Abstract
Background
Regarding the low penetration of biosimilars in the French market, in 2018, the government introduced two mutually exclusive financial incentives to increase biosimilar use. They redirect 20% (general case) or 30% (experimental case) of the price difference between the reference product and its biosimilar to hospitals for every biosimilar delivered in retail pharmacies from these hospital prescriptions. The experimental case specifically targets prescribing clinical units.
Objectives
Our study aimed to assess whether the new payment scheme closer to physicians (experimental case) improved etanercept biosimilar penetration after 25 months.
Method
We evaluated hospital prescriptions using IQVIA Xponent data. The monthly number of etanercept boxes delivered in retail pharmacies was linked to the corresponding hospital prescription. The impact of the experimental case on the etanercept biosimilar rate was assessed by a difference-in-difference method.
Results
Among the 39 hospitals studied in the experimental case compared with the 169 belonging to the general case, a similar growing trend of etanercept biosimilar use was observed before October 2018. At the start of the experiment, there was an acceleration of biosimilar penetration in the experimental group, until both groups reached a plateau. A significant double difference estimator of 9.72 percentage points in favor of the experiment confirmed this (p < 2.2.10−16).
Conclusion
The French experimental incentive appeared to be more effective at increasing biosimilar use. As it is expected to be implemented in all hospitals, the knowledge gained during this testing phase should allow adjustment of some of its terms and increase physician engagement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Directly rewarding clinical units is more effective in increasing biosimilar uptake than incentivizing an entire hospital structure (hospital administration) as physicians are deeply engaged. |
The influence of the experimental incentive appeared to be greater at the beginning of its implementation. To keep physicians and their clinical units motivated, regular follow-up on the performance of their objectives and close communication are needed. |
Experimentation for testing novel hospital remuneration systems prior to generalization is relevant for identifying points that need to be improved. |
1 Introduction
Biopharmaceuticals take an increasingly important place in the global pharmaceutical spending, and their worldwide market was expected to reach approximately US$250 billion in 2020 (i.e., €244 billion) [1]. In Europe, they represented 34% (€78.6 billion) of pharmaceutical expenditure in 2021. Biosimilars are biological medicines highly similar to another biological product already approved, called the reference product [2]. They are therapeutically comparable to this reference product, allowing them to foster competition and lower prices. For this reason, biosimilars are an essential way to reduce health care expenses [3]. With the loss of patents of major biopharmaceuticals such as adalimumab (Humira®) and infliximab (Remicade®), the economic issues surrounding the spread of biosimilars are more than ever at the heart of policies regulating health care expenditure [4, 5]. Thus, IQVIA reported that the biosimilars list price savings in Europe accounted for €5.7 billion in 2020 and will potentially reach over $100 billion worldwide in the next 5 years [6, 7]. In the EU5 countries (France, Germany, Italy, Spain, and the UK), savings of more than €10 billion have been made between 2016 and 2020 [8]. Among the major top-selling biopharmaceuticals that lost their exclusivity between 2016 and 2020, etanercept (Enbrel®) sales accounted for €9.6 billion in the EU5 and the US [9] in 2015.
Policy design has an impact on biosimilar uptake [10]. However, states in Europe have been slow to encourage switching between biosimilars and reference products. According to Rémuzat et al. [5], only Poland promoted patients to switch in 2017, although France was one of the first EU countries to adopt legislation authorizing biosimilars switching. It was initially permitted only for naïve patients in 2014 [11] and since 2017, at any time during treatment [12]. However, in terms of biosimilar rates, France lags behind Poland, with an etanercept biosimilar rate of 50% in 2022 versus 68% in Poland [13]. The European Medicines Agency (EMA) is responsible for the market authorization of biologic products in the EU but has not provided guidance on the interchangeability of a reference product and its biosimilars. Prescribing practices and recommendations to prescribers have been considered the domain of member states [14, 15]. In September 2022, with the Heads of Medicines Agencies (HMA), the EMA finally took a stand on the interchangeability of biosimilar medicines and provided a rationale for the use of a biosimilar in place of its reference product and vice versa [15].
Incentives are implemented within these regulatory frameworks. Physicians are the first link in the chain for biosimilar uptake. For this reason, most European countries’ educational policies primarily target physicians with clinical guidelines or prescribing recommendations [16]. Due to a lack of understanding about the consequences of switching, they initially preferred to use biosimilars for naïve patients [16, 17]. In Europe, payers have implemented different policies targeting prescribing behavior to promote biosimilar uptake and ensure the ‘best-value biologic’ [18]. Physicians in Austria and Belgium are encouraged to rationally use medicines. In Germany, there are biosimilar prescribing quotas, as well as in Belgium, where physicians are obliged to prescribe minimum quotas by specialty of ‘cheap medicines’Footnote 1 [5, 16, 18, 19]. In Sweden, policy measures to increase biosimilar uptake among physicians vary by county. Because county councils have overall responsibility for all health care services provided in their primary health care centers, they may establish differing regional treatment recommendations. One of the main incentives, such as the French scheme presented in this article, is a profit-sharing of savings between hospitals and county councils [16, 18].
Since 2000, new types of funding known as ‘pay-for-performance’ (P4P) or ‘benefit-sharing programs’ have grown in popularity. They entail rewarding health care providers who meet predetermined quality-of-care targets [20, 21]. These programs can affect individuals (e.g., physicians, GPs, patients) or a group (e.g., hospital, clinical unit) and can provide incentives either positive, negative or both [22]. More recently, they focus on the sustainability of health care systems and target efficiency indicators [21].
Biopharmaceuticals are commonly injected during inpatient hospital care in France. Some of them, such as etanercept, can be used in outpatient settings as well, but they must be prescribed by hospital specialists, at the very least for treatment initiation. Patients in the outpatient circuit purchase their treatment from retail pharmacies, and prescriptions are renewed either by the hospital specialists or a local specialist. In both cases, as most of these treatments fall under the scope of long-term and disabling illnesses, patients are totally covered for these treatments by the French National Health Insurance (NHI).
In addition, hospital prescriptionsFootnote 2 account for a rising share of outpatient care spending (mean growth of +3.5% per year between 2014 and 2019), with 17.8 billion euros (21%) reimbursed by the NHI in 2016 and 19.5 billion euros (22 %) in 2019 [23]. Among these, biopharmaceuticals accounted for 1.5 billion euros in 2016 and 1.4 billion euros in 2019 [24]. In 2017, the NHI paid approximately €110 million for etanercept hospital prescriptions delivered in retail pharmacies. According to the study by Robinson and Jarrion [25], the market penetration of three anti-TNFα (tumor necrosis factor alpha) (infliximab, etanercept, adalimumab) biosimilars used in hospital ambulatory care in France moved up to 75% by 2020. However, considering both hospital and community settings and considering all active substances with available biosimilars, biosimilar penetration was still found to be limited in France: approximately 10% in volume in 2016 versus 60% compared with generic medicines, and even less for enoxaparin, teriparatide, and etanercept [24, 26]. Thus, hospital prescriptions of biosimilars provide a promising opportunity for additional cost savings, particularly for city care expenditures. Lechat estimated that French NHI savings would reach €300 million in 2019 [27].
Box 1: French hospital funding and hospital influence on city care expenditures French hospitals are mostly funded by the NHI based on the activity payment model for both public and private health facilities with medicine, surgery and obstetric (MSO) activity. By way of exception, costly or cutting-edge medications as well as a few medical devices (registered on an ‘addition list’) are covered in addition to service fees. Over the past two decades, the government has become more involved in controlling health expenditures funded by the NHI system, particularly for hospital prescriptions delivered in retail pharmacies2 (PHEV). The Social Security Funding Act for 2016 introduced a contract (CAQES)Footnote 3 that links health facilities to the French NHI and their health regional agency. It focuses on the quality and the efficiency of care (proper use of medicines and health products, improvement of care organization and promotion of the relevance of procedures and prescriptions) and gives rise to financial interest or sanction, depending on whether the objectives set are achieved or not. Part of the main objectives are about prescription rates of generics and biosimilars, and a contribution to PHEV spending savings. |
Therefore, in 2018, the French government introduced two new mutually exclusive incentives to increase biosimilars use. The first incentive (the so-called ‘general case’) is applicable to all French health facilities that are under a CAQES3 agreement (see Box 1) [28] (mostly all health facilities). For every biosimilar provided in a retail pharmacy, 20% of the price difference between the reference product and this biosimilar is redirected to the hospital from which the first prescription originates. The second incentive (the so-called ‘experimental case’) is a national experiment in which 40 hospitals were selected following a voluntary answer to a national call for proposals. It raises the first incentive to 30% of the price difference between the reference product and its biosimilars and directly targets the clinical units that have prescribed the biosimilar. According to the legal text, at least 50% of the compensation received goes toward the clinical unit budget at the origin of the prescription; the balance is reassigned to the hospital's general budget. Clinical units should have provided an application file explaining how they planned to prescribe more biosimilars [26]. Fig. 1 illustrates the rules for linking a patient prescription to a health facility.
Patient circuit and rules for linking a patient prescription to a facility. In the French health system, patients can obtain prescriptions from hospital physicians who are mostly specialists or from local doctors (GPs or independent specialists). Treatments are then dispensed by retail pharmacies. As health facilities and physicians are registered with an official number (FINESS number and repository of practitioners’ word number [RPPS], respectively), prescriptions could be linked to them. According to incentive instructions, the last hospital to issue the prescription receives the remuneration
The experimental case specifically targets clinicians because currently in France only clinicians are permitted to switch from one biopharmaceutical to another. Pharmacists cannot change a reference product to its biosimilars as they can do for generic medicines with the original chemical ones. To modify their patients’ chronic treatment, clinicians and more generally clinical units may reorganize the patient care, sometimes supported by consultation with specific pharmacists, to set aside time to explain and persuade them to switch to biosimilars. As a result, two new incentives were introduced to stimulate and reward these efforts.
Both incentives were launched a few months apart in 2018 for two active substances: etanercept and insulin glargine. The general case was introduced in February 2018 [28]; the experimental case started in October 2018, with the publication of the 40 health facilities selected in the Official Journal of the French Republic, and was scheduled to last 3 years (from October 2018 to October 2021) [29]. However, the end date was postponed to September 2022 by a new decree on 30 March 2022.
The aim of our research is to evaluate the experiment for etanercept after 25 months and assess if directly incentivizing clinicians compared with the general case improves the share of biosimilars for this active substance.
2 Methods
2.1 Design of the Study
This retrospective observational study was conducted between February 2018 and October 2020. An analysis of IQVIA Xponent data, a database compiled from a panel of 14,000 retail pharmacies (60% of the French retail pharmacies), allowed us to estimate the number of medicine boxes supplied in retail pharmacies linked to the initial hospital prescription (the hospital is identified by the pharmacy in the NHI reimbursement system).
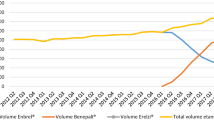
The study focuses on the anti-TNFα etanercept group that includes three biologic products: Enbrel® (reference product), Benepali® (biosimilar, launched in October 2016) and Erelzi® (biosimilar, launched in December 2017). The delivered product is identified in the dataset by its brand name, active substance (i.e., etanercept) and whether it is a reference product (Ref) or a biosimilar (see the timeline of the study in Fig. 2). The quantity of boxes delivered is weighted according to the number of vials that each box contains and according to the dosage in milligrams [28]. A box of four injections of etanercept 50 mg is weighted 1, four injections of etanercept 10 mg are weighted 0.2, and four injections of etanercept 25 mg are weighted 0.5, as indicated in the official text.
Hospitals are identified by their FINESSFootnote 4 number, as well as their name, locality (county), type of establishment (public facilities, teaching and nonteaching, chartered private nonprofit facilities [that could be private foundations, cancer treatment centers or dialysis centers, etc.], and private for-profit facilities), and whether they are in the experimental case according to the official list published in October 2018 [29]. The volume of MSO activity and the volume of medicine activityFootnote 5 measured for the year 2018 were added to the data frame thanks to HospiDiag opendata [30]. From the original dataset, our main analysis focused on health facilities with an average delivery of three or more boxes of etanercept in retail pharmacies during the period before incentives kick-off (period P0, from November 2017 to January 2018). We analyzed data for 39 out of the 40 establishments involved in the experimental case and for 169 hospitals in the general caseFootnote 6.
2.2 Biosimilar Prescription Rate
First, we focus on the trend of etanercept consumption and the market share of its biosimilars. The monthly mean volume of boxes was calculated globally for all hospitals and by incentive group for this purpose. Then, we compared the rate of biosimilars dispensed for each hospital, aggregated by incentive group.
The monthly (t) prescription rate of biosimilars was calculated by the hospital (h) using the following Eq. (1):
where t is the month of dispensation and h is the prescribing hospital
2.3 Average Effect of the Experimental Case
A difference-in-difference (DID) estimation was performed with the R Studio program to assess the impact of the experimental case. The more critical assumption to ensure the internal validity of the DID is that temporal effects are assumed to be comparable between the two groups [31]. Thus, the main assumption of the test is that if the experimental case had not been introduced, all hospitals would have fallen under the general case, and the trends of biosimilar rate would have been parallel for both hospital groups (the group exposed to the experimental case and the control group under the general case) (Fig. 3).
Difference-in-difference estimation explanation scheme (Source: https://www.publichealth.columbia.edu/research/population-health-methods/difference-difference-estimation). From November 2017 to September 2018 (period before the introduction of the experiment), the mean biosimilar rate (mBSr) difference was constant between the two hospital groups (parallel trend). After the introduction of the experiment, from October 2018 to October 2020, the mBSr rose faster for hospitals in the experimental case than in the general case. The difference-in-difference estimator consists of the difference between the observed outcome trend in the experimental group and the unobserved counterfactual outcome trend in the experimental group
This temporal trend of etanercept biosimilars was graphically observed during the first period. The difference in mean biosimilar rate (mBSr) in the experimental case before and after its introduction was compared with the same difference (before/after) for hospitals in the general case.
The mBSr was calculated by month for each hospital group. Then, the average by period was calculated (mBSrP1 and mBSrP2).
The DID estimator, corresponding to the average treatment effect (ATT, Eq. 2), was estimated via a linear regression (Eq. 3). The significance level was fixed at 5% risk. We assume that our variable is not transformed because the linear regression is robust to the linearity assumption.
Y = mBSr, T = dummy variable for the intervention group (hospitals under the experimental case), P = dummy variable for the second period (Oct. 2018–Oct. 2020), PT = dummy variable for the intervention effect on the treatment group, β3 = DID indicator.
2.4 Propensity Score Matching
To improve the comparability of the two groups by controlling for observable variables, we introduced propensity score matching on period P0 (November 2017 to January 2018) [32, 33]. The sample consists of treatment-group health facilities that are matched one to one (1:1) with control-group facilities based on their estimated propensity score. Because of the small size of the intervention group, which may cause loss of test power if further reduced, we use the nearest neighbor matching estimator [34] without replacement and without caliper. The MatchIt function [35] was used in the R Studio program. The matching variables included in the logistic estimation of the propensity score are the county, the type of establishment, the level of MSO and medicine activity and the total volume of etanercept boxes during the pretreatment period P0. These variables are chosen because they can influence the level of etanercept prescription: larger hospitals should have more activities and should be most interested in these incentives. In fact, the remuneration received by the hospitals is directly proportional to the total volume of etanercept dispensation (biosimilars and the original biologic medicine) (equation in Fig. 4).
Calculation of hospital remuneration by incentives. The amount of hospital renumeration paid out each year is calculated by multiplying the volume of etanercept boxes delivered in retail pharmacies from the same hospital prescription by the biosimilar prescription rate (considering the private practitioner renewals) and a Factor R that is the annual compensation by box of biosimilar delivered. The R factor is given in the table for years 2018–2020 and differs in each group, as it corresponds to 20% (general case) or 30% (experimental case) of the price difference between the reference product and biosimilar for French national health insurance
After a first DID analysis on the entire sample described previously, a second analysis was performed on health facilities selected after matching.
3 Results
As observed in Table 1, some differences exist between the two hospital groups without matching. The majority of hospitals are public but there are more teaching hospitals in the experimental case group. The volume of activity emphasizes that hospitals in the experimental case group are mainly larger hospitals. Compared with 23% in the general case group, more than 70% of the experimental group's health care facilities are classified as having the highest MSO activity, with more than 40,400 patients a year. More specifically, in the medical field, 47% of the general case group and nearly 80% of the experimental case group fall into the ‘M4, M5’ category and have a minimum of 20,000 patients a year. Every county of France is represented in both incentives except Corse, which is the smallest, and the geographical distribution of facilities is homogenous (Table 1). The total volume of etanercept dispensation was significantly higher for the experimental case than for the general case group, with 380.93 boxes versus 100.35 on average by hospital over P0.
Between November 2017 and October 2020, the monthly total volume of etanercept (biosimilars and reference product) delivered in retail pharmacies from hospital prescriptions remained consistent (Fig. 5), with a median of 10,585 boxes per month (min [February 2018]: 9337; max [February 2019]: 12,006). Hospitals in the experimental case group accounted for 47.4% (min: 46.1 %; max: 48.5 %] of the total market share with a monthly average of 5048 boxes of etanercept (Fig. 5).
Etanercept market share (global volume of boxes) from November 2017 to October 2020 by hospital group. The total volume of etanercept boxes delivered by retail pharmacies is given for the months from November 2017 to October 2020. Each month, the total quantity of etanercept delivered comes from prescriptions from hospitals in the experimental case (black bars) and from hospitals in the general case and was consistent among months
Meanwhile, the etanercept biosimilars market increased sharply in both groups (+8.5% mean monthly growth between October 2018 and July 2019, just after the start of the experiment) and then seemed to plateau from the end of 2019 (+1.1% mean monthly growth between August 2019 and October 2020) (Fig. 6). In October 2020, more than 5100 boxes of etanercept biosimilars were delivered, compared with approximately 1100 boxes in November 2017. As Benepali® was the first etanercept biosimilar authorized; it was the most commonly used biosimilar throughout the study (77% of total biosimilar boxes delivered in October 2020).
Etanercept biosimilar market (global volume of boxes) from November 2017 to October 2020 for the 208 hospitals studied. The total volume of etanercept biosimilar (BS) boxes delivered by retail pharmacies is given for the months from November 2017 to October 2020. Each month, the total quantity of BS delivered comes from prescriptions from hospitals in the experimental case (black bars) and from hospitals in the general case
The biosimilar rate increased each month for both hospital groups, but the health facilities participating in the experiment always had a higher market share of biosimilars than those under the general case (Fig. 7). The gap between them widened since October 2018. The boxplot shows significant differences in biosimilar rates between hospitals in both groups. This variability appears to be more important for the general case group (Fig. 7).
Monthly distribution of the rate of etanercept biosimilar (BSr) by incentive group. Dark boxplots illustrate the distribution of the etanercept BS rate for hospitals in the experimental case each month, from February 2018 to October 2020. White boxplots are the same for hospitals in the general case. Their concomitant observation allows us to observe that mean BS rates by group both grew until a plateau in the last 4 months and a difference in the BS rate distribution that is more concentrated in the experimental case
As the temporal trend of etanercept biosimilars was graphically parallel during the first period (P1) (Fig. 8 in the electronic supplementary material [ESM]), the DID method was applied to assess the difference between the two incentives during period 1 and period 2. According to the regression, the DID estimator was 0.0972. This indicates that the experimental case led, on average, to a significant increase in the biosimilars rate of 9.72% (p < 2.2.10−16). The adjusted R-square was high (0.7621), meaning that the model mostly explains the observed difference in biosimilar rate changes between the two periods (Table 2), which means that more than three quarters of the differences between the two groups can be attributed to the fact that hospitals were in the experimental group.
The second analysis focuses on the 78 matched facilities. After the propensity score matching was achieved (mean propensity score was 0.1875), the distribution by county remained homogenous. Although the total volume of etanercept dispensation at P0 remained different (p = 0.033 after matching versus < 0.001 before), the global difference in hospital type and size (i.e., measure of activities) between the two groups appears to be not significantly different (Table 3).
The trend of biosimilar rate over the months for this sample is similar to the previously studied population, but with less variability in the general case group (Fig. 9 and Fig. 10 in the ESM). The P1 period also shows a parallel trend between the two groups (Fig. 10 in ESM).
Thus, the DID analysis was rerun (Table 4) and reveals that the experimental case led, on average, to a significant increase of 8.75% in the biosimilar rate (p < 2.2.10−16). The adjusted R-square was slightly lower (0.7288) but was still able to explain the observed biosimilar rate difference between the two groups (Table 4).
4 Discussion
The experimental case resulted in a significant increase in biosimilars use of 9.71 points compared with the general case and 8.75 if we compare the two hospital groups after matching. These findings are consistent with an intermediate health ministry report, which found a + 8- to + 10-point difference in the rate of biosimilar prescriptions delivered in retail pharmacies in 2021 between hospitals under the experimental case and a group of hospitals in the general case [36]. They support the public authorities’ hypothesis that incentives directly targeting physicians (i.e., prescribers) are a better way to improve biosimilars use than hospital-oriented incentives. They confirm that physicians are deeply engaged when their efforts are directly recounted. However, the observed difference between the two incentives is less than what was expected (15 points) by public authorities. These results and their representativeness should be confirmed with the most recent data at the end of the experiment.
The major bias of this type of incentive and its assessment is a selection bias inherent to the design of the experimental case because hospitals that participated in it answered a call for proposals beforehand. These 39 hospitals out of the 208 studied are specifically concerned about issues surrounding etanercept biosimilars because they accounted for nearly half of the etanercept prescriptions, and the remuneration is proportional to the volume of prescription.
To consider this issue, we have excluded facilities with fewer than three etanercept boxes by month on average during the preanalysis period (P0). The secondary analysis was performed after matching hospitals from each group.
Moreover, we observed a lower explanatory power after matching hospital groups than without matching. We assume that the number of prescriptions affects the biosimilar rate as the experimental group has more prescriptions than the general case group.
Throughout the study, hospitals in the experiment group maintained a larger share of the biosimilar market than the other hospitals. They may have already taken steps to increase their etanercept biosimilar prescriptions before the experiment began. If the DID method is known to partially eliminate the nonobservable bias, the matching method helps to select comparable populations as if randomization has been carried out [31]. This method can also introduce a kind of bias because constraining the general case to be similar to the experiment group is likely to be selective [37]. It is not representative of the reality where facilities most interested in biosimilar prescriptions were those trying to enter the experimental case.
Globally, the influence of the experimental incentive appears to be greater at the beginning of its implementation. The promised remuneration may have boosted the dynamism of care teams, allowing for rapid effectiveness. Some hospital management most likely anticipated part of the expected remuneration. They may have funded some of the clinical unit needs and requests (equipment, staff, etc.), which provided them with additional motivation. Switching patients can take some time because physicians have to inform them and obtain their consent beforehand. Thus, one of the recommendations by Moorkens et al. is that physicians must be encouraged [16].
However, the plateau observed from July 2019 to October 2020 most likely reveals a ceiling effect. It may illustrate the limits of clinical units’ ability to switch more patients, the loss of enthusiasm for the experiment, and/or the impact of the COVID-19 pandemic, because the same curve inflexion is observed in both incentive groups.
Moreover, as the official instruction specifies only that the “majority of the hospital remuneration should be given to the clinical units” [28, 36], different levels of remuneration for clinical units are observed depending on negotiations with their hospital management, and other units such as pharmacies that may have been involved. This sticking point could also be one reason that has discouraged prescribers from continuing their efforts when clinical units were not always paid the full amount they had expected.
Last, a lack of information provided to prescribers could influence their involvement. Prescribers whose hospital belongs (by default) to the general case are not always well informed of such incentives. In contrast, the experimental case needs the involvement of clinical units and prescribers to answer the call for proposals. However, prescribers do not often have easy access to their results and the amount of remuneration perceived, which is paid per semester a year later. Those who kept their enthusiasm may have introduced common use of biosimilars into clinical unit practice. As a result, even if this enthusiasm wanes over time, biosimilar prescription will become more systematic, and part of the management of patients on biosimilars will become habitual.
Patients treated with biologic products typically have severe and chronic diseases such as immunology and inflammatory diseases or metabolic disorders and are generally familiar with the treatment they have been taking for a long time. As a result, they could be reluctant to switch to another product [38]. Nonetheless, Scherlinger et al. observed a high rate of patient acceptance of switching from etanercept reference product to biosimilars in their study conducted in 2017 in the rheumatology department of Bordeaux University Hospital (France). Patients who refused, on the other hand, were found to be older and have had the disease for a longer period. They were also hesitant to use generics [39]. It may be assumed that when clinicians take the time to explain, patients are confident in their doctors and are more likely to accept the change.
To reach the expected performance, Glickman and Peterson and Mathes et al. advocate for encouraging health care providers to develop innovative solutions that benefit all stakeholders, including patients [20, 21]. Moreover, they advise testing new programs in real-world settings before generalizing them, as the French government has done with the new profit-sharing scheme to increase etanercept biosimilar uptake.
This French experiment must be financed to ensure the clinical units' compensation. Funding of 2.5 million euros for 2018 and 5 million euros for the following years was estimated at the start of the experiment for the two biosimilar groups. Meanwhile, it should save between 6 and 12 million euros for each biosimilar group, depending on the year [26].
There are not yet official data on the financial impact of the two incentives at this time. The 2019 NHI annual report predicted a savings of 50 million euros as a result of the new biosimilar national incentive for hospital prescriptions delivered in retail pharmacies [40]. After hospitals have received their remuneration, the expected economy provided by the experimental case is estimated to be €6 M in 2018 and €12 M in 2019 and 2020 [28]. A short preliminary report was sent to the hospitals under the experimental case in 2021. It indicates a mean etanercept biosimilar remuneration of €20,402 (min: €1743; max: €98,966) for the 2020 first semester.
While the major French teaching hospitals (higher prescribers) are under the experimental case, the dataset provides only a sample of health facilities included in the general case. Taking into account that private practitioners’ renewals were not included, the financial impact was not calculated.
Confirming the appeal for these types of measures, a similar incentive targeting adalimumab was implemented 6 months after the experimental case began with etanercept and insulin glargine. This other anti-TNFα represents a major financial issue because its reference product (Humira®) was the highest selling product in the world with $20 billion in 2018 [41]. Its biosimilar was launched into the French market in October 2018. In light of these promising preliminary results, the following further analyses should be performed: (1) on adalimumab biosimilar penetration thanks to the new incentive scheme, and (2) comparison of hospitals under experimentation, and identification of indicators that favor biosimilar prescriptions in this incentive scheme.
5 Conclusion
This study found that hospitals in the experimental incentive program were more likely to prescribe biosimilars. As a result, it suggests that the demand-side policy that targets physicians by rewarding clinical units is effective. Twenty-five months after the scheme's implementation, a difference of approximately 8–10% was observed between the two groups. The findings support the hypothesis made by public authorities that the closer the incentive is to the physicians, the more likely they are to participate.
Otherwise, this experience emphasizes wide disparities among hospitals, with a high concern for the genuine access of physicians to comprehensive information, money they should obtain and direct usage of it by care units. The assessment and correction of these limiting points should be performed before rolling out this incentive.
Notes
This policy measure concerns both generics and biosimilar medicine. Prescribers should prescribe using international nonproprietary names (INNs), and pharmacists must deliver the cheapest medicines when they have this type of prescription.
Hospital prescriptions delivered in retail pharmacies (PHEV in French) are written by hospital physicians for patient discharge and are given in community pharmacies. The prescribed treatments are mostly covered by the French NHI.
CAQES: (in French) contrat d’amélioration de la qualité et de l’efficience des soins.
All French social and health care establishments are registered in a national file with a corresponding number called the ‘FINESS’ number (in French: fichier national des établissements sanitaires et sociaux). It is the same for physicians who are identified by their number from the repository of practitioners (i.e., RPPS). Thanks to these numbers, all prescriptions made by facilities or independent physicians can be linked to them.
Health facility activity is quantified by the number of patients discharged (included deaths) thanks to an anonymized discharge summary with the primary diagnosis. This summary is forwarded to Technical Agency for Information on Hospital Care (ATIH).
The general case applies to all French hospitals that have signed the contract (CAQES) with NHI and their regional agency. In France, there are over 3000 health facilities, 1354 of which are public.
References
Global medicines use in 2020: outlook and implications. IMS Health for Healthcare Informatics Report, 2015. https://www.imshealth.com/en/thought-leadership/ims-institute/reports/global-medicines-use-in-2020#ims-form. Accessed 21 Feb 2016.
European Medicines Agency. Biosimilar medicines: Overview [Internet]. 2019 Oct. EMA/627319/2022. https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview#regulatory-guidance-section. Accessed Oct 2022.
IQVIA. The Impact of Biosimilar Competition in Europe 2021. White paper online 2021, Dec. https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2021. Accessed Sept 2022.
Kim Y, Kwon HY, Godman B, Moorkens E, Simoens S, Bae S. Uptake of biosimilar infliximab in the UK, France, Japan, and Korea: budget savings or market expansion across countries? Front Pharmacol. 2020;11.
Rémuzat C, Dorey J, Cristeau O, Ionescu D, Radière G, Toumi M. Key drivers for market penetration of biosimilars in Europe. J Mark Access Health Policy. 2017;5(1):1272308.
IQVIA. Biosimilars reach inflection point – on track to reduce drug costs by $100 bn over next five years. 2020, Oct. https://www.iqvia.com/newsroom/2020/10/iqvia-institute-for-human-data-science-study-biosimilars-reach-inflection-point--on-track-to-reduce. Accessed Sept 2022.
Elyse Muñoz. A positive road ahead for biosimilars? IQVIA institute reports. 2021, Jan. https://www.iqvia.com/insights/the-iqvia-institute/reports/a-positive-road-ahead-for-biosimilars. Accessed Sept 2022.
IQVIA. Advancing biosimilar sustainability in Europe. 2018, Sept. IQVIA institute report. https://www.iqvia.com/insights/the-iqvia-institute/reports/advancing-biosimilar-sustainability-in-europe. Accessed Oct 2022.
IMS health. Delivering on the Potential of Biosimilar Medicines. 2016, March. https://www.medicinesforeurope.com/wp-content/uploads/2016/03/IMS-Institute-Biosimilar-Report-March-2016-FINAL.pdf. Accessed Oct 2022.
Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PLoS ONE. 2017;12(12): e0190147. https://doi.org/10.1371/journal.pone.0190147.
LOI n° 2013-1203 du 23 décembre 2013 de financement de la sécurité sociale pour 2014 [Internet]. JORF n°0298, NOR EFIX1324269L décembre, 2013. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000028372809. Accessed Sept 2022.
Ministère des solidarités et de la santé, ministère de l’Action et des comptes publics. INSTRUCTION N° DGOS/PF2/DSS/1C/DGS/PP2/2017/244 du 3 août 2017 relative aux médicaments biologiques, à leurs similaires ou « biosimilaires », et à l’interchangeabilité en cours des traitements - Légifrance [Internet]. NOR SSAH1722975J Dec 10, 2017. https://www.legifrance.gouv.fr/circulaire/id/42638. Accessed Nov 2022.
IQVIA. The Impact of Biosimilar Competition in Europe 2022. White paper online 2022, Dec. https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2022. Accessed Mar 2022.
European Medicines Agency, European Commission. Biosimilars in the EU - Information guide for healthcare professionals [Internet]. European Medicines Agency; 2019 Feb p. 40. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed May 2022.
European Medicines Agency. Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU [Internet]. 2022 Sept. EMA/627319/2022. https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf. Accessed Nov 2022.
Moorkens E, Simoens S, Troein P, Declerck P, Vulto AG, Huys I. Different policy measures and practices between swedish counties influence market dynamics: part 2—biosimilar and originator etanercept in the outpatient setting. BioDrugs. 2019;33(3):299–306.
Gibofsky A, McCabe D. US rheumatologists’ beliefs and knowledge about biosimilars: a survey. Rheumatol Oxf Engl. 2020;60(2):896–901.
Barcina Lacosta T, Vulto AG, Turcu-Stiolica A, Huys I, Simoens S. Qualitative analysis of the design and implementation of benefit-sharing programs for biologics across Europe. BioDrugs. 2022;36(2):217–29. https://doi.org/10.1007/s40259-022-00523-z. (Epub 2022 Mar 18).
INAMI (Belgian National Insurance Institute for Maladies and Invalidity) Prescrire « bon marché » [Internet] last update October 13, 2021. https://www.riziv.fgov.be/fr/professionnels/sante/medecins/soins/Pages/prescrire-bon-marche-20150101.aspx. Accessed Apr 2023.
Mathes T, Pieper D, Morche J, Polus S, Jaschinski T, Eikermann M. Pay for performance for hospitals. Cochrane Database Syst Rev. 2019;(7).
Seth W. Glickman MD, Eric D. Peterson MD. Innovative health reform models: pay-for-performance initiatives. Am J Manag Care. 2009.
Eijkenaar F. Pay for performance in health care: an international overview of initiatives. Med Care Res Rev. 2012;69(3):251–76.
Sécurité sociale. Les prescriptions hospitalières exécutées en ville [Internet]. Les Comptes de la sécurité sociale; 2020 Sep [cited 2022 Mar 2] p. 130–3. https://www.securite-sociale.fr/files/live/sites/SSFR/files/medias/CCSS/2020/FICHE_ECLAIRAGE/CCSS-FICHE_ECLAIRAGE-SEPT_2020-%20Les%20prescriptions%20hospitali%C3%A8res%20ex%C3%A9cut%C3%A9es%20en%20ville.pdf. Accessed Nov 2018.
Assurance Maladie. Rapport charges et produits, propositions de l’assurance maladie pour 2022.pdf [Internet]. 2021 juillet [cited 2022 Jun 10] pp. 65–77. https://assurance-maladie.ameli.fr/sites/default/files/rapport_charges_et_produits_-_propositions_de_lassurance_maladie_pour_2022_juillet_2021.pdf. Accessed June 2022.
Robinson JC, Jarrion Q. Competition from biosimilars drives price reductions for biologics in the French single-payer health system. Health Aff. 2021;40(8):1190–7.
Ministère des solidarités et de la santé. Arrêté du 3 août 2018 relatif à l’expérimentation pour l’incitation à la prescription hospitalière de médicaments biologiques similaires délivrés en ville [Internet]. JORF n°0188, NOR SSAS1821431A août, 2018. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000037316661 Accessed Sept 2020.
Lechat P. Médicaments biosimilaires: enjeux réglementaires et impacts médicoéconomiques. Bull Acad Natl Med. 2020;204(8):877–83.
Ministère des solidarités et de la santé. INSTRUCTION N° DSS/1C/DGOS/PF2/2018/42 du 19 février 2018 relative à l’incitation à la prescription hospitalière de médicaments biologiques similaires, lorsqu’ils sont délivrés en ville [Internet]. circulaire.legifrance.gouv.fr, NOR SSAS1805085J Feb 19, 2018. https://www.omedit-grand-est.ars.sante.fr/media/29966/download?inline. Accessed Sept 2020.
Ministère des solidarités et de la santé, ministère de l’Économie, des finances et de la relance. Arrêté du 2 octobre 2018 fixant la liste des établissements retenus dans le cadre de l’expérimentation pour l’incitation à la prescription hospitalière de médicaments biologiques similaires délivrés en ville [Internet]. JORF n°0233, NOR SSAS1826777A Oct 9, 2018. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000037477126. Accessed Sept 2022.
ATIH. Open data available from https://hospidiag.atih.sante.fr/. Accessed May 2022.
Fougère D. Econometric evaluation methods. Rev Francaise Aff Soc. 2010;1:105–28.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Abadie A. Semiparametric difference-in-differences estimators. Rev Econ Stud. 2005;72:1–19. https://doi.org/10.2307/3700681.
Rubin DB. Matching to remove bias in observational studies. Biometrics. 1973;29:159–83.
Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. https://doi.org/10.18637/jss.v042.i08.
Lemaire N. Avis du Comité technique de l’innovation en santé sur le projet d’expérimentation nationale « Incitation à la prescription hospitalière de médicaments biologiques similaires, lorsqu’ils sont délivrés en ville» [Internet]. 2022 Mar p. 2. https://solidarites-sante.gouv.fr/IMG/pdf/biosimilaires-avis_ctis-mars_2022.pdf. Accessed Apr 2022.
Daw JR, Hatfield LA. Matching and regression to the mean in difference-in-differences analysis. Health Serv Res. 2018;53(6):4138–56. https://doi.org/10.1111/1475-6773.12993.
Rezk MF, Pieper B. To See or NOsee: the debate on the nocebo effect and optimizing the use of biosimilars. Adv Ther. 2018;35(6):749–53.
Scherlinger M, Langlois E, Germain V, Schaeverbeke T. Acceptance rate and sociological factors involved in the switch from originator to biosimilar etanercept (SB4). Semin Arthritis Rheum. 2019;48(5):927–32.
Assurance Maladie. Rapport charges et produits-propositions de l’assurance maladie pour-2019.pdf [Internet]. 2018 Jul. https://assurance-maladie.ameli.fr/sites/default/files/2018-07_rapport-propositions-pour-2019_assurance-maladie.pdf. Accessed 10 June 2022.
Abbvie. AbbVie reports full-year and fourth-quarter 2018 financial results|AbbVie News Center [Internet]. 2019. https://news.abbvie.com/news/abbvie-reports-full-year-and-fourth-quarter-2018-financial-results.htm. Accessed 21 Feb 2022.
Acknowledgements
We would like to acknowledge IQVIA and Biogen France who provided us with the dataset. We also thank all of the reviewers who helped us to improve the quality of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest, Corporate-sponsored research or other substantive relationships
IQVIA and Biogen France provided us with the dataset for the analysis.
Data availability
The main dataset that supports the findings of this study is available from IQVIA but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of IQVIA and Biogen France.
Code availability
The R code used in this analysis is available from the authors upon request.
Authors’ contributions
All authors contributed to the study concept and design. Data processing was handled by MT and MR; MT and ADT undertook the data analyses. The first draft of the manuscript was written by MT and all authors commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tano, M., Paubel, P., Ribault, M. et al. What About Offering a Financial Incentive Directly to Clinical Units to Encourage the Use of Biosimilars? Results of a Two-Year National Experiment in France. Appl Health Econ Health Policy 21, 799–811 (2023). https://doi.org/10.1007/s40258-023-00812-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00812-w