Abstract
Background
In states in the USA without in vitro fertilzation coverage (IVF) insurance coverage, more embryos are transferred per cycle leading to higher risks of multi-fetal pregnancies and adverse pregnancy outcomes.
Objective
To determine frequency and cost of selected adverse perinatal complications based on number of embryos transferred during IVF, and calculate incremental cost per IVF live birth.
Methods
Medical records of patients who conceived with IVF (n = 116) and delivered at >20 weeks gestational age between 2007 and 2011 were evaluated. Gestational age at delivery, low birth weight (LBW) term births, and delivery mode were tabulated. Healthcare costs per cohort, extrapolated costs assuming 100 patients per cohort, and incremental costs per infant delivered were calculated.
Results
The highest prematurity and cesarean section rates were recorded after double embryo transfers (DET), while the lowest rates were found in single embryo transfers (SET). Premature singleton deliveries increased directly with number of transferred embryos [6.3 % (SET), 9.1 % (DET) and 10.0 % for ≥3 embryos transferred]. This trend was also noted for rate of cesarean delivery [26.7 % (SET), 36.6 % (DET), and 47.1 % for ≥3 embryos transferred]. The proportion of LBW infants among deliveries after DET and for ≥3 embryos transferred was 3.9 and 9.1 %, respectively. Extrapolated costs per cohort were US$718,616, US$1,713,470 and US$1,227,396 for SET, DET, and ≥3 embryos transferred, respectively.
Conclusion
Attempting to improve IVF pregnancy rates by permitting multiple embryo transfers results in sharply increased rates of multiple gestation and preterm delivery. This practice yields a greater frequency of adverse perinatal outcomes and substantially increased healthcare spending. Better efforts to encourage SET are necessary to normalize healthcare expenditures considering the frequency of very high cost sequela associated with IVF where multiple embryo transfers occur.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Multiple gestation and preterm delivery rates, but not necessarily pregnancy rates, increase with clinical IVF practices that encourage multiple embryo transfers. |
Given the high cost sequela associated with IVF where multiple embryo transfers occur, single embryo transfers should be performed whenever possible. |
1 Introduction
Currently, 11 US states mandate insurance coverage for infertility diagnosis and treatment; only five of these states have in vitro fertilization (IVF) explicitly covered [1]. Studies have demonstrated statistically significant decreases in the number of embryos transferred per cycle and in multiple birth rates in states where mandated insurance coverage for IVF exists; without IVF insurance coverage, more embryos are transferred per cycle [2]. Thus in 2013, approximately 21 % of assisted reproductive technology (ART) cycles incorporating fresh non-donor oocytes culminated in transfer of ≥3 embryos, approximately 6 % of cycles involved transfer of ≥4 embryos, and 2 % of cycles involved transfer of ≥5 embryos [3]. While such high-order embryo transfers are intended to help improve pregnancy rates, the concern with transferring so many embryos at the same time has been well documented [4–10]. In 2013, 57.8 % of twins and 95.8 % of triplets (or other higher-order multiple gestations) were born preterm, and singletons born from multi-fetal pregnancies had a higher preterm birth rate (17.3 %) than singletons from single-fetus pregnancies (11.3 %) [3]. In USA, the downstream economic burden of these ART-associated preterm deliveries is estimated at US$1 billion annually [6].
Twins, triplets and singletons from multi-fetal pregnancies were also at an increased risk of being born with low birth weight (LBW) (<2500 g); approximately 56.2 % of twins and 92.3 % of triplets resulting from ART cycles in 2013 were LBW. Of note, the proportion of LBW infants is higher for singletons deriving from multi-fetal pregnancies where reduction occurred (17.1 %) compared to those from single-fetus pregnancies (9.0 %). LBW infants are at an increased risk of death and short- and long-term disabilities including cerebral palsy, intellectual disabilities, and limitations in motor and cognitive skills [3].
Multi-fetal pregnancies pose dangers to both mother and fetuses. Other risks such as intrauterine growth restriction, maternal hypertension, pre-eclampsia, and postpartum hemorrhage occur three to seven times more than in singleton pregnancies, while perinatal mortality rates are estimated to be fourfold higher for twins and sixfold higher for triplets than for singletons [8].
Given these adverse outcomes, it is surprising that multiple-embryo transfers remain so common in current IVF practice. The majority of patients in USA self-fund their IVF cycles, and for the average American household in states without mandates in 2007/2008, the cost of one fresh IVF cycle represented 52 % of the annual disposable income, compared to 13 % of the annual disposable income in states with mandates [11]. Thus, patients often elect to transfer multiple embryos to increase the likelihood that one embryo will implant and result in a live birth [2]. However, the associated complications conflict with the goal of pursuing infertility therapy in order to have a healthy child under normative definitions of health [8].
Even though insurance does not usually cover IVF in the USA, these poor perinatal outcomes are nevertheless paid for from national health expenditures. The mean hospital cost per preterm infant and LBW infant, compared to mean cost of all infants is US$21,500 and US$27,200 versus US$3200, respectively [12].
This retrospective cohort study examined pregnancy outcomes and incremental costs based on the number of embryos transferred during IVF at an academic medical center in Vermont, a state where no insurance mandate for IVF currently exists. Based on associated pregnancy outcomes per cohort, the estimated hospital costs at time of delivery per cohort and incremental cost per baby gained were calculated. We hypothesized that as the number of embryos transferred increased, rates of adverse perinatal outcomes such as cesarean sections (C-sections), preterm births, and LBW infants would rise.
2 Methods
Data for IVF transfers were previously collected by faculty in the Vermont Center for Reproductive Medicine at Fletcher Allen Health Care (FAHC). After receiving Institutional Review Board (IRB) approval, a list of patients who had successfully conceived with IVF between 2007 and 2011 was generated. These records were divided into three groups which were determined by the number of embryos transferred during IVF (stratified by 1, 2 or ≥3 embryos). Follow-up data for each cohort were then collected from the FAHC delivery database (“OB Net”) which registered maternal and fetal outcomes for those who delivered at FAHC at >20 weeks gestation. Outcomes examined included gestational age at delivery, birth weight, and delivery method. Risk of each patient group with 95 % confidence interval (CI) was calculated. Patients with prior preterm deliveries and those who had elective C-sections were not included. Each group with multiple embryos transferred was compared against the single-embryo transfer group using Fisher’s exact test. A p value <0.05 was considered statistically significant.
Direct inpatient costs of vaginal deliveries, C-sections, premature infants, term infants, and LBW term infants (as the majority of preterm infants are also LBW) were estimated across cohorts using published data [13, 14] and adjusted for the cost of medical inflation through 2015 [15]. Neonatal costs of initial hospitalization from 2003 with inflation adjustment were utilized for estimates because, to our knowledge, this is the most recent study that quantifies, in dollar amounts, the cost of prematurity based on week of gestation at delivery and birth weight. The cost of each outcome was summarized by sum, mean, and standard deviation (SD). The mean and sum of total cost for each group was calculated as was the 95 % CI of the mean via Monte Carlo simulations. There were 10,000 replicates simulated assuming the total cost of each patient followed a gamma distribution. The distributional parameters were estimated by the moment estimates.
Outcomes and costs were then extrapolated to 100 patients per cohort. Extrapolated cost equaled the mean cost multiplied by the outcome rate, where both mean cost and outcome rate were estimated from the study population. The incremental cost between cohorts was calculated using the extrapolated totals for each group. Incremental costs were then divided by the incremental benefit (“babies gained”) to compute incremental cost per additional baby gained in each cohort of 100 patients [16].
The 95 % CI associated with each incremental cost was calculated by the bootstrap method described as follows. We simulated 100 data (including total cost and number of infants) with replacement from the data that we had for each group. We summed the cost and number of infants by group, and then calculated incremental cost for DET and the group with ≥3 embryos transferred. The same steps were repeated for 10,000 times and the collected incremental costs were used to calculate the 95 % CIs.
In a cost-effectiveness analysis, we focused on the comparisons between SET and DET and between ≥3ET and SET. The differences between groups in hospital costs and number of additional infants born (“effectiveness”) were calculated. Further, we estimated the cost-effectiveness acceptability curves using the method of Van Hout et al. [17] via the R package ICEInfer [18]. All the statistical analyses were carried out using the statistical software R 3.1.2 [19].
The outcome of additional baby gained was chosen instead of the more often used “live birth” (where multiples count as one live birth) to reflect that each child born as a result of ART has individual costs associated with its initial hospitalization, its lifetime costs and its future earning potential. Thus, to truly evaluate the difference in cost between cohorts, costs associated with each baby gained should be evaluated rather than live birth. However, in terms of treatment effectiveness, all individuals in this study did achieve a live birth, and thus, were considered a treatment success.
3 Results
Of all IVF conceptions occurring between 2007 and 2011, a total of 116 patients proceeded to deliver at >20 weeks’ gestation at FAHC where complete perinatal records were available for analysis. In this population, 17 deliveries were from SET, 73 were from DET, while 26 resulted from transfer of ≥3 embryos. Of the SET cases, all deliveries were singleton. Among IVF patients who underwent DET, 44 singletons and 29 sets of twins were born. For IVF patients who had transfer of ≥3 embryos, a review of delivery records showed that 20 singletons, 5 sets of twins and 1 set of triplets were born. For the entire study sample, these 116 pregnancies resulted in a total of 152 babies.
3.1 Demographics
OB NET contained demographic information regarding maternal age. Mean maternal age at delivery for SET was 37.5 ± 4.9 years of age, DET 34.2 ± 4.8 years of age and ≥3ET 36.8 ± 4.2 years of age. The Vermont population is mostly caucasian, and as nearly all patients pay for IVF out of pocket, the study population it is limited to middle and upper class populations.
3.2 Gestational Age at Delivery
Preterm delivery was defined as gestational age at delivery of <37 weeks. The lowest rate of preterm delivery was found in IVF patients who had SET (6.3 %), while those patients having DET had the highest rate of preterm pregnancies (27.4 %). The latter circumstance resulted in 35.3 % of deliveries after DET being preterm (95 % CI = 26.3, 45.4, p = 0.020; see Table 1). These data were controlled for prior preterm pregnancies by excluding patients with prior preterm deliveries.
In this sample, 26.9 % of all preterm deliveries were singletons while most babies (73.1 %) born prematurely were from multiple gestation. The proportion of preterm singletons increased with the number of embryos transferred per cohort, such that 6.3 % followed SET, 9.1 % followed DET (95 % CI = 3, 22.6) and 10.0 % followed transfer of ≥3 embryos (95 % CI = 1.8, 33.1; see Table 1). Data estimating the associated hospital costs for preterm infants were used to calculate approximate cost of the preterm neonates registered in our sample, as previously described [13]. Records revealed that preterm infants of SET cost FAHC US$8106 (or US$8106/infant), while preterm infants of DET cost FAHC US$673,570 (or US$18,710/infant). Of note, preterm deliveries following transfer of ≥3 embryos resulted in hospital costs of US$110,396 (or US$12,226/infant). Using rate of prematurity found in each patient group, the observed costs were extrapolated to a 100-patient cohort. From this it was estimated that total cost of preterm infants from SET would be equal to US$50,663, while the total cost from DET and transfer of ≥3 embryos would be US$924,494 and US$424,592, respectively (see Table 2).
To project the total expenditure for each group more accurately, medical costs for normal-weight, term infants per cohort were also calculated. Actual cost to FAHC based on term infants delivered after SET was US$35,319 (mean = US$2355/infant), while cost of term infants born after 2 and ≥3 embryos transferred was US$137,416 (mean = $2216/infant) and US$49,215 (mean = US$2344/infant), respectively. Extrapolating to 100 patients per cohort yielded cost of all normal-weight, term infants delivered after SET, DET and ≥3 embryos transferred to be US$220,774, US$188,577 and US$189,438, respectively (see Table 2). Infants born at term with low or very low birth weights were not included in this analysis.
3.3 Low Birth Weight Term Infants
Low birth weight (LBW) babies were defined as a weight at delivery of <2500 g. Very low birth weight (VLBW) babies were defined as delivery weight of <1500 g. Extremely low birth weight babies (ELBW) were defined as delivery weight of <1000 g. Of note, no deliveries after SET were LBW although 27 babies (26.5 %, p = 0.021) after DET were LBW or VLBW, and 11 babies (33.3 %, p = 0.009) delivered after transfer of ≥3 embryos were LBW. After excluding preterm deliveries, as costs for those babies were accounted for in the previous paragraphs, four babies (3.9 %) from DET and three babies (9.1 %) following transfer of ≥3 embryos were LBW (see Table 1). In this sample, all remaining infants with low, very low, or extremely low birth weights were preterm.
Hospital costs per infant were also tabulated based on birth weight [13]. After excluding preterm deliveries, the estimated institutional cost per cohort of term LBW or VLBW babies was US$0 for SET, US$54,812 for DET and US$24,897 for deliveries after ≥3 embryos transferred. After extrapolating these findings to 100-patient cohorts for each group, estimated costs were calculated at US$0 for SET, US$75,232 for DET and US$95,816 for deliveries after ≥3 embryos transferred (see Table 2).
3.4 Delivery Mode: Utilization of Cesarean Section
After controlling for elective abdominal deliveries (elective C-sections), the highest C-section rate was found in DET (54.9 %) (95 % CI = 42.7, 66.6), while the lowest C-section rate was for IVF patients who underwent SET (26.7 %). When comparing singleton versus multiple gestations, 100 % of C-sections in SET were for singleton gestations, while 38.5 % of C-sections performed in DET and 66.7 % of those performed for deliveries after ≥3 embryos transferred were for singletons. The C-section rate for singletons increased as the number of embryos per cohort increased: 26.7 % for SET, 36.6 % for DET (95 % CI = 22.6, 53.1), and 47.1 % for deliveries after ≥3 embryos transferred (see Table 1).
The next phase of this analysis assumed that the cost for each vaginal delivery was US$3400 while cost for each C-section was US$5900 [14]. After adjusting for inflation, estimated cost for C-section for deliveries following SET was US$25,960, while C-sections from DET and for deliveries after ≥3 embryos transferred resulted in institutional costs of US$253,110 and US$77,880, respectively (see Table 2). After including estimated costs of vaginal deliveries, total delivery costs (to hospital) were US$67,100 for SET, US$372,790 for DET and US$119,020 for deliveries after ≥3 embryos transferred. Extrapolating these observations to 100 patients per cohort, the estimated delivery expenditure per cohort was US$447,209 for SET, US$525,167 for DET and US$517,550 for those who delivered after ≥3 embryos transferred.
3.5 Aggregate Analysis
Combining the cost data associated with preterm infants, term infants (excluding LBW/VLBW), LBW and VLBW term infants, as well as costs associated with mode of delivery, the estimated hospital costs per cohort were US$110,525 for SET (95 % CI of mean total cost: US$5,738–US$7,293), US$1,238,588 for DET (95 % CI of mean total cost: US$6,684–US$31,528) and US$303,528 for deliveries after ≥3 embryos transferred (95 % CI of mean total cost: US$7,786–US$16,308). Using extrapolated estimates to better compare the three IVF treatment groups, projected costs per cohort were calculated at US$718,616 for SET, US$1,713,470 for DET and US$1,227,396 for deliveries after ≥3 embryos transferred assuming each cohort consisted of 100 patients (see Table 2).
3.6 Incremental Cost Evaluation
Costs were compared between cohorts (DET vs. SET, and ≥3ET vs. SET) using extrapolated totals for each group. The totals were then divided by the incremental benefit (“babies gained”) to calculate incremental cost per additional baby gained in each cohort. Thus, each baby gained through DET cost US$24,871 (95 % CI: US$15,827–US$39,724) while each infant gained after ≥3 embryos transferred cost US$18,844 (95 % CI: US$14,568–US$24,161) (see Table 3).
3.7 Cost-Effectiveness Analysis
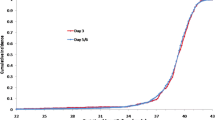
While each group was considered effective from a treatment perspective given the outcome of a live birth, the observed effectiveness difference in terms of numbers of babies born was 0.397 between DET and SET and their mean difference in cost was US$10,465.488 per infant. Similarly, the two differences between ≥3ET and SET were 0.269 and US$5,172.683 per infant, respectively. Given an incremental cost-effectiveness willingness to pay (acceptable maximum amount to pay in addition to the cost of SET), the corresponding acceptability, or probability that the treatment is cost effective, was calculated and presented in a cost-effectiveness acceptability curve (CEAC). The CEACs for DET versus SET and for ≥3ET versus SET were nearly identical and the single curve is presented in Fig. 1. The curve shows that SET should be absolutely favored over DET and ≥3ET if the incremental cost-effectiveness (ICE) willingness to pay is ≤US$5,000–US$10,000 above the cost of SET; the probability that DET/≥3ET is cost effective starts increasing after that point and reaches 0.5 when the ICE willingness to pay is US$20,000 above SET.
The acceptability curve by the method of Van Hout et al. [17] where the incremental cost effectiveness (ICE) willingness to pay is in thousands of US dollars and acceptability is the probability that DET/≥3ET is more cost effective than SET (the two curves are nearly identical)
4 Discussion
This paper examines the direct, inpatient costs of maternal deliveries, hospital stays and costs of initial neonatal hospitalizations based on the number of embryos transferred during in vitro fertilization. To the knowledge of the authors, it is the first paper to do so. This investigation contributes additional evidence to support earlier findings that rates of preterm births, LBW babies, and C-sections performed increase with DET and when ≥3 embryos are transferred, compared to SET. However, our study shows that this increase is not completely attributable to the negative effect of multiple gestations alone. Indeed, as the number of embryos transferred increased, higher rates of preterm births and C-sections were recorded even among singleton pregnancies. The reason for this observation is unknown. Perhaps it is related to transfer of multiple lower-quality or less “fit” embryos, which are more likely to result in relatively poorer perinatal outcomes. Unfortunately our analysis could not test this hypothesis as morphology scores were not available for evaluation and chromosomal screening of embryos was not uniformly available. Alternatively, negative effects of uterine crowding (during placentation) when multiple embryos are transferred simultaneously could explain our findings. In any case, costs associated with deliveries from DET and deliveries after ≥3 embryos transferred were substantially higher than costs associated with SET. After normalizing for equal numbers of patients in each cohort (n = 100), the estimated hospital costs associated with births from DET were nearly 2.5 times greater than for births following SET (US$1,713,470 vs. US$718,616).
Additionally, as seen in Fig. 1, if a patient sees it as an advantage to have more than one baby in a cycle, they (or society) need to be willing to pay more than an additional US$5,000–US$10,000 (above the cost of a SET), with increasing costs for increasing likelihood of having an additional baby. As mentioned previously, the acceptability of 0.0 is actually a reasonable target because, through SET, she can have a live singleton, which is a highly desired outcome. It is important to remember that the costs are estimated hospital costs, associated only with maternal delivery, hospitalization and initial neonatal admission. Thus, the ICE willingness to pay would actually be much higher for associated acceptability if all costs were included.
This work supports the position that IVF cycles involving appropriate embryo transfer policies should be covered by insurance companies [2, 4, 7, 9, 10]. Many developed countries such as Australia, the UK, Finland, Austria, Spain, the Netherlands, Israel and parts of Canada already provide coverage for IVF cycles [20]. Without insurance coverage, couples may feel financially pressured to transfer more embryos per cycle to increase their chances of having a successful pregnancy [2]. Even women who would be candidates for SET often transfer multiple embryos, such that 63.2 % of IVF twin births in USA (2006) and 54.9 % of IVF triplets were born to women aged <35 years [6]. Similarly in our study population, 54.3 % of women who gave birth to twins or triplets were aged <35 years.
It might be argued that the expense of doing consecutive SET cycles to successfully conceive and deliver a baby will exceed the cost of one successful IVF treatment where more than one embryo is transferred. Yet, not only is the incremental cost per baby gained (US$24,871 for DET and $18,844 for ≥3 embryos transferred) significantly greater than the cost of an IVF cycle in the USA (average US$12,400) [2, 21], but IVF techniques and success rates have continued to improve so drastically that SET now has nearly the same success rates as double embryo transfers [22–26]. Data from 2013 demonstrated that among good-prognosis women (<35 years old who had extra embryos available), live birth rates using fresh, non-donor eggs or embryos resulted in a SET live birth rate of 51.0 %, and a DET live birth rate of 55.8 %. However, 44 % of the live births for DET were either twins or triplets compared to the 1.8 % of multiple gestations that resulted from embryo splitting after SET [3]. Thus the difference in the cost of treatment between cohort groups can now be considered nominal, while the small increase in live birth rate resulted in a substantial increase in multiples. These data support the conclusion that insurance companies would save a significant amount by covering IVF given the cost savings achieved by decreasing the number of multiple embryo transfers that are performed.
Others may claim that offering insurance coverage for an expensive procedure will drive up demand so much that any cost savings gained through coverage will be negated by increased IVF uptake. However, the number of individuals who require IVF to treat infertility is actually a small part of the background population. Even in countries with very high utilization rates, ART does not exceed 0.25 % of public or private spending on healthcare. Thus, the aftermath of multiple embryo transfers already accounts for a much larger proportion of healthcare spending than healthcare spending on IVF in high-utilization countries [7]. Interestingly, the lifetime tax contribution of a child conceived through IVF in USA would exceed by seven-fold the amount of any government subsidy needed to access this treatment [4]. Likewise, a UK analysis demonstrated that an initial investment of GBP12,931 to achieve an IVF singleton was worth 8.5 times that amount to the UK Treasury in discounted future tax revenue [27].
By providing insurance coverage, the process of IVF can be more regulated as to the number of embryos that are actually transferred. For example, the Belgian government agrees to pay for six IVF cycles in women aged <43 years, if they followed particular embryo transfer guidelines. IVF data from before and after this law was implemented have been compared, and the percentage of SET increased from 14 to 49 % while implantation rates were 25.9 % before implementation and 23 % afterwards. While there was no significant change in overall pregnancy rates (36 vs. 37 %), twin pregnancies declined sharply from 19 to 3 %. Thus, elective SET made feasible by insurance coverage for IVF can significantly decrease the multiple gestation rate and associated costs, without reducing overall pregnancy rates [28].
The interconnected challenges of preterm birth and multiple gestations are mutually exacerbated by IVF practices where multiple embryo transfers predominate [29]. A recent analysis based on Californian data found that if partial subsidization were provided for every IVF cycle initiated there, a net surplus of at least US$20M per year would still be realized by stabilizing the IVF multiple birth rate at ~3.2 %. Such coverage would remain net revenue-positive for California, because although IVF is expensive, the price to provide this technology is always less than the cost for one high-risk preterm/multiple birth [29].
The major strengths of our study include the fact that results were controlled for both prior preterm deliveries and elective C-sections so that such patients did not skew the data. Additionally, all IVF pregnancies in the state of Vermont that met inclusion criteria were included in this analysis. This investigation is the first to present comprehensive, longitudinal data on the Vermont IVF population and resulting expenses encountered here. Given Vermont’s rural nature, the state has only one IVF center so patients must either attend this unit or obtain treatment out of state.
As with any study, there are limitations to these findings and ours should be acknowledged. First, the number of patients in each group was unequal. Cohorts were extrapolated to 100 patients each, but the cost estimates are less accurate than if there had actually been 100 patients per cohort. The most important limitation is that the sample population was small despite the fact that all IVF pregnancies that met inclusion criteria were included; statistically significant p values were seen in select circumstances, but overall the population was not large enough to demonstrate statistical significance. Despite this, our findings parallel those reported by the US Centers for Disease Control [3], and the associated hospital costs cannot be ignored. Indeed, we expect that costs computed here are actually underestimates due to the exclusion of costs associated with other adverse outcomes such as maternal hypertension, pre-eclampsia, interuterine growth restriction, and the increased perinatal surveillance required with multiple gestations. Additional indirect medical costs associated with preterm birth such as developmental delay, chronic respiratory problems, and vision and hearing impairments were also not included in our estimate. Preterm and LBW infants are significantly more likely to be rehospitalized and require more acute care visits over the first year of life than infants born full term and at normal birth weight. The cost of providing healthcare to LBW infants through the first year of life is estimated to approach US$5 billion per year [30]. Another weakness is that data entered into “OB Net” are transcribed from the original medical record by an individual, rather than entered directly. Thus, the results are dependent on faithful transcription and documentation.
In conclusion, our study demonstrates that with multiple embryo transfers there may be higher rates of preterm birth, more LBW term babies born, and higher rates of cesarean delivery. Such outcomes lead to very large medical expenditures and present a substantial economic burden to healthcare delivery systems. Moreover, the medical bills continue to accrue as long-term disabilities from perinatal sequela are treated over the lifetime of IVF offspring. In USA, the current insurance system does not make it financially feasible for IVF patients to elect single embryo transfer; in the absence of satisfactory insurance coverage for IVF, these high-cost trends will continue.
References
American Society for Reproductive Medicine. State infertility insurance laws. http://www.asrm.org/insurance.aspx. Accessed 27 Nov 2015.
Martin JR, Bromer JG, Sakkas D, Patrizio P. Insurance coverage and in vitro fertilization outcomes: a U.S. perspective. Fertil Steril. 2011;95:964–9.
United States. Centers for Disease Control and Prevention: Division of Reproductive Health. 2013 Assisted Reproductive Technology National Summary Report, 2013.
Ata B, Seli E. Economics of assisted reproductive technologies. Curr Opin Obstet Gynecol. 2010;22:183–8.
Blickstein I, Jones C, Keith LG. Zygotic-splitting rates after single-embryo transfers in in vitro fertilization. N Engl J Med. 2003;348:2366–7.
Bromer JG, Ata B, Seli M, Lockwood CJ, Seli E. Preterm deliveries that result from multiple pregnancies associated with assisted reproductive technologies in the USA: a cost analysis. Curr Opin Obstet Gynecol. 2011;23:168–73.
Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91:2281–94.
ESHRE Capri Workshop Group. Multiple gestation pregnancy. Hum Reprod. 2000;15:1856–64.
Forte L. Funding of IVF in Quebec: a cost-benefit analysis. Infertility Awareness Association of Canada. March 13, 2009.
Janvier A, Spelke B, Barrington KJ. The epidemic of multiple gestations and neonatal intensive care unit use: the cost of irresponsibility. J Pediatr. 2011;159:409–13.
Chambers GM, Adamson GD, Eijkemans MJ. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–27. doi:10.1016/j.fertnstert.2013.06.017.
Kowlessar NM, Jiang HJ, Steiner C. Hospital stays for newborns, 2011. Healthcare Cost and Utilization Project (HCUP) Statistical Brief # 163. October 2013. Rockville, MD.
Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: quantification by gestational age and birth weight. Obstet Gynecol. 2003;102:488–92.
Moore JE, Witt WP, Elixhauser A. Complicating conditions associated with childbirth, by delivery method and payer, 2011. Healthcare Cost and Utilization Project (HCUP) Statistical Brief #173. May 2014. Rockville, MD.
Consumer Price Index, Medical Care. Bureau of Labor Statistics. Databases, tables & calculators. http://data.bls.gov/cgi-bin/surveymost. Accessed 27 Nov 2015.
Jones CA. Economic evaluation of alternative embryo transfer policies in in vitro fertilisation (IVF). PhD thesis; 2005, Univ Oxford. British Library EthOS uk.bl.ethos.426399.
Van Hout BA, Al MJ, Gordon GS, Rutten FFH. Costs, effects and C/E ratios alongside a clinical trial. (VAGR curve). Health Econ. 1994;3:309–19.
Obenchain B. ICEinfer: incremental cost-effectiveness (ICE) Statistical Inference from two unbiased samples. R package version 1.0-1. 2014. http://CRAN.R-project.org/package=ICEinfer. Accessed 10 Dec 2015.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. http://www.R-project.org/.
Ndegwa S, Kelly S. Status of public funding for in vitro fertilization in Canada and internationally [Environmental Scan Issue 14]. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2010.
American Society for Reproductive Medicine. Resources. “Is in vitro fertilization expensive?” https://www.asrm.org/detail.aspx?id=3023. Accessed 4 Dec 2015.
Straughen JK, Salihu HM, Keith L, Petrozzino J, Jones C. Obligatory versus elective single embryo transfer in in vitro fertilization: a population-based analysis of data from the U.K. Human Fertilisation and Embryology Authority. J Reprod Med. 2013;58:95–100.
Lukassen HGM, Braat DD, Wetzels AMM, Zielhuis GA, Adang EMM, Scheenjes E, Kremer JAM. Two cycles with single embryo transfer versus one cycle with double embryo transfer: a randomized controlled trial. Hum Reprod. 2005;20:702–8.
Kjellberg AT, Carlsson P, Bergh C. Randomized single versus double embryos transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Hum Reprod. 2006;21:210–6.
Gerris J, De Sutter P, De Neubourg D, Van Royen E, Vander Elst J, Mangelschots K, et al. A real-life prospective health economic study of elective single embryo transfer versus two-embryo transfer in first IVF/ICSI cycles. Hum Reprod. 2004;19:917–23.
Fauque P, Jouannet P, Davy C, Guibert J, Viallon V, Epelboin S, et al. Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril. 2010;94:927–35.
Connolly M, Gallo F, Hoorens S, Ledger W. Assessing long-run economic benefits attributed to an IVF-conceived singleton based on projected lifetime net tax contributions in the UK. Hum Reprod. 2009;24(3):626–32. doi:10.1093/humrep/den435.
Gordts S, Campo R, Puttermans P, Brosens I, Valkenburg M, Norre J, et al. Belgian legislation and the effect of elective single embryo transfer on IVF outcome. Reprod Biomed Online. 2005;10(4):436–41.
Sills ES. An evidence-based policy for the provision of subsidised fertility treatment in California: integration of array comparative genomic hybridisation with IVF and mandatory single embryo transfer to lower multiple gestation and preterm birth rates. PhD thesis (2013), Univ Westminster-London. British Library EthOS uk.bl.ethos.576982.
Cuevas KD, Silver DR, Brooten D, Youngblut JM, Bobo CM. Hospital charges at birth and frequency of rehospitalizations and acute care visits over the first year of life: a comparison by gestational age and birth weight. Am J Nurs. 2005;105(7):56–65.
Acknowledgments
The authors are grateful to Sue O’Brien (Embryologist) for her assistance with this research.
Authors’ contributions
OJC was principal investigator, conceived the research, and prepared the initial manuscript; CLK performed statistical analysis and contributed to the manuscript. PRC, RSR and ESS contributed to study design, assisted in data analysis, and developed drafts of the work; CAJ was lead faculty, assisted with research design, data collection, and provided oversight on the entire project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source
None.
Conflict of interest
Olivia J. Carpinello, Peter R. Casson, Chia-Ling Kuo, Renju S. Raj, E. Scott Sills and Christopher A. Jones declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Carpinello, O.J., Casson, P.R., Kuo, CL. et al. Cost Implications for Subsequent Perinatal Outcomes After IVF Stratified by Number of Embryos Transferred: A Five Year Analysis of Vermont Data. Appl Health Econ Health Policy 14, 387–395 (2016). https://doi.org/10.1007/s40258-016-0237-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0237-2