-utility analysis of deep brain stimulation surgery plus best medical therapy versus best medical therapy in patients with Parkinson’s: economic evaluation alongside the PD SURG trial. Mov Disord. 2016;31(8):1173–82.
Dang TTH, Rowell D, Connelly LB. Cost-effectiveness of deep brain stimulation with movement disorders: a systematic review. Move Disord Clin Pract. 2019;6(5):348–58.
Afentou N, Jarl J, Gerdtham UG, Saha S. Economic evaluation of interventions in Parkinson’s disease: a systematic literature review. Move Disord Clin Pract. 2019;6(4):282–90.
Marsili L, Bologna M, Miyasaki JM, Colosimo C. Parkinson’s disease advanced therapies-a systematic review: more unanswered questions than guidance. Parkinsonism Relat Disord. 2021;83:132–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
CEA Registry. Center for the Evaluation of Value and Risk in Health. https://cevr.tuftsmedicalcenter.org/databases/cea-registry. Accessed May 2021.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Ankit R. WebPlotDigitizer. 2021. https://automeris.io/WebPlotDigitizer. Accessed 12 Nov 2023.
Paulden M. Why it’s time to abandon the ICER. Pharmacoeconomics. 2020;38(8):781–4.
International Monetary Fund. World Economic Outlook database, April 2023. 2023. https://www.imf.org/en/Publications/WEO/weo-database/2023/April/download-entire-database. Accessed 12 Nov 2023.
Microsoft Corporation. Microsoft Excel. 2018. https://office.microsoft.com/excel. Accessed 12 Nov 2023.
StataCorp. Stata statistical software: release 16. 2019;16. https://www.stata.com/. Accessed 12 Nov 2023.
Adarkwah CC, van Gils PF, Hiligsmann M, Evers SM. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):513–23.
Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, et al. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson's disease. Eur J Neurol. 2006;13(11):1186–202.
Walter E, Odin P. Cost-effectiveness of continuous subcutaneous apomorphine in the treatment of Parkinson’s disease in the UK and Germany. J Med Econ. 2015;18(2):155–65.
Valldeoriola F, Morsi O, Tolosa E, Rumià J, Martí MJ, Martínez-Martín P. Prospective comparative study on cost-effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson’s disease. Mov Disord. 2007;22(15):2183–91.
Vivancos-Matellano F, Garcia-Ruiz A, Garcia-Agua SN. Pharmacoeconomic study of the treatment of advanced Parkinson’s disease. Rev Neurol. 2016;63(12):529–36.
Pietzsch JB, Garner AM, Marks WJJ. Cost-effectiveness of deep brain stimulation for advanced Parkinson’s disease in the United States. Neuromodulation. 2016;19(7):689–97.
Fann JC, Chang KC, Yen AM, Chen SL, Chiu SY, Chen HH, et al. Cost-effectiveness analysis of deep brain stimulation for Parkinson disease in Taiwan. World Neurosurg. 2020;138:e459–68.
Norlin JM, Willis M, Persson U, Andersson E, Pålhagen E, Odin P. Swedish guidelines for device-aided therapies in Parkinson’s disease: economic evaluation and implementation. Acta Neurol Scand. 2021;144(2):170–8.
Tomaszewski KJ, Holloway RG. Deep brain stimulation in the treatment of Parkinson’s disease: a cost-effectiveness analysis. Neurology. 2001;57(4):663–71.
Mahajan UV, Ravikumar VK, Kumar KK, Ku S, Ojukwu DI, Kilbane C, et al. Bilateral deep brain stimulation is the procedure to beat for advanced Parkinson disease: a meta-analytic, cost-effective threshold analysis for focused ultrasound. Neurosurgery. 2021;88(3):487–96.
Zhu XL, Chan DT, Lau CK, Poon WS, Mok VC, Chan AY, et al. Cost-effectiveness of subthalmic nucleus deep brain stimulation for the treatment of advanced Parkinson disease in Hong Kong: a prospective study. World Neurosurg. 2014;82(6):987–93.
Kawamoto Y, Mouri M, Taira T, Iseki H, Masamune K. Cost-effectiveness analysis of deep brain stimulation in patients with Parkinson’s disease in Japan. World Neurosurg. 2016;89:628-35.e1.
Meng Y, Pople CB, Kalia SK, Kalia L, Davidson B, Bigioni L, et al. Cost-effectiveness analysis of MR-guided focused ultrasound thalamotomy for tremor-dominant Parkinson’s disease. J Neurosurg. 2020;136(1):273–8.
Young MK, Ng SK, Mellick G, Scuffham PA. Mapping of the PDQ-39 to EQ-5D scores in patients with Parkinson’s disease. Qual Life Res. 2013;22(5):1065–72.
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–22.
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908.
Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9(6):581–91.
Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68(2):165.
Fasano A, Antonini A, Katzenschlager R, Krack P, Odin P, Evans AH, et al. Management of advanced therapies in Parkinson’s disease patients in times of humanitarian crisis: the COVID-19 experience. Mov Disord Clin Pract. 2020;7(4):361–72.
Bagepally BS, Chaikledkaew U, Chaiyakunapruk N, Attia J, Thakkinstian A. Meta-analysis of economic evaluation studies: data harmonisation and methodological issues. BMC Health Serv Res. 2022;22(1):202.
Crespo C, Monleon A, Díaz W, Ríos M. Comparative efficiency research (COMER): meta-analysis of cost-effectiveness studies. BMC Med Res Methodol. 2014;22(14):139.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Department of Health Research, Government of India funds the HTARC, ICMR-NIE grant no. T.11011/08/2017-HR(Part-1)/E-office-8025571 dated 27 November, 2019. The funders played no role in the study’s conception, execution, or manuscript preparation.
Conflict of interest
Akhil Sasidharan, Bhavani Shankara Bagepally, and S. Sajith Kumar have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All relevant data and materials supporting the findings of this study are included in the submitted files. Additional data or materials, if required, can be obtained upon reasonable request by contacting the corresponding author.
Code availability
Not applicable.
Author contributions
BSB and AS designed the study. BSB, AS, and SK conducted the literature search, screening, and data extraction. AS and SK performed the quality assessment, and AS performed the data synthesis. AS drafted the manuscript. All authors contributed to the interpretation of the results and critically revised the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasidharan, A., Bagepally, B.S. & Kumar, S. Cost Effectiveness of Deep Brain Stimulation for Parkinson’s Disease: A Systematic Review. Appl Health Econ Health Policy 22, 181–192 (2024). https://doi.org/10.1007/s40258-023-00848-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00848-y