Abstract
Many countries have considered telemedicine and home monitoring of patients as a solution to the demographic challenges that health-care systems face. However, reviews of economic evaluations of telemedicine have identified methodological problems in many studies as they do not comply with guidelines. The aim of this study was to examine economic evaluations alongside randomised controlled trials of home monitoring in chronic disease management and hereby to explore the resources included in the programme costs, the types of health-care utilisation that change as a result of home monitoring and discuss the value of economic evaluation alongside randomised controlled trials of home monitoring on the basis of the studies identified. A scoping review of economic evaluations of home monitoring of patients with chronic disease based on randomised controlled trials and including information on the programme costs and the costs of equipment was carried out based on a Medline (PubMed) search. Nine studies met the inclusion criteria. All studies include both costs of equipment and use of staff, but there is large variation in the types of equipment and types of tasks for the staff included in the costs. Equipment costs constituted 16–73% of the total programme costs. In six of the nine studies, home monitoring resulted in a reduction in primary care or emergency contacts. However, in total, home monitoring resulted in increased average costs per patient in six studies and reduced costs in three of the nine studies. The review is limited by the small number of studies found and the restriction to randomised controlled trials, which can be problematic in this area due to lack of blinding of patients and healthcare professionals and the difficulty of implementing organisational changes in hospital departments for the limited period of a trial. Furthermore, our results may be based on assessments of older telemedicine interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This review of economic evaluations alongside randomised controlled trials of home monitoring in chronic disease management describes nine studies and includes both costs of equipment and use of staff. Large variation was found in the types of equipment and types of tasks for the staff included in the costs. Equipment costs constituted 16–73% of the total programme costs. |
Future studies should pay special attention to equipment costs and the possibilities for using the patient’s own devices or other elements that could reduce the costs of telemedicine interventions. |
1 Introduction
Many countries and international institutions have considered telemedicine and home monitoring of patients as a solution to the demographic challenges that healthcare systems face including an increasing number of patients with chronic disease [1]. Telemedicine is defined broadly here as the delivery of healthcare services through the use of information and communication technologies (ICT) in situations where the patients and the health professionals are in different locations. Home monitoring of patients via ICT is an important part of telemedicine interventions, and a number of studies have shown that it can reduce the number of bed days and emergency department visits for patients with chronic disease [2]. This has led to the hope that telemedicine and home monitoring can reduce the healthcare costs per patient [3].
However, reviews of economic evaluations of telemedicine and home monitoring do not provide a clear result. A 2002 systematic review of cost-effectiveness studies of telemedicine interventions [4] included 55 articles, but only 44% meet all the quality criteria applied in the review. Only 60% of the studies included equipment costs and many were small-scale and short-term, pragmatic studies. Therefore, the authors concluded that there was no solid evidence that telemedicine was cost effective.
Similarly, a 2009 systematic review [5] identified 33 economic evaluations of telemedicine and found that most lacked information on perspective and costing method. Few studies used sensitivity analysis and statistical analysis to assess the validity of the results and under half provided details on resources consumed in physical units and reported prices separately from quantities. The authors concluded that most of the economic evaluations had not been undertaken in accordance with standard evaluation techniques.
A 2012 systematic review that included 80 full economic evaluations of telemedicine, found that most studies had inadequate details about study design and methodology, including how costs were collected, calculated and reported [6]. The author thus found no further evidence that telemedicine interventions were cost effective. A 2014 review of nine published systematic reviews of cost-effectiveness analysis of telemedicine studies [7] also identified methodological flaws and joined the general consensus that most of the telemedicine studies do not follow the guidelines for economic evaluation.
Two other reviews from 2014 have found similar results from studies of telemedicine for specific patient groups. A review of six cost-effectiveness studies of telemedicine for patients suffering from chronic obstructive pulmonary disease (COPD) [8] found a potential for cost savings, but also noted the poor quality of the economic evidence and that decision makers seeking large-scale implementation of telemedicine in clinical practice should be cautious. A literature review of 32 cost-effectiveness studies of telemedicine intervention for patients with chronic heart failure [9] again concluded that most studies were not comprehensive economic evaluations (e.g. 72% did not include relevant costs, and many lacked information on investment costs). However, the few studies with a proper economic evaluation showed that telemedicine was cost saving and slightly improved effectiveness.
Most reviews of economic evaluations of telemedicine refer to the handbook on methods for economic evaluation of healthcare programmes [10] when assessing the economic evaluations. The handbook recommends economic evaluations to include estimation of the costs of organising and operating the programme (the programme costs) as well as the economic and health consequences. The programme costs should include both the variable costs (that vary with the level of outputs, e.g. supplies and health professionals’ time) and the fixed costs (that do not vary with the quantity of output in the short term, e.g. 1 year). Typical examples of fixed costs are capital costs, which are the costs of purchasing the major capital assets required by the programme (e.g. equipment and buildings). Fixed costs typically represent investments at a single point in time, often at the beginning of the programme. The recommended method for estimating capital costs is to annuitise the initial capital outlay over the expected number of years of use of the asset and calculate the equivalent annual cost [10].
The handbook [10] also includes a 10-item check-list for assessing economic evaluations of healthcare programmes. According to the check-list economic evaluation should include all relevant costs and consequences including capital costs (item 4), information about costs and consequences should be measured accurately in appropriate physical units (item 5) and give a credible valuation of costs (i.e. prices) and consequences (item 6).
These items are often not included in the estimated costs of telemedicine. Their importance has been underlined, however, in a 2016 guideline on economic evaluation of health IT programmes [11] as considerable costs can be associated with health IT implementation, maintenance, required infrastructure and time spent on training in the use of the technology. A 2015 guideline for measuring costs and benefits of eHealth intervention [12] also noted that implementation of eHealth often incurs equipment costs that can be spread over the expected life time of the equipment.
Reviews of telemedicine evaluations have so far focused on scientific methods and study quality. However, researchers involved in assessment of telemedicine and home-monitoring programmes need information about which healthcare resources to include in the estimation of the programme costs of home monitoring and which types of use of healthcare that may change as a result of the use of home monitoring. Here, a review limited to health economic evaluations based on randomised controlled trials can be useful because randomised studies can be designed to include the information needed, and because randomised studies have the highest level of internal validity. The aim of this study was to examine economic evaluations alongside randomised controlled trials of home monitoring in chronic disease management and hereby to explore the resources included in the programme costs, the types of healthcare utilisation that change as a result of home monitoring and to discuss the value of economic evaluation alongside randomised controlled trials of home monitoring on the basis of the studies identified.
2 Methods
The method used in this study is a scoping review, which is a rapid form of knowledge synthesis where the aim is to map the key concepts underpinning a research area and the main sources of evidence available [13]. This type of review can be done as a stand-alone project in a complex area with limited evidence, or it can be done prior to a systematic review. A scoping review differs from a systematic review as it addresses broader research questions, permits inclusion of different study designs, often does not assess the quality of the included studies, has a less structured data extraction, and typically uses a qualitative synthesis of the evidence [14]. The typical stages of a scoping review are: (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collecting, summarising, and reporting the results [13]. We have identified our research question in the study aim above and describe the other stages in the following sections.
2.1 Identifying Relevant Studies
The review includes studies with the three following criteria: (1) economic evaluations based on randomised controlled trials (RCTs) of home monitoring; (2) for patients with one of the chronic diseases: asthma, COPD, diabetes, heart failure, hypertension; and (3) reporting the estimated costs per patient in the intervention and the control group, the home-monitoring programme costs and the costs per patient related to investment and use of home-monitoring equipment.
To identify studies, we used a search strategy inspired by a comprehensive review by Wootton [15] that included 141 RCTs of telemedicine in the management of chronic diseases (asthma, COPD, diabetes, heart failure, hypertension). One the basis of this review, the specific search terms were:
(Telemedicine OR telehealth OR tele monitoring OR home monitoring) AND (Costs OR cost-effectiveness OR cost-utility) AND (Asthma OR COPD OR diabetes OR heart failure OR hypertension). The search was limited regarding article type for RCTs, and only articles written in English were included.
2.2 Study Selection
Studies were selected in two steps. First, titles and abstracts identified from the Medline search were screened for their eligibility for inclusion. Then the full text of potentially relevant studies was obtained and examined to confirm that the studies included information on home monitoring, programme costs and types of costs (as this information was rarely available in the abstract). The two authors selected the studies independently, and any discrepancies were resolved through discussion.
2.3 Charting the Data
The following information from the selected studies (including appendices and online supplements) was entered into a data chart using the Microsoft Excel 2010:
-
Authors
-
Year of publication
-
Country in which the study took place
-
Study population
-
Content of the home-monitoring intervention
-
Perspective of the economic evaluation
-
Types of costs included in the programme costs
-
Types of costs included in the economic consequences of home monitoring (in addition to the programme costs)
-
Number of subjects
-
Mean cost per patient in the home-monitoring group
-
Mean cost per patient in the control group
-
Mean programme costs per patient of the home-monitoring intervention
-
Mean cost of home-monitoring equipment per patient.
Equipment costs were defined as the costs of investment, leasing, or rental of home-monitoring equipment (e.g. spirometer, pulse oximeter, weight scale, heart rate and blood-pressure monitor, and other wireless technology) as well as the costs of submission and collection of information (e.g. use of telephone lines, broadband connection, web-based portal, etc.). Thus, costs related to use of healthcare professionals were not included in the costs of equipment.
To improve the comparability of results from the different studies, the estimated costs in the local currency was transferred into Euro (€) using the exchange rate of the year concerned [16]. We subsequently adjusted for inflation by taking into account changes in the consumer prices from the year of the study to 2016. This was done using the Harmonised Index of Consumer Prices for the European Union estimated by Eurostat [17]. Our results are thus price level 2016.
2.4 Summarising and Reporting the Results
In summarising the identified studies, we first described the range of home-monitoring interventions, the patient groups, and the geographical distribution. We then compared the types of costs included in the estimated programme costs and the economic consequences of home monitoring. Two tables were made, one with information about the patients, the interventions and the included costs, and another with information about the estimated average costs per patient, the home-monitoring programme costs and the equipment costs. Our analysis was especially focused on differences in the types of costs included, costs of equipment and use of healthcare professionals, and the need for further research.
3 Results
3.1 Search Results
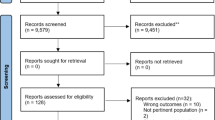
The Medline search was carried out June 20th 2017 and identified 630 publications. Based on screening of abstracts, 577 were excluded mainly because they were not based on RCTs or did not describe reviews of home monitoring. After assessing 53 full-text articles for eligibility, 44 articles were excluded, mostly because the articles did not describe the programme costs per patient or the costs of equipment (Fig. 1). The remaining nine articles [18,19,20,21,22,23,24,25,26] were included in the review.
3.2 General Characteristics of Included Studies
The nine studies were published between 2012 and 2017 (Table 1) and were mostly undertaken in Europe. The nine studies comprised the following: interventions for patients only with COPD (n = 4 studies), patients with hypertension (1), patients with diabetes (1), patients with chronic heart failure (1), patients with asthma (1) and one study included three patient groups. The intervention periods were 4–12 months and between one and six different devices were installed in the patients’ homes. Data were submitted to a website or web-based portal, and the number of weekly readings varied between studies. Often, clinical teams assessed the data in monitoring teams and contacted the patients by phone based on alerts. Thus, although the home-monitoring interventions were similar, the studies differed in the specific devices used, the duration of the interventions, and the patient groups.
3.2.1 Resources Included in Programme Costs and Economic Consequences of Home Monitoring
As described in Table 1, there is variation in the types of equipment and types of tasks for the staff included in the estimated costs. For example, some studies include costs of hosting and installation in their estimated equipment costs, whereas others only included costs of hardware and peripherals. Similarly, some studies included costs of training of staff and patients, whereas others only included time used for monitoring of patients. Except for two studies [25, 26] all studies used the annuitisation method to estimate annual costs and assumed that the equipment would last for 3–5 years.
The different kinds of healthcare utilisation included in the estimated economic consequences of home monitoring are also described in Table 1. The perspective of the economic evaluations in all studies had a healthcare sector perspective, and seven studies included both patients’ utilisation of hospital services, emergency department service, general practitioner (GP) and some form of primary care. The table also shows that the highest (percentage) cost reductions among the home-monitoring patients were found with regard to the patients’ use of GP [20, 23, 25], district nursing [19, 22], emergency department [21], hospitalisations [18, 24] and drugs [26]. Thus, a reduction in the costs per patient was often found with regard to primary and acute care.
3.3 The Estimated Home-Monitoring Costs
The home-monitoring programme costs varied between €71 and €3,323 (Table 2). This reflects mainly the differences between the patient groups but also the differences in content and duration of the interventions and the types of costs included. Equipment costs varied between €16 and €1277 per patient (Table 2) and constituted 16–73% of the total programme costs. Equipment costs thus had a substantial impact on the total programme costs in most of the studies.
In three of the nine studies, the mean cost per patient using home monitoring was lower than the cost per patient in the control group, by €681 to €2765. In six studies, the mean costs were higher in the home-monitoring group, by €98 to €2718 per patient and in two of these six studies, this difference in the mean costs per patient between the telemedicine group and the control group was statistically significant [22, 25].
Table 2 also shows the home-monitoring programme costs. In five studies, home monitoring led to a reduction in other types of costs as the change in mean cost was less than the home-monitoring programme cost [18, 20, 24,25,26]. However, in four studies [19, 21,22,23] the costs of other types of healthcare were increased, as the absolute change in mean cost was higher than the home-monitoring programme cost.
4 Discussion
This scoping review of nine economic evaluations based on RCTs has shown that there was large variation in the types of equipment and types of tasks for the staff included in the costs. Some studies include costs of hosting and installation of equipment, whereas others only included costs of hardware and peripherals. Similar, some studies included costs of training of staff and patients, whereas others only included time used for monitoring. Equipment costs constituted 16–73% of the total programme costs. In six of the nine studies home monitoring resulted in reduction in primary care or emergency contacts. However, in total, home monitoring resulted in increased average costs per patient in six of nine studies. The main reason seems to be that home monitoring may involve substantial programme costs (i.e. the costs of running the home-monitoring intervention), and it does not necessarily reduce other costs (e.g. hospital admission or primary care). Choice of hardware and devices is thus a crucial economic factor when implementing home monitoring for patients with chronic disease. It should also be noted that three studies found statistically significantly improved clinical outcomes [18, 22, 25]. Therefore, these studies could demonstrate that even though telemedicine is increasing the costs per patient, these technologies may be cost effective. Two of the studies concluded that this was the case [22, 25].
4.1 The Value of Economic Evaluation Alongside RCTs of Home Monitoring
A main advantage of the RCT design is the high level of internal validity as it minimises the risk of systematic error (bias) by ensuring that the intervention and the control groups have similar observed and unobserved characteristics [27]. In addition, RCTs allow collection of the data that is considered necessary at patient level. However, several critical issues have been raised against the use of RCTs in studies of telemedicine and digital health.
First, the cost-effective introduction of home monitoring may require organisational changes (e.g. within a clinical department), but these changes are unlikely to occur if the same department is going to offer conventional treatment for a control group and a telemedicine service for an intervention group over a time-limited trial period [28]. Cluster randomisation, as used in two of the nine studies [18, 23], or observational before-after studies may thus demonstrate more positive results at patient level than studies with randomisation at patient level if large organisational changes are a condition for realising the full benefits of a telemedicine service.
Second, an RCT is also time-consuming when assessing clinical information systems, and this can be a problem in studies of rapidly developing IT applications. As an example, the RCT of home monitoring of patients with diabetic foot ulcer described in Table 1 [24], took 4 years to carry out. The duration of the RCT itself can lead to a less positive result in studies of telemedicine, but, as pointed out in a discussion of the pros and cons of RCT in clinical information systems, other designs such as prospective observational studies may also take several years [29].
Third, RCT guidelines state that blinding of patients and healthcare professionals is needed to avoid bias. This may be impossible in practice and may bias the results if both groups have positive expectations toward telemedicine and home monitoring [30]. The nine studies described in this review did not blind the patients. Studies of digital health interventions suggest that if a person who has sought help for a particular problem is randomised to the control group, he/she might find a digital solution online and thereby reduce the estimated impact of the intervention [27]. The lack of blinding may thus lead to positive or negative bias.
Finally, it is important to be aware that using RCTs in studies of home monitoring with the objective of minimising the risk of bias (and thereby ensuring internal validity) may be at the expense of a low degree of transferability or external validity [27]. For example, if only highly motivated patients are included in an RCT to ensure high compliance, the level of transferability of the results will be low. If expensive IT solutions are used to engage patients and health professionals and thereby increase the success of the trial, the home monitoring may also be more expensive than otherwise needed.
Based on these potential problems with RCTs, it has been argued that observational studies can provide valuable evidence on the cost effectiveness of interventions if RCTs are impractical [10]. Others have pointed out that although RCTs are an important part of the toolkit for evaluation of digital health interventions, they make up only one part [29]. Parallel to this, it has been suggested that RCTs should be undertaken only when the intervention has reached an acceptable degree of stability, the implementation of the intervention is expected to have a high degree of fidelity, and it is likely that clinical outcomes will be improved [27]. However, it should be noted that potential problems with the duration of a trial, the transferability of the results, and implementation problems can also occur in observational studies. Differences between results from RCTs and observational studies are an important evidence gap that needs to be considered in future studies of telemedicine and home monitoring.
4.2 Strengths and Limitations
The strength of this review is that it only includes studies based on RCTs that report information on both programme and equipment costs. Thus, we avoided a large number of potentially misleading economic evaluations based on studies with low levels of evidence or not reporting programme costs.
The inclusion of only nine studies may be considered a limitation because most of the existing economic evaluations have been excluded. Even with our highly selected sample of studies, few studies fully complied with the checklist for reporting of health-economic evaluations [10] and included detailed information about the resources used, prices for each resource, and the different types of costs. This review originally aimed to collect information about fixed costs, variable costs and capital costs from each study, but this level of detail was only available in six of the nine studies.
Assessment of eligibility of the articles was difficult because of a large variation in the reporting of the economic evaluations. Especially, assessment of whether the programme costs and the equipment costs are included in the estimated costs was difficult. As an example, two articles [31, 32] were not included because, even though the use of health professionals in the home monitoring for (e.g.) training of patients was described, the estimated programme costs did not include these costs. Similar, another randomised study [33] excluded the equipment costs, because both the intervention and the control group where implanted with the same device with the possibility to do home monitoring. Finally, a study [34] did not included device costs related to home monitoring because the equipment was provided free of charge, consequently, the study was not included in the review.
This review was limited to studies of home monitoring for patients with one of five different chronic diseases. A substantial part of the variation in the estimated costs per patient therefore could be explained by the differences between the patient groups and the interventions. Inclusion of other types of telemedicine, other patient groups, or other databases for the literature search may have altered the results. Furthermore, the studies included in the review were published in 2012–2017, with data collection starting before 2011 in seven of the nine studies. Moreover, the home-monitoring equipment was probably selected months before the studies started, and thus the results of this review may reflect the costs of older telemedicine technologies. The more recent technical developments may have reduced the prices and costs of telemedicine equipment.
5 Conclusion
This scoping review of economic evaluations alongside RCTs of home monitoring in chronic disease management has shown that there was large variation in the types of equipment and types of tasks for the staff included in the costs. Equipment costs constituted 16–73% of the total programme costs. The studies did demonstrate that the interventions resulted in reduction in, for example, primary care or emergency contacts; however, overall home monitoring resulted in increased average costs per patient in six of the nine studies. Those who design future studies of home monitoring should be aware of this risk of increased costs per patient, and special attention should be given to equipment costs. The use of the patient’s own devices or other elements that may reduce costs should be considered when designing future interventions.
Change history
13 October 2017
Table 2, ‘Stoddart [19]’ row: The cell entry in the ‘Mean cost per control patient (SE)’ column,
References
Commission Communication. Telemedicine for the benefit of patients, healthcare systems and societies. COM/2008/689 final. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2008:0689:FIN:EN:PDF. Accessed 01 Aug 2011.
Henderson C, Knapp M, Fernández J, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ. 2013;346:f1035.
AlDossary S, Martin-Khan MG, Bradford NK, Smith AC. A systematic review of the methodologies used to evaluate telemedicine service initiatives in hospital facilities. Int J Med Inform. 2017;97:171–94.
Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S. Systematic review of cost effectiveness studies of telemedicine interventions. BMJ. 2002;324(7351):1434–7.
Bergmo TS. Can economic evaluation in telemedicine be trusted? A systematic review of the literature. Cost Eff Resour Alloc. 2009;7(1):1.
Mistry H. Systematic review of studies of the cost-effectiveness of telemedicine and telecare. Changes in the economic evidence over twenty years. J Telemed Telecare. 2012;18(1):1–6.
Mistry H, Hyeladzira G, Oppong R. Critical appraisal of published systematic reviews assessing the cost-effectiveness of telemedicine studies. Telemed e-Health. 2014;20(7):609–18.
Udsen FW, Hejlesen O, Ehlers LH. A systematic review of the cost and cost-effectiveness of telehealth for patients suffering from chronic obstructive pulmonary disease. J Telemed Telecare. 2014;20:212–20.
Grustam AS, Severens JL, Nijnatten J, Koymans R, Vrijhoef HJ, et al. Cost-effectiveness of telehealth interventions for chronic heart failure patients: a literature review. Int J Technol Assess Health Care. 2014;30(01):59–68.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
Luzi D, Pecoraro F, Tamburis O. Economic evaluation of health IT. Page 165–180. In: Ammenwerth E, Rigby M, Eds. Evidence-based Health Informatics and the Scientific Development of the Field. Evidence-Based Health Informatics: Promoting Safety and Efficiency Through Scientific Methods and Ethical Policy (2016).
Bergmo TS. How to measure costs and benefits of ehealth interventions: an overview of methods and frameworks. J Med Internet Res. 2015;17(11):e254. doi:10.2196/jmir.4521.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi:10.1080/1364557032000119616.
Levati S, Campbell P, Frost R, et al. Optimisation of complex health interventions prior to a randomised controlled trial: a scoping review of strategies used. Pilot Feasibility Stud. 2016;2:17. doi:10.1186/s40814-016-0058-y.
Wootton R. Twenty years of telemedicine in chronic disease management–an evidence synthesis. J Telemed Telecare. 2012;18:211–20.
European Central Bank. Exchange rates. http://sdw.ecb.europa.eu/browse.do?node=9691296. Accessed 29 Dec 2016.
Eurostat. Harmonised Index of Consumer prices (HICP). http://ec.europa.eu/eurostat/web/hicp/data/database?p_p_id=NavTreeportletprod_WAR_NavTreeportletprod_INSTANCE_BO6Fgp25CkI9&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column-2&p_p_col_count=3. Accessed 29 Dec 2016.
Henderson C, Knapp M, Fernandex J-L, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ. 2013;20(346):f103.
Stoddart A, van der Pol M, Pinnock H, Hanley J, et al. Telemonitoring for chronic obstructive pulmonary disease: a cost and cost-utility analysis of a randomised controlled trial. J Telemed Telecare. 2015;21(2):108–18.
De San Miguel K, Smith J, Lewin G. Telehealth remote monitoring for community-dwelling older adults with chronic obstructive pulmonary disease. Telemed e-Health. 2013;19(9):652–7.
Jódar-Sánchez F, Ortega F, Parra C, et al. Cost-utility analysis of a telehealth programme for patients with severe chronic obstructive pulmonary disease treated with long-term oxygen therapy. J Telemed Telecare. 2014;20(6):307–16.
Stoddart A, Hanley J, Wild S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomised controlled trial. BMJ. 2013;3(5):e002681.
Udsen FW, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish ‘TeleCare North’ cluster-randomised trial. BMJ Open. 2017;7(5):e014616.
Fasterholdt I, Gerstrøm M, Rasmussen BSB, et al. Cost-effectiveness of telemonitoring of diabetic foot ulcer patients. Health Inform J 2016;1460458216663026. doi:10.1177/1460458216663026.
Ryan D, Price D, Musgrave SD, et al. Clinical and cost effectiveness of mobile phone supported self-monitoring of asthma: multicenter, randomised controlled trial. BMJ. 2012;344:e1756.
Cui Y, Doupe M, Katz A, Nyhof P, Forget EL. Economic evaluation of Manitoba Health Lines in the management of congestive heart failure. Healthc Policy. 2013;9(2):36–50.
Murray E, Hekler EB, Andersson G, et al. Evaluating digital health interventions: key questions and approaches. Am J Prev Med. 2016;51(5):843–51.
Kidholm K, Jensen LK, Kjølhede T, Nielsen E, Horup MB. Validity of the model for assessment of telemedicine: a Delphi study. J Telemed Telecare. 2016;1:357633X16686553. doi:10.1177/1357633X16686553.
Liu JLY, Wyatt JC. The case for randomized controlled trials to assess the impact of clinical information systems. J Am Med Inform Assoc. 2011;18:173–80.
Craig JA, Carr L, Hutton J, Glanville J, Iglesias CP, Sims AJ. A review of the economic tools for assessing new medical devices. Appl Health Econ Health Policy. 2015;13(1):15–27.
Madsen LB, Christiansen T, Kirkegaard P, Pedersen EB. Economic evaluation of home blood pressure telemonitoring: a randomized controlled trial. Blood Press. 2011;20(2):117–25.
Haesum LK, Soerensen N, Dinesen B, et al. Cost-utility analysis of a tele-rehabilitation program: a case study of COPD patients. Telemed e-Health. 2012;18(9):688–92.
Zanaboni P, Landolina M, Marzegalli M, et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res. 2013;15(5):e106. doi:10.2196/jmir.2587.
Calò L, Gargaro A, De Ruvo E, et al. Economic impact of remote monitoring on ordinary follow-up of implantable cardioverter defibrillators as compared with conventional in-hospital visits. A single-center prospective and randomized study. J Interv Card Electrophysiol. 2013;37(1):69–78.
Acknowledgements
The authors thank Lise Kvistgaard Jensen and Claire Gudex, Odense University Hospital, for language editing of the manuscript and Christian Kronborg, COHERE, University of Southern Denmark for valuable comments on behalf of the Center for Innovative Medical Technology, Odense University Hospital.
Author information
Authors and Affiliations
Contributions
KK contributed to development of the aim of the study, selection of the method for the review, literature search, assessment of the articles, charting of data, analysis and interpretation of data. MBDK contributed to development of the aim of the study, literature search, assessment of the articles, charting of data, analysis and interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
The authors Kristian Kidholm and Mie Borch Dahl Kristensen declare that there is no conflict of interest.
Funding
The review was funded by the Research Council at Odense University Hospital.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s40258-017-0356-4.
Rights and permissions
About this article
Cite this article
Kidholm, K., Kristensen, M.B.D. A Scoping Review of Economic Evaluations Alongside Randomised Controlled Trials of Home Monitoring in Chronic Disease Management. Appl Health Econ Health Policy 16, 167–176 (2018). https://doi.org/10.1007/s40258-017-0351-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-017-0351-9