Abstract
Therapeutic options for people with moderate or severe atopic dermatitis refractory to topical therapy have rapidly expanded in recent years. These new targeted immunomodulatory agents—biologics and Janus kinase (JAK) inhibitors—have each demonstrated high levels of efficacy and acceptable safety in mostly placebo-controlled clinical trials for atopic dermatitis, but there is no universally applicable algorithm to help choose between them for a given patient. Hence, patients and physicians should utilize shared decision making, discussing efficacy, safety, mode of delivery, monitoring, costs, speed of onset, and other factors to reach individualized treatment decisions. In this review, we try to aid shared decision making by summarizing the efficacy, safety, and monitoring of biologics and oral JAK inhibitors for adults with atopic dermatitis. Network meta-analyses suggest that higher doses of abrocitinib and upadacitinib are more effective than biologics. They also show that, among biologics, dupilumab is likely more effective than tralokinumab and lebrikizumab. Biologics are generally considered safer than JAK inhibitors, although concerns about JAK inhibitors are mainly extrapolated from older generation JAK inhibitors used in higher-risk populations. We also outline evidence and considerations for choosing and using systemic immunomodulatory treatments for special populations including pregnant individuals, those with human immunodeficiency virus (HIV), hepatitis B and C, end stage kidney disease, and older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atopic dermatitis is a complex, chronic inflammatory skin condition that can be effectively managed with systemic, targeted therapies such as biologics and Janus kinase inhibitors. |
Shared decision making, considering relative efficacy, safety, and individual patient characteristics and values are important when choosing between these medications. |
1 Introduction
Atopic dermatitis is a heterogeneous disease primarily characterized by chronic, eczematous pruritic skin lesions [1]. Severe pruritus, skin pain, swelling, and xerosis, along with sleep disturbances, mental health comorbidities and associated atopy contribute to a decreased quality of life, particularly in patients with moderate-to-severe disease [2, 3]. For people whose atopic dermatitis is inadequately controlled or whose quality of life is substantially lowered despite appropriate topical therapy, ultraviolet phototherapy or systemic therapy can be used [4]. While phototherapy can be effective for many patients, the evidence base underlying it is of low certainty, and the need for frequent clinic visits is often not feasible for patients [5]. Until recently, only broad-acting systemic immunomodulators such as corticosteroids, methotrexate, mycophenolate mofetil, cyclosporine, and azathioprine were available to treat atopic dermatitis. Those agents are limited in many cases by poor tolerability, the need for ongoing bloodwork monitoring, and limited efficacy [6]. Additionally, cyclosporine, which is the most effective of the older agents [7], is not recommended for long-term use, which is problematic given the chronic nature of atopic dermatitis.
Over the last decade, substantial progress has been made to meet the therapeutic needs of people with refractory, severe atopic dermatitis. While the role of Th2 cytokines in atopic dermatitis pathogenesis has been known since the 1990s, only recently has this pathway been targeted as therapy [8, 9]. There are now four targeted systemic agents approved by the US Food and Drug Administration (FDA) for atopic dermatitis, including two biologics and two Janus kinase (JAK) inhibitors, with several other agents at various stages of development. These targeted treatments give patients and clinicians multiple effective treatment options for more severe atopic dermatitis, leading to improved quality of life for many patients. Patients and clinicians considering systemic therapy must choose between these agents, taking various medication- and patient-specific factors into account.
The objective of this review is to help inform clinical decision-making for adults with atopic dermatitis considering systemic therapy. As this review focuses on treatment for adults, we have not included in-depth data and suggestions regarding pediatric populations. We present the mechanism of action, efficacy and safety profiles, monitoring, and considerations for special patient populations for biologics and oral JAK inhibitors recently approved and those likely to be approved soon for the treatment of atopic dermatitis in adults.
2 Considerations When Choosing Systemic Therapy
Once a patient and their clinician make the decision to pursue systemic treatment for atopic dermatitis, there are several considerations that may influence their choice of first-line agent. Efficacy, including rapid onset of action, is important [10]. Safety, including avoiding both nuisance and more severe adverse effects, is essential. Patients may prefer oral versus injectable therapy, or a specific agent based on their values or their own research of treatment options. Patients and clinician should discuss the benefits and risks of different treatments to enable individualized shared decision making [11, 12].
Patient characteristics, such as age, comorbidities, pregnancy status, and family planning also factor into treatment decisions. Atopic dermatitis impacts individuals of all ages. Although historically thought to occur primarily in early childhood and resolve by adolescence, studies with longer duration of follow-up show that atopic dermatitis can occur at any age, and there is increasing recognition that atopic dermatitis is common among older adults over 60 years of age [13, 14]. Unfortunately, high-quality clinical evidence for the use of systemic treatment in older patients is lacking, as older individuals are often excluded from clinical trials [15], along with pregnant individuals and people with comorbidities including kidney or hepatic disease [16].
Costs and medication coverage (either through private or public payors) may limit options for some patients. Newer targeted agents are more expensive than older conventional systemic agents [17,18,19]. As a result, access to new targeted therapies such as biologics and small molecules are often limited [16, 20]. In one US study, denial or lack of insurance coverage was reported as the most common reason why patients who were candidates for biologic treatment did not initiate therapy [21]. Conventional systemic agents remain viable treatment options for patients with moderate-to-severe atopic dermatitis, particularly when cost or insurance coverage is an issue.
Ultimately, while there is no universally applicable therapeutic algorithm for systemic treatment of atopic dermatitis, utilizing a shared decision-making process that incorporates patient preferences and characteristics, as well as a discussion of benefits, risks, costs, and availability of therapies, will be important in formulating a therapeutic plan. Given the large evidence base supporting their efficacy and safety, biologics and oral JAK inhibitors are generally preferred, when available, over conventional systemic agents. As such, the focus of this article is on those newer targeted agents, and we do not discuss conventional agents in detail.
3 Biologics
3.1 Dupilumab
Dupilumab is a human IgG-4 monoclonal antibody that blocks the interleukin-4 receptor α (IL-4Rα), ultimately downregulating the effects of IL-4 and IL-13 cytokines (Table 1) [22]. It has been approved by the FDA and European Medicines Agency (EMA) for atopic dermatitis since 2017 and is now used in a variety of conditions including atopic dermatitis for children as young as 6 months old, asthma, rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis. For adults with atopic dermatitis, an initial loading dose of 600 mg followed by 300 mg every 2 weeks is administered by subcutaneous injections. For pediatric patients aged 6 months to 17 years of age, dosing regimens differ depending on age and weight.
Compared with placebo, dupilumab has shown to improve or clear atopic dermatitis and associated symptoms as well as improve quality of life in both 16 and 52 week phase 3 clinical trials [23,24,25,26,27]. The most common adverse events associated with dupilumab are conjunctivitis and injection-site reactions with few serious adverse events reported in clinical trials (Table 2) [27, 28]. Dupilumab-associated conjunctivitis can be managed with supportive therapies including warm compresses and lubricating or antihistamine eyedrops [29]. If patients are experiencing more severe conjunctivitis, referral to ophthalmology and antiinflammatory eyedrops and topical agents can be considered.
Pharmacovigilance and observational studies from use in routine clinical practice have identified that dupilumab may be associated with some rare side effects including arthritis, cutaneous T-cell lymphoma, head and neck dermatitis, and psoriasiform reactions [30,31,32,33,34]. Limited studies investigating arthritis associated with dupilumab postulate that IL-4α receptor antagonism leads to immune dysregulation favoring IL-23 and IL-17 effects, and consequent inflammatory arthritis [35]. Cessation of therapy is reported to result in resolution of dupilumab associated arthritis. Long-standing atopic dermatitis has been observed to evolve into mycosis fungoides in the absence of targeted systemic therapy [36, 37]. Further, mycosis fungoides can mimic atopic dermatitis clinically and histologically, thus making the causal connection between dupilumab and mycosis fungoides difficult to dissect. More research is needed to understand the relationship between dupilumab and cutaneous lymphoma [38]. There are limited studies examining the cause of dupilumab associated head and neck dermatitis. It has been linked to varying etiologies including Malassezia (supported by some cases improving with antifungal therapy), rosacea, allergic contact dermatitis, and steroid withdrawal; treatment commonly involves topical corticosteroids and tacrolimus [39,40,41]. Psoriasiform reactions induced by dupilumab are thought to be secondary to upregulation of the Th17 pathway, which is described in pathogenesis of psoriasis [42]. Management of de novo psoriasis should follow general guidelines on classical and reactive psoriasis [43]. At this time, these manifestations of immune dysregulation associated with dupilumab, and whether and how they ought to change management strategies for individual patients, remain poorly understood.
No routine screening or monitoring investigations are required for people initiating or taking dupilumab (Table 2). Contraindications include hamster protein hypersensitivity and helminth infections [44, 45].
3.2 Tralokinumab
Tralokinumab is a human IgG4 monoclonal antibody that directly binds to IL-13, preventing interaction with IL-13Rα1 and IL-13Rα2 [46, 47]. In addition to downregulating JAK1 and tyrosine kinase 2 pathways by blocking interaction with Type II receptors, constituting IL-13Rα1 and IL-4Rα, tralokinumab inhibits signaling mediated by IL-13Rα2. It has been FDA- and EMA-approved since 2021. In the USA it is approved for adults (age 18 and over) with atopic dermatitis, and in Europe for patients 12 years of age and older. The dose for adolescents and adults is 600 mg then 300 mg subcutaneous injections every 2 weeks. Tralokinumab has shown to improve atopic dermatitis clearance, sleep, pruritus, and quality of life in 16 and 52 week phase 3 clinical trials [48, 49, 50].
These trials demonstrated comparable overall rates of adverse effects between treatment groups and placebo groups [51]. Conjunctivitis was a common adverse event, as was seen with dupilumab (Table 2). No routine screening or monitoring is necessary for tralokinumab. It is contraindicated in patients with previous hypersensitivity reactions and those with helminth infections.
3.3 Lebrikizumab
Lebrikizumab is a human IgG4 monoclonal antibody that binds to a different epitope of IL-13 than tralokinumab, and blocks interaction with type II receptor consisting of IL-4Rα/IL-13Rα1 subunits (Table 1) [46, 47]. This ultimately results in inhibition of JAK1 and tyrosine kinase 2 pathways. Phase 3 trials for treatment of atopic dermatitis have been completed and it has been approved for use by the EMA. Phase 3 trials have revealed that 500 mg of lebrikizumab at week 0 and 2 and then followed by 250 mg every 2 weeks improves clearance of atopic dermatitis and associated symptoms at 16 and 52 week endpoints [52, 53]. While lebrikizumab showed early promise for the treatment of asthma [54], it was not effective in larger trials [55].
In the replicate atopic dermatitis phase 3 trials, ADvocate 1 and ADvocate 2, the frequency of adverse events in the lebrikizumab and placebo arms were similar [52]. Conjunctivitis was the most commonly reported adverse effect, and was reported at higher rates in the treatment arm. In phase 3 trials, rates of conjunctivitis appear numerically lower with lebrikizumab than with tralokinumab and dupilumab; however, conjunctivitis adverse event reporting differs between trials, and formal comparisons have not been made in either head-to-head trials or network meta-analysis. In the ADhere trial, higher rates of adverse events were reported for those receiving lebrikizumab and topical corticosteroids compared with those receiving placebo and topical corticosteroids (43.4% versus 34.8%). Conjunctivitis, headache, herpes infection, hypertension, and injection site reaction were the most commonly reported events (Table 2). While official guidance has not yet been released, it is expected that no routine screening or monitoring will be recommended for lebrikizumab treatment.
3.4 Nemolizumab
Nemolizumab is a human monoclonal antibody that binds to IL-31Rα, preventing IL-31 signaling, thereby reducing pruritus (Table 1) [56, 57]. It was approved in Japan in 2022 to treat itch in atopic dermatitis for patients 13 years of age and older. Standard dosing of nemolizumab in Japan is 60 mg subcutaneous injections given every 4 weeks. It is also in phase 3 trials to treat itch in prurigo nodularis. In a phase 3 clinical trial, nemolizumab 60 mg improved severity of atopic dermatitis, quality of life, and pruritus compared with placebo, although there was no difference in achieving clear or almost clear skin between the two groups [58].
More patients on nemolizumab compared with placebo had injection-related reactions (8% versus 3%) [58]. A meta-analysis including six randomized controlled trials found that nemolizumab had similar rates of adverse events overall compared to placebo [59].
4 Oral JAK Inhibitors
4.1 Background and Uses
JAK inhibitors interfere with the JAK signal transducer and activator of transcription (STAT) signaling pathway by preventing ATP binding, inhibiting downstream effects of transcription induction of various genes [60]. Four JAK kinases and seven STAT proteins, which exist in all cell types, work in conjunction to affect the regulation of numerous cytokines [61].
JAK inhibitors were first approved for rheumatoid arthritis, myelofibrosis, and polycythemia vera, but further clinical research revealed their therapeutic effects on additional conditions including atopic dermatitis, psoriasis, inflammatory bowel disease, vitiligo, spondylarthritis, and systemic lupus erythematous [62, 63].
First generation JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, act broadly in that they non-selectively inhibit multiple JAK kinases. Second generation inhibitors, including abrocitinib and upadacitinib, preferentially target specific JAK kinases, which could result in potential differences in efficacy and side effects profiles [64]. Although JAK inhibitors can act selectively, in vitro and laboratory studies have demonstrated that even highly selective JAK inhibitors can broadly inhibit signaling pathways with increased concentration [65].
4.2 Abrocitinib
Abrocitinib preferentially inhibits JAK1, thereby inhibiting IL-4, IL-13, TSLP, and IL-31 signaling and Th2 differentiation (Table 1) [66, 67]. It has been FDA approved for atopic dermatitis since 2022 for people 12 years and older, and it has also been EMA approved since 2021 for use in adults. It is approved at doses ranging from 50–200 mg by mouth daily. Abrocitinib has been shown to improve clearance and symptoms of atopic dermatitis compared to placebo in various phase 3 clinical trials [68,69,70].
Integrated safety analysis for abrocitinib from five clinical trials, consisting of participants receiving either placebo, 100 mg, or 200 mg of abrocitinib, revealed that nausea, headache, and acne were the most common adverse events (Table 2) [71]. Though rates of serious infections were similar between placebo and treatment arms, a dose-related increase was seen in herpes zoster and herpes simplex infections. The most frequent serious infections included pneumonia, herpes simplex, and herpes zoster.
4.3 Upadacitinib
Upadacitinib is a second-generation selective JAK-1 inhibitor (Table 1) [72]. It is approved for a variety of conditions such as rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, ankylosing spondylitis, and axial spondylitis. It was approved in or after 2021 in Europe, USA, Japan, and other countries for the treatment of atopic dermatitis for those 12 years and older. It is available in 15 mg and 30 mg tablets, with both doses approved for once daily use. Phase 3 clinical trials have demonstrated that upadacitinib improves clearance of atopic dermatitis and associated symptoms at 16 week endpoints [73, 74].
Pooled analysis from four clinical trials, consisting of participants receiving placebo, 15 mg, or 30 mg of upadacitinib, revealed that most commonly reported adverse events were acne, nasopharyngitis, upper respiratory tract infection, and headache (Table 2) [75]. Rates of serious infections were similar between placebo and treatment arms. The most common serious infections were eczema herpeticum, herpes zoster, pneumonia, and coronavirus infection.
4.4 Baricitinib
Baricitinib is a JAK1/JAK2 inhibitor (Table 1) [76]. JAK2 plays a role in IL-5 signaling and Th2 differentiation, contributing to atopic dermatitis (AD) pathogenesis [66, 77]. It has been FDA and EMA approved for conditions such as rheumatoid arthritis, coronavirus disease 2019 (COVID-19), and alopecia areata. It has been approved in Europe, but not the USA, since 2020 for atopic dermatitis in adults in 2 mg or 4 mg daily oral dosing. Compared with placebo, baricitinib has been shown to improve clearance and associated symptoms in phase 3 clinical trials [78,79,80,81,82]
In an integrated analysis of eight clinical trials of patients receiving placebo, 2 mg, or 4 mg of baricitinib, the most common adverse events were upper respiratory tract infections and headaches (Table 2) [83]. The most common serious infections were eczema herpeticum, cellulitis, erysipelas, pneumonia, and coronavirus infections.
4.5 Monitoring Recommendations for JAK Inhibitor Use
Clinical trial results are not sufficient to support specific evidence-based recommendations for screening and monitoring investigations for JAK inhibitors used in atopic dermatitis, but there appears to be consensus that some investigations are necessary [84,85,86,87]. Prior to initiating abrocitinib, upadacitinib, and baricitinib, we recommend tuberculosis testing, complete blood count (CBC), lipid profile, hepatitis B and C serology, and complete metabolic panel (CMP), with monitoring of the CBC and lipid profile at 4–12 weeks, and pregnancy testing for those able to become pregnant. It is unclear whether ongoing monitoring is necessary, but could be considered every 3–12 months or after any dose increase. Human immunodeficiency virus (HIV) can also be considered at baseline.
As JAK inhibitors are immunosuppressive, patients should be aware of the associated increased risk of infection, including serious infection. Additionally, live vaccines while on treatment should be avoided. Because of the risk of herpes zoster (shingles) associated with JAK inhibitors, consideration should be given to initiating the vaccination series with the recombinant zoster vaccine prior to starting therapy.
4.6 The FDA’s Black-Boxed Warning for JAK Inhibitors
The FDA has mandated “black-boxed warnings” for all JAK inhibitors, indicating increased risk of serious infections, cardiovascular disease, malignancies, thrombotic events, and mortality.
Warnings regarding cardiovascular risk, malignancies, and thrombotic events have been extrapolated from concerns and evidence related to oral ruxolitinib and tofacitinib. In a large randomized controlled trial of rheumatoid arthritis patients aged 50 and older with cardiovascular risk factors, treatment with tofacitinib was not non-inferior to tumor necrosis factor-α inhibition with regards to major adverse cardiovascular events and cancer risk [88]. Studies have also shown associations with malignancies in myelofibrosis patients on ruxolitinib [89]. Those patient populations are at higher baseline risk for serious adverse events than most patients with atopic dermatitis, and tofacitinib and ruxolitinib are different, less selective JAK inhibitors than abrocitinib and upadacitinib. Still, the evidence and the FDA’s warning should not be ignored. Recent meta-analyses of clinical trials of JAK inhibitors for atopic dermatitis have noted no increased risk for venous thromboembolisms, but the included trials are all short-term [90]. Ongoing pharmacovigilance is warranted regarding the risk of serious adverse events related to JAK inhibition for atopic dermatitis.
5 Relative Efficacy and Safety of Targeted Systemic Therapies
Most clinical trials of systemic therapy for atopic dermatitis compare new targeted agents against placebo, with limited head-to-head trials comparing outcomes between systemic therapies. Head to head trials comparing dupilumab against abrocitinib and upadacitinib have been conducted [91,92,93].
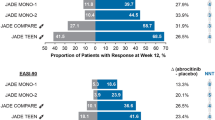
JADE COMPARE randomized 838 adult participants into 200 mg or 100 mg of oral abrocitinib daily, 300 mg of subcutaneous injections of dupilumab every 2 weeks, and placebo groups [91]. Abrocitinib 200 mg daily was found to be more effective than dupilumab in itch reduction at 2 weeks with no other formal statistical comparisons performed. JADE DARE, another trial comparing abrocitinib 200 mg daily versus dupilumab, found 200 mg of abrocitinib to be statistically superior to dupilumab in itch reduction at 2 weeks and skin clearance at 4 weeks, but only modestly superior numerically in these outcomes by 16 weeks with no formal statistical testing performed [92].
Heads Up compared upadacitinib 30 mg daily versus dupilumab versus placebo among 692 adults with moderate-to-severe atopic dermatitis [93]. Upadacitinib 30 mg daily was found to be more effective than dupilumab at improving the signs and symptoms of atopic dermatitis at 16- and 24-week endpoints. No head-to-head studies of upadacitinib 15 mg versus dupilumab have been published.
To improve the precision of those head-to-head comparisons and to enable comparisons between treatments that have not been compared in head-to-head trials, network meta-analysis, which incorporates direct and indirect clinical trial evidence, is a useful technique [94]. In a living systematic review and network meta-analysis of systemic immunomodulatory treatments used up to 16 weeks for atopic dermatitis, the results for upadacitinib 30 mg daily and abrocitinib 200 mg daily versus dupilumab are similar to those seen in head-to-head trials [95, 96]. Compared with dupilumab 600 mg then 300 mg every 2 weeks, abrocitinib 200 mg daily and oral upadacitinib 30 mg daily are both somewhat more efficacious with regards to improving the signs, symptoms, and health-related quality of life of atopic dermatitis [Eczema Area and Severity Index (EASI) mean difference (MD) − 2.1, 95% credible interval (CrI) − 4.1 to 0.0; EASI MD − 2.7, 95% CrI − 4.8 to − 0.5; respectively] [95, 97]. Upadacitinib 15 mg daily had similar efficacy to dupilumab (EASI MD − 0.2, 95% CrI − 2.3 to 2.1), while baricitinib 2–4 mg daily (EASI MD 5.7, 95% CrI 3.5–8.0; EASI MD 3.3, 95% CrI 1.0–5.7; respectively), abrocitinib 100 mg daily (EASI MD 2.2, 95% CrI 0.3–4.3) and tralokinumab 600 mg then 300 mg every 2 weeks (EASI MD 4.6, 95% CrI 2.6–6.8) were less efficacious than dupilumab.
The pattern of results is similar when comparing binary outcomes such as the proportion of participants achieving at least 75% improvement in EASI (EASI-75). Compared with dupilumab, abrocitinib 200 mg daily [odds ratio (OR) 1.6, 95% CrI 1.2–2.1], upadacitinib 30 mg daily (OR 2.1, 95% CrI 1.6–2.7), and 15 mg daily (OR 1.2, 95% CrI 0.9–1.7) have higher odds of achieving EASI-75, whereas abrocitinib 100 mg daily (OR 0.7, 95% CrI 0.5–1.0), baricitinib 2 mg (OR 0.4, 95% CrI 0.3–0.6) and 4 mg daily (OR 0.5, 95% CrI 0.3–0.7), and tralokinumab (OR 0.4, 95% CrI 0.3–0.5) had lower odds of achieving EASI-75 [98]. Lebrikizumab 500 mg at week 0 and 2 then 250 mg every 2 weeks is associated with similar but somewhat lower odds of achieving EASI-75 than dupilumab (OR 0.8, 95% CrI 0.5–1.1), and nemolizumab 60 mg every 4 weeks is associated with lower odds of achieving EASI-75 than dupilumab (OR 0.3, 95% CrI 0.1–0.6).
In a network meta-analysis analyzing potential harm between different systemic therapies, there were higher rates of adverse events in upadacitinib 30 mg daily [risk difference (RD) 108 more per 1000 patients, 95% confidence interval (CI) 72–141] compared with placebo [99]. Abrocitinib 200 mg daily, baricitinib 2–4 mg daily and upadacitinib 15 mg daily were rated as intermediate harm compared with placebo (RD 85 more per 1000 patients, 95% CI 45–122; RD 60 more per 1000 patients, 95% CI 18–99; RD 55 more per 1000 patients, 95% CI 14–95). Dupilumab and tralokinumab were deemed to be similar in their risk of adverse events compared to placebo (RD 20 fewer per 1000 patients, 95% CI − 50 to 10; RD 1 fewer per 1000 patient, 95% CI − 43 to 40). However, general adverse event outcomes in clinical trials are heterogeneous, and in atopic dermatitis trials, they sometimes include flares of atopic dermatitis as an adverse event, making these comparisons difficult to interpret.
Of note, comparisons of efficacy and safety of biologics and systemic JAK inhibitors in network meta-analysis reflect short-term data. Relative comparisons of systemic therapies in long-term studies have yet to be published.
6 Special Considerations for Use of Targeted Systemic Agents in Special Populations
6.1 Pregnancy
There are currently insufficient clinical data available to clarify drug-associated risks for targeted systemic medications for atopic dermatitis in pregnancy (Table 3). Randomized controlled trials (RCTs) for dupilumab, tralokinumab, lebrikizumab, and nemolizumab excluded pregnant and breastfeeding individuals, and to date, few data from interventional studies have been published on exposure to biologics during pregnancy in atopic disease [100]. In vivo data from animal studies for dupilumab and tralokinumab are not suggestive of enhanced risk in pregnancy [101]. Several case reports and limited case series demonstrate good birth outcomes with maternal dupilumab exposure during pregnancy [102,103,104,105,106,107,108,109,110,111,112]. There are currently two observational studies underway aiming to evaluate pregnancy outcomes after dupilumab use [110, 111]. There are no data published on the use of tralokinumab, lebrikizumab, or nemolizumab during pregnancy or breastfeeding. The European Task Force on Atopic Dermatitis does not recommend the use of dupilumab in pregnant or lactating patients, but note that available clinical data describing dupilumab use in pregnancy does not indicate teratogenicity of dupilumab [102]. In contrast, LactMed, a National Institutes of Health (NIH)-sponsored database of medications and risks in lactation, states, “evidence indicates that dupilumab is acceptable to use during breastfeeding” [112]. This is due to the large molecular weight and the protein composition of the therapy that would be rapidly digested in the gastrointestinal tract.
JAK inhibitors for atopic dermatitis are contraindicated in pregnancy. Preclinical data from in vivo animal studies suggest potential effects on fetal development and pregnancy outcomes [113]. It is recommended that patients with atopic dermatitis at risk of becoming pregnant should use effective contraception while taking JAK inhibitors for the duration of treatment and at least 4 weeks after the last dose [114]. JAK inhibitors for atopic dermatitis are also contraindicated during lactation [115]. In vivo animal studies demonstrate that JAK inhibitors are present in breastmilk. An observational registry is planned to assess the safety of abrocitinib in pregnant patients and their offspring [116].
6.2 Human Immunodeficiency Virus
There are multiple case reports of people living with HIV on antiretroviral therapy successfully treated with dupilumab [117,118,119,120,121,122,123,124], including two patients with acquired immunodeficiency syndrome (AIDS) [105]. Theoretically, the IL-4 inhibition could be beneficial in patients with HIV, as IL-4 upregulates chemokine receptor CXCR4, an important mediator of HIV cellular entry. This is further supported by genetic studies that demonstrate an association between decreased IL-4 activity and decreased rates of HIV infection [117]. A survey of the International Eczema Council (IEC), conducted when dupilumab was still the only targeted agent approved for atopic dermatitis, found 67% of members preferred dupilumab as first-line systemic treatment for patients with HIV infection over older immunomodulators [16].
Data on treatment with other biologics in atopic dermatitis for patients with HIV are lacking.
There are currently no clinical data on safety of JAK inhibitors for atopic dermatitis in patients with HIV. Case series have suggested that JAK inhibitors can be used safely for other immune-mediated inflammatory diseases in people with HIV [125]. Ex vivo and in vitro analyses demonstrate JAK inhibitors use several mechanisms to impede the seeding and maintenance of the HIV reservoir. RCTs are currently underway to understand the impact of the JAK inhibitor ruxolitinib on inflammation associated with HIV infection [126].
6.3 Hepatitis B and C
Several case reports and retrospective study data suggest safe and effective use of dupilumab in patients with hepatitis B and C [16, 105], including cases of concurrent treatment of chronic hepatitis B virus at initiation of dupilumab [127]. We did not identify reports of other biologics used for atopic dermatitis among people with hepatitis B or C.
Hepatitis B and C screening is recommended for all patients prior to initiating JAK inhibitors. Several case reports have reported reactivation of hepatitis B virus or hepatitis C virus chronic infections in other immune-mediated inflammatory diseases [128]. JAK inhibitors should not be used in patients with evidence of active or chronic hepatitis B or hepatitis C infections until treatment is completed, although treatment with an oral JAK inhibitor concurrently with treatment of chronic hepatitis B virus infection may be considered in consultation with a hepatologist [129, 130].
6.4 Liver Disease
Limited case reports and cohort studies show successful treatment with dupilumab in patients with acute liver failure and hepatosplenomegaly [16]. We did not identify reports to date of other biologics for atopic dermatitis used in the setting of hepatic impairment.
There are limited clinical studies on safety of JAK inhibitors for atopic dermatitis in patients with severe hepatic impairment. Although pharmacokinetic studies demonstrate that mild to moderate hepatic impairment does not result in clinically significant consequences, JAK inhibitors are contraindicated in severe liver disease [131,132,133].
6.5 End-Stage Kidney Disease
Dupilumab has been used successfully in people with end-stage kidney disease (also known as end stage renal disease) and kidney transplants [134,135,136]. A retrospective observational study found that dupilumab was effective and safe among patients with chronic kidney disease [137]. We did not identify reports of other biologics used for atopic dermatitis among people with end-stage kidney disease.
The safety of JAK inhibitors in patients with severe kidney impairment or end-stage kidney disease is unknown [63]. Elimination of JAK inhibitor baricitinib occurs primarily by renal excretion [138], whereas abrocitinib and upadacitinib is excreted primarily via hepatic metabolism [139,140,141]. Baricitinib is not recommended in patients with severe or end-stage kidney disease. Dose adjustments may be needed for patients with kidney disease.
6.6 Older Adults
Older adults are generally at increased risk for medication-related adverse events due to altered drug metabolism and increased rates of frailty, comorbidities, and polypharmacy [142]. Pooled data from four randomized controlled trials of dupilumab for atopic dermatitis, including a total of 2444 participants aged 25 years and older, demonstrated that treatment emergent adverse events were comparable across age groups, although older adults (aged 60 years and older) were found to more commonly experience adverse events of arthralgias, urinary tract infections, and conjunctivitis [143]. Secondary analysis of three randomized controlled trials of tralokinumab in atopic dermatitis also found that in 104 participants aged 65 years and older, those treated with tralokinumab experienced similar rates of adverse events compared to placebo [144]. Data for other biologics used to treat AD among older adults are limited.
JAK inhibitors for atopic dermatitis should be used with caution in older adults. Older adults have increased baseline risks of serious infection, malignancy, major adverse cardiovascular events, thrombosis, and mortality relative to younger people. Starting with the lower doses of abrocitinib and upadacitinib is recommended for older adults, and dose reductions below standard dosing of 100 mg and 15 mg, respectively, should be considered in older patients greater than 70 years of age [145].
6.7 Drug–Drug Interactions
While drug–drug interactions are not a particular concern for biologic medications, JAK inhibitors broadly impact cytochrome P450 and transporter proteins, suggesting potential drug–drug interactions [146, 147]. Although extensive assessment of clinical significance has yet to be published, in vitro studies suggest that abrocitinib levels are affected by cytochrome P450 inhibitors and inducers, and appropriate dose adjustments should be made with coadministration [148]. In vivo and in vitro studies demonstrate low concern of cytochrome P450 or transporter mediated drug–drug interactions with baricitinib and upadacitinib, although coadministration of an OAT 3 inhibitor, probenecid, decreased renal clearance of baricitinib through OAT3 inhibition [147, 149].
7 Summary: How to Choose the Right Medication for the Right Patient
New targeted systemic immunomodulators, including biologics and JAK inhibitors, are highly effective for people with moderate-to-severe atopic dermatitis. They improve the symptoms of atopic dermatitis and alleviate its impact on quality of life, reducing the burden of disease. Based on available studies, including randomized trials, meta-analyses, observational studies, mechanistic data, and our clinical experience, we recommend the following considerations when choosing between different targeted systemic treatment options.
For most adults with moderate-to-severe atopic dermatitis considering systemic therapy, dupilumab is a good choice given its efficacy, longer safety track record and ease of use, without any laboratory screening or monitoring required. Despite a paucity of data, it may also be suitable for special populations, including people with HIV, viral hepatitis, kidney, and liver disease and older adults. Dupilumab is also the only targeted agent approved to treat both atopic dermatitis and asthma, so may have additional benefits for patients living with both conditions.
Higher doses of upadacitinib and abrocitinib are faster acting and somewhat more effective than dupilumab, and their lower doses are very effective as well. Many of the safety concerns for these JAK inhibitors are, at this point, extrapolated from other JAK inhibitors used in higher-risk populations. Those safety concerns do not preclude their use, but caution is warranted, particularly in special populations at higher risk for adverse events. Further, regulators, including the FDA, advise that JAK inhibitors are indicated only after patients have had an inadequate response (or contraindication to) other systemic medications, including biologics.
Tralokinumab, lebrikizumab, and nemolizumab 60 mg appear to have similar benign safety profiles to dupilumab, but with less long-term safety data in real-world clinical practice to date. They are effective, but somewhat less so than dupilumab in network meta-analysis of trials up to 16 weeks. Of note, patients may benefit from switching to a different biologic if dupilumab therapy is unsuccessful; in one study, approximately 50% of patients achieved improved Investigator’s Global Assessment, numeric rating scale peak pruritus scores, and patient satisfaction when treated with tralokinumab after not tolerating or responding to dupilumab [150].
When choosing among targeted systemic treatments for atopic dermatitis, tradeoffs between efficacy and safety concerns, as well as history of prior treatments, cost, and patient preferences, should be discussed with patients, enabling informed shared decision making. For most patients using systemic treatments, particularly when they are first initiated, concomitant topical antiinflammatory medications are also recommended.
The last decade has seen a dramatic improvement in the treatments we can offer people with more severe atopic dermatitis, and we anticipate more targeted agents will be approved in the coming years. Ongoing assessment of the relative efficacy and safety of approved and upcoming medications, incorporating head-to-head trials and long-term observational studies that include special patient populations, will be essential to keeping clinicians and patients informed as they choose between systemic treatment options.
References
Guttman-Yassky E, Waldman A, Ahluwalia J, Ong PY, Eichenfield LF. Atopic dermatitis: pathogenesis. Semin Cutan Med Surg. 2017;36:100–3.
Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic dermatitis in america study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the us adult population. J Investig Dermatol. 2019;139:583–90.
Kim DH, Li K, Seo SJ, Jo SJ, Yim HW, Kim CM, et al. Quality of life and disease severity are correlated in patients with atopic dermatitis. J Korean Med Sci. 2012;27:1327–32.
Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623–33.
Musters AH, Mashayekhi S, Harvey J, Axon E, Lax SJ, Flohr C, et al. Phototherapy for atopic eczema. Cochrane Database Syst Rev. 2021;10:CD013870.
Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis: the era of biologics and emerging therapeutic approaches. Exp Dermatol. 2019;28:756–68.
Drucker AM, Ellis AG, Bohdanowicz M, Mashayekhi S, Yiu ZZN, Rochwerg B, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 2020;156:659–67.
Chan SC, Li SH, Hanifin JM. Increased interleukin-4 production by atopic mononuclear leukocytes correlates with increased cyclic adenosine monophosphate-phosphodiesterase activity and is reversible by phosphodiesterase inhibition. J Investig Dermatol. 1993;100:681–4.
Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31.
Augustin M, Langenbruch A, Blome C, Gutknecht M, Werfel T, Ständer S, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34:142–52.
Boeri M, Sutphin J, Hauber B, Cappelleri JC, Romero W, Di Bonaventura M. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatol Treat. 2022;33:1449–58.
Thomas C, Raibouaa A, Wollenberg A, Capron J-P, Krucien N, Karn H, et al. Patient preferences for atopic dermatitis medications in the UK, France and Spain: a discrete choice experiment. BMJ Open. 2022;12: e058799.
Abuabara K, Magyari A, McCulloch CE, Linos E, Margolis DJ, Langan SM. Prevalence of atopic eczema among patients seen in primary care: data from the health improvement network. Ann Intern Med. 2019;170:354–6.
Abuabara K, Langan SM. Atopic dermatitis across the life course. Br J Dermatol. 2023;188:709–17.
Lam M, Zhu JW, Maqbool T, Adam G, Tadrous M, Rochon P, et al. Inclusion of older adults in randomized clinical trials for systemic medications for atopic dermatitis: a systematic review. JAMA Dermatol. 2020;156:1240–5.
Drucker AM, Lam M, Flohr C, Thyssen JP, Kabashima K, Bissonnette R, et al. Systemic therapy for atopic dermatitis in older adults and adults with comorbidities: a scoping review and International Eczema Council Survey. Dermatitis. 2022;33:200–6.
Eichenfield LF, DiBonaventura M, Xenakis J, Lafeuille M-H, Duh MS, Fakih I, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther. 2020;10:791–806.
Joensuu JT, Aaltonen KJ, Aronen P, Sokka T, Puolakka K, Tuompo R, et al. Cost-effectiveness of biologic compared with conventional synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a Register study. Rheumatol Oxf Engl. 2016;55:1803–11.
Pharmacoeconomic Report: Dupilumab (Dupixent): (Sanofi Genzyme, a division of sanofi-aventis Canada Inc.): Indication: Indicated for the treatment of patients aged 12 years and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2020 [cited 2023 Dec 24]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK566135/
Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2018;120:10-22.e2.
Wang C, Kraus CN, Patel KG, Ganesan AK, Grando SA. Real-world experience of dupilumab treatment for atopic dermatitis in adults: a retrospective analysis of patients’ records. Int J Dermatol. 2020;59:253–6.
Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2020;50:5–14.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Zhao Y, Wu L, Lu Q, Gao X, Zhu X, Yao X, et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2022;186:633–41.
de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083–101.
Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2017;389:2287–303.
Deleuran M, Thaçi D, Beck LA, de Bruin-Weller M, Blauvelt A, Forman S, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377–88.
Achten R, Bakker D, Ariens L, Lans A, Thijs J, van der Schaft J, et al. Long-term follow-up and treatment outcomes of conjunctivitis during dupilumab treatment in patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol Pract. 2021;9:1389-1392.e2.
Agnihotri G, Shi K, Lio PA. A clinician’s guide to the recognition and management of dupilumab-associated conjunctivitis. Drugs RD. 2019;19:311–8.
Faiz S, Giovannelli J, Podevin C, Jachiet M, Bouaziz J-D, Reguiai Z, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019;81:143–51.
Jang DH, Lee JI, Bae JY, Jung HJ, Park MY, Ahn J. Facial erythema after the treatment of dupilumab in SLE patient. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. 2020;16:60.
Jay R, Rodger J, Zirwas M. Review of dupilumab-associated inflammatory arthritis: an approach to clinical analysis and management. JAAD Case Rep. 2022;21:14–8.
Bettuzzi T, Drucker A, Staumont-Sallé D, Bihan K, Lebrun-Vignes B, Sbidian E. Adverse events associated with dupilumab in the World Health Organization pharmacovigilance database. J Am Acad Dermatol. 2022;86:431–3.
Soria A, Du-Thanh A, Seneschal J, Jachiet M, Staumont-Sallé D, Barbarot S, et al. Development or exacerbation of head and neck dermatitis in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155:1312–5.
Bridgewood C, Sharif K, Freeston J, Saleem B, Russell T, Watad A, et al. Regulation of entheseal IL-23 expression by IL-4 and IL-13 as an explanation for arthropathy development under dupilumab therapy. Rheumatol Oxf Engl. 2021;60:2461–6.
Rajka G, Winkelmann RK. Atopic dermatitis and Sézary syndrome. Arch Dermatol. 1984;120:83–4.
Van Haselen CW, Toonstra J, Preesman AH, Van Der Putte SC, Bruijnzeel-Koomen CA, Van Vloten WA. Sézary syndrome in a young man with severe atopic dermatitis. Br J Dermatol. 1999;140:704–7.
Park A, Wong L, Lang A, Kraus C, Anderson N, Elsensohn A. Cutaneous T-cell lymphoma following dupilumab use: a systematic review. Int J Dermatol. 2023;62:862–76.
Kozera E, Stewart T, Gill K, De La Vega MA, Frew JW. Dupilumab-associated head and neck dermatitis is associated with elevated pretreatment serum Malassezia-specific IgE: a multicentre, prospective cohort study. Br J Dermatol. 2022;186:1050–2.
Bax CE, Khurana MC, Treat JR, Castelo-Soccio L, Rubin AI, McMahon PJ. New-onset head and neck dermatitis in adolescent patients after dupilumab therapy for atopic dermatitis. Pediatr Dermatol. 2021;38:390–4.
Jo CE, Finstad A, Georgakopoulos JR, Piguet V, Yeung J, Drucker AM. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84:1339–47.
Su Z, Zeng Y-P. Dupilumab-associated psoriasis and psoriasiform manifestations: a scoping review. Dermatol Basel Switz. 2023;239:646–57.
Murphy MJ, Cohen JM, Vesely MD, Damsky W. Paradoxical eruptions to targeted therapies in dermatology: a systematic review and analysis. J Am Acad Dermatol. 2022;86:1080–91.
Kreeshan FC, Al-Janabi A, Warren RB, Hunter HJA. Real-world experience and laboratory monitoring of dupilumab in patients with moderate to severe atopic dermatitis in a tertiary centre. Dermatol Ther. 2021;11:149–60.
Din ATU, Malik I, Arshad D, Din ATU. Dupilumab for atopic dermatitis: the silver bullet we have been searching for? Cureus. 2020;12:e7565.
Popovic B, Breed J, Rees DG, Gardener MJ, Vinall LMK, Kemp B, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13Rα1 and IL-13Rα2. J Mol Biol. 2017;429:208–19.
Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75:54–62.
Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour J-P, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184:437–49.
Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450–63.
Gutermuth J, Pink AE, Worm M, Soldbro L, Bjerregård Øland C, Weidinger S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br J Dermatol. 2022;186:440–52.
Simpson EL, Merola JF, Silverberg JI, Reich K, Warren RB, Staumont-Sallé D, et al. Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials. Br J Dermatol. 2022;187:888–99.
Blauvelt A, Thyssen JP, Guttman-Yassky E, Bieber T, Serra-Baldrich E, Simpson E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023;188:740–8.
Simpson EL, Gooderham M, Wollenberg A, Weidinger S, Armstrong A, Soung J, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol. 2023;159:182–91.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98.
Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–96.
Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. 2012;91:552–66.
Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826–35.
Kabashima K, Matsumura T, Komazaki H, Kawashima M, Nemolizumab-JP01 Study Group. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383:141–50.
Liang J, Hu F, Dan M, Sang Y, Abulikemu K, Wang Q, et al. Safety and efficacy of nemolizumab for atopic dermatitis with pruritus: a systematic review and meta-regression analysis of randomized controlled trials. Front Immunol. 2022;13: 825312.
Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10.
Furumoto Y, Gadina M. The arrival of JAK inhibitors: advancing the treatment of immune and hematologic disorders. BioDrugs Clin Immunother Biopharm Gene Ther. 2013;27:431–8.
Zhao M-Y, Zhang W, Rao G-W. Targeting Janus kinase (JAK) for fighting diseases: the research of JAK inhibitor drugs. Curr Med Chem. 2022;29:5010–40.
McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet Lond Engl. 2021;398:803–16.
Cinats A, Heck E, Robertson L. Janus kinase inhibitors: a review of their emerging applications in dermatology. Skin Ther Lett. 2018;23:5–9.
Lin CM, Cooles FA, Isaacs JD. Basic mechanisms of JAK inhibition. Mediterr J Rheumatol. 2020;31:100–4.
O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–50.
Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol. 2020;21:783–98.
Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863–73.
Blauvelt A, Silverberg JI, Lynde CW, Bieber T, Eisman S, Zdybski J, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86:104–12.
Shi VY, Bhutani T, Fonacier L, Deleuran M, Shumack S, Valdez H, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351–8.
Simpson EL, Silverberg JI, Nosbaum A, Winthrop KL, Guttman-Yassky E, Hoffmeister KM, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22:693–707.
Kotyla PJ, Islam MA, Engelmann M. Clinical Aspects of Janus kinase (JAK) inhibitors in the cardiovascular system in patients with rheumatoid arthritis. Int J Mol Sci. 2020;21:7390.
Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet Lond Engl. 2021;397:2151–68.
Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2021;397:2169–81.
Guttman-Yassky E, Thyssen JP, Silverberg JI, Papp KA, Paller AS, Weidinger S, et al. Safety of upadacitinib in moderate-to-severe atopic dermatitis: an integrated analysis of phase 3 studies. J Allergy Clin Immunol. 2023;151:172–81.
Kubo S, Nakayamada S, Sakata K, Kitanaga Y, Ma X, Lee S, et al. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol. 2018;9:1510.
He H, Guttman-Yassky E. JAK Inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20:181–92.
Simpson EL, Lacour J-P, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242–55.
Silverberg JI, Simpson EL, Wollenberg A, Bissonnette R, Kabashima K, DeLozier AM, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691–9.
Bieber T, Reich K, Paul C, Tsunemi Y, Augustin M, Lacour J-P, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4). Br J Dermatol. 2022;187:338–52.
Wollenberg A, Nakahara T, Maari C, Peris K, Lio P, Augustin M, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 Phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543–52.
Simpson EL, Forman S, Silverberg JI, Zirwas M, Maverakis E, Han G, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62–70.
Bieber T, Katoh N, Simpson EL, de Bruin-Weller M, Thaçi D, Torrelo A, et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: an updated integrated analysis of eight clinical trials. J Dermatol Treat. 2023;34:2161812.
Iznardo H, Roé E, Serra-Baldrich E, Puig L. Efficacy and safety of JAK1 inhibitor abrocitinib in atopic dermatitis. Pharmaceutics. 2023;15:385.
Sandborn WJ, Feagan BG, Loftus EV, Peyrin-Biroulet L, Van Assche G, D’Haens G, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology. 2020;158:2123-2138.e8.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Baricitinib. [Updated 2022 Aug 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548012/
Winthrop KL, Harigai M, Genovese MC, Lindsey S, Takeuchi T, Fleischmann R, et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis. 2020;79:1290–7.
Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26.
Polverelli N, Elli EM, Abruzzese E, Palumbo GA, Benevolo G, Tiribelli M, et al. Second primary malignancy in myelofibrosis patients treated with ruxolitinib. Br J Haematol. 2021;193:356–68.
Chen T-L, Lee L-L, Huang H-K, Chen L-Y, Loh C-H, Chi C-C. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254–61.
Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101–12.
Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet Lond Engl. 2022;400:273–82.
Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–55.
Watt J, Tricco AC, Straus S, Veroniki AA, Naglie G, Drucker AM. Research techniques made simple: network meta-analysis. J Investig Dermatol. 2019;139:4-12.e1.
Drucker AM, Morra DE, Prieto-Merino D, Ellis AG, Yiu ZZN, Rochwerg B, et al. Systemic immunomodulatory treatments for atopic dermatitis. JAMA Dermatol. 2022;158:523–32.
Gao Q, Zhao Y, Zhang J. Efficacy and safety of abrocitinib and upadacitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a systematic review and meta-analysis. Heliyon. 2023;9: e16704.
Silverberg JI, Thyssen JP, Fahrbach K, Mickle K, Cappelleri JC, Romero W, et al. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: a systematic literature review and network meta-analysis. J Eur Acad Dermatol Venereol. 2021;35:1797–810.
Drucker AM. Comparing binary efficacy outcomes for systemic immunomodulatory treatments for atopic dermatitis in a living systematic review and network meta-analysis. Unpublished manuscript.
Chu AWL, Wong MM, Rayner DG, Guyatt GH, Martinez JPD, Ceccacci R, et al. Systemic treatments for atopic dermatitis (eczema): systematic review and network meta-analysis of randomized trials. J Allergy Clin Immunol. 2023;152:1470–92.
Pfaller B, José Yepes-Nuñez J, Agache I, Akdis CA, Alsalamah M, Bavbek S, et al. Biologicals in atopic disease in pregnancy: an EAACI position paper. Allergy. 2021;76:71–89.
Shakuntulla F, Chiarella SE. Safety of biologics for atopic diseases during pregnancy. J Allergy Clin Immunol Pract. 2022;10:3149–55.
Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248–56.
Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:e256–7.
Kage P, Simon J-C, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484–5.
Patruno C, Potestio L, Scalvenzi M, Battista T, Raia F, Picone V, et al. Dupilumab for the treatment of adult atopic dermatitis in special populations. J Dermatol Treat. 2022;33:3028–33.
Gracia-Darder I, Pons De Ves J, Reyero Cortina M, Martín-Santiago A. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:e15237.
Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960–1.
Akhtar NH, Khosravi-Hafshejani T, Akhtar D, Dhadwal G, Kanani A. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. 2022;18:9.
Riquelme-Mc Loughlin C, Mascaró JM. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578–9.
Post-authorization Safety Study in North America to Monitor Pregnancy and Infant Outcomes Following Administration of Dupilumab During Planned or Unexpected Pregnancy—Full Text View—ClinicalTrials.gov [Internet]. [cited 2023 Jul 15]. https://clinicaltrials.gov/ct2/show/NCT04173442
An Observational Retrospective Cohort Study Being Conducted in Women With Atopic Dermatitis (AD)—Full Text View—ClinicalTrials.gov [Internet]. [cited 2023 Jul 15]. https://clinicaltrials.gov/ct2/show/NCT03936335
Dupilumab. Drugs Lact Database Lact [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006 [cited 2023 Sep 23]. http://www.ncbi.nlm.nih.gov/books/NBK500904/
Gisbert JP, Chaparro M. Safety of new biologics (vedolizumab and ustekinumab) and small molecules (tofacitinib) during pregnancy: a review. Drugs. 2020;80:1085–100.
Napolitano M, Caiazzo G, Fabbrocini G, Balato A, Di Caprio R, Scala E, et al. Increased expression of interleukin-23A in lesional skin of patients with atopic dermatitis with psoriasiform reaction during dupilumab treatment. Br J Dermatol. 2021;184:341–3.
Napolitano M, Ruggiero A, Fontanella G, Fabbrocini G, Patruno C. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34: e14475.
Pfizer. CIBINQOTM Pregnancy Registry: an observational study of the safety of abrocitinib exposure in pregnant women and their offspring [Internet]. clinicaltrials.gov; 2023 May. Report No.: NCT05721937. https://clinicaltrials.gov/study/NCT05721937
Avallone G, Trunfio M, Giura MT, Siliquini N, Viola R, Orofino G, et al. Dupilumab in HIV-positive patients with atopic dermatitis: a long-term follow-up patient and a literature review. Dermatol Online J. 2021;27.
Nusbaum KB, Kaffenberger BH, Paradiso Bs MM, Sopkovich JA, Daou H, Seminario-Vidal L, et al. Dupilumab for treatment of atopic dermatitis in patients living with HIV: a case series. Int J Dermatol. 2021;60:e344–6.
Ordóñez-Rubiano MF, Rubiano-Mojica PC, Casas M. Young HIV-positive male patient with severe atopic dermatitis on dupilumab and SARS-CoV-2 infection, a pioneer hypothesis. Int J Dermatol. 2021;60:514–5.
Olbricht N, Kromer C, Forkel S, Schön MP, Buhl T. Effective treatment of atopic dermatitis with dupilumab in an HIV-positive patient. J Dtsch Dermatol Ges J Ger Soc Dermatol. 2020;18:1488–90.
Lor M, Villa N, Holland V. Safe and effective treatment of atopic dermatitis using dupilumab over 23 months in a patient with HIV. Dermatol Ther. 2020;33: e14271.
Brodska P, Panzner P, Sedlacek D, Terl M, Cetkovska P. Use of dupilumab in a patient with atopic dermatitis, severe asthma, and HIV infection. Dermatol Ther. 2020;33: e14159.
Romagnuolo M, Angileri L, Tavecchio S, Marzano AV, Ferrucci S. Safety and efficacy of dupilumab in a patient with severe atopic dermatitis and HIV infection, with 15 months of follow-up. Clin Exp Dermatol. 2020;45:762–3.
Tanner JF, Knutsen AP, Siegfried E. Safety and efficacy of dupilumab in an atopic adolescent with primary immune deficiency. Dermat Contact Atopic Occup Drug. 2021;32:e91–2.
Naovarat BS, Salazar G, Ishimori M, Williams FM, Reveille JD. Biological treatment usage in patients with HIV and rheumatic disease, 2003–2021: long-term safety and follow-up. RMD Open. 2022;8: e002282.
Gavegnano C, Brehm JH, Dupuy FP, Talla A, Ribeiro SP, Kulpa DA, et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog. 2017;13: e1006740.
Ly K, Smith MP, Thibodeaux Q, Beck K, Bhutani T, Liao W. Dupilumab in patients with chronic hepatitis B on concomitant entecavir. JAAD Case Rep. 2019;5:624.
Hong X, Xiao Y, Xu L, Liu L, Mo H, Mo H. Risk of hepatitis B reactivation in HBsAg−/HBcAb+ patients after biologic or JAK inhibitor therapy for rheumatoid arthritis: a meta-analysis. Immun Inflamm Dis. 2023;11: e780.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
Harigai M, Winthrop K, Takeuchi T, Hsieh T-Y, Chen Y-M, Smolen JS, et al. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open. 2020;6: e001095.
Veeravalli V, Dash RP, Thomas JA, Babu RJ, Madgula LMV, Srinivas NR. Critical assessment of pharmacokinetic drug–drug interaction potential of tofacitinib, baricitinib and upadacitinib, the three approved Janus kinase inhibitors for rheumatoid arthritis treatment. Drug Saf. 2020;43:711–25.
Wang EQ, Le V, O’Gorman M, Tripathy S, Dowty ME, Wang L, et al. Effects of hepatic impairment on the pharmacokinetics of abrocitinib and its metabolites. J Clin Pharmacol. 2021;61:1311–23.
Trueman S, Mohamed MF, Feng T, Lacerda AP, Marbury T, Othman AA. Characterization of the effect of hepatic impairment on upadacitinib pharmacokinetics. J Clin Pharmacol. 2019;59:1188–94.
Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339–41.
Elamin S, Murphy B. Dupilumab in the management of atopic dermatitis in an immunosuppressed renal transplant patient. Clin Exp Dermatol. 2022;47:1191–3.
Waldman RA, DeWane ME, Sloan B, Grant-Kels JM, Lu J. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251–2.
Lukac D, Pagani K, McGee JS. Overview of use, efficacy, and safety of dupilumab in complex patients: a retrospective, case-series study from a large, urban academic center. Arch Dermatol Res. 2023;315:1777–81.
Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Sel. 2021;5:293–304.
Wojciechowski J, Malhotra BK, Wang X, Fostvedt L, Valdez H, Nicholas T. Population pharmacokinetics of abrocitinib in healthy individuals and patients with psoriasis or atopic dermatitis. Clin Pharmacokinet. 2022;61:709–23.
Mohamed M-EF, Trueman S, Feng T, Anderson J, Marbury TC, Othman AA. Characterization of the effect of renal impairment on upadacitinib pharmacokinetics. J Clin Pharmacol. 2019;59:856–62.
Wang EQ, Le V, Winton JA, Tripathy S, Raje S, Wang L, et al. Effects of renal impairment on the pharmacokinetics of abrocitinib and its metabolites. J Clin Pharmacol. 2022;62:505–19.
Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7:11–22.
Silverberg JI, Lynde CW, Abuabara K, Patruno C, de Benedetto A, Zhang H, et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023;24:469–83.
Merola JF, Butler DC, Mark T, Schneider S, Kim Y, Abuabara K. Safety and efficacy of tralokinumab in older adults with moderate-to-severe atopic dermatitis: a secondary analysis. JAMA Dermatol. 2023;159:1119–23.
Nash P, Kerschbaumer A, Dörner T, Dougados M, Fleischmann RM, Geissler K, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71–87.
Alim K, Bruyère A, Lescoat A, Jouan E, Lecureur V, Le Vée M, et al. Interactions of Janus kinase inhibitors with drug transporters and consequences for pharmacokinetics and toxicity. Expert Opin Drug Metab Toxicol. 2021;17:259–71.
Veeravalli V, Dash RP, Thomas JA, Babu RJ, Madgula LMV, Srinivas NR. Critical assessment of pharmacokinetic drug–drug interaction potential of tofacitinib, baricitinib and upadacitinib, the three approved Janus kinase inhibitors for rheumatoid arthritis treatment. Drug Saf [Internet]. 2020 [cited 2023 Nov 20];43. https://pubmed.ncbi.nlm.nih.gov/32367507/.
Wang X, Dowty ME, Wouters A, Tatulych S, Connell CA, Le VH, et al. Assessment of the effects of inhibition or induction of CYP2C19 and CYP2C9 enzymes, or inhibition of OAT3, on the pharmacokinetics of abrocitinib and its metabolites in healthy individuals. Eur J Drug Metab Pharmacokinet. 2022;47:419–29.
Payne C, Zhang X, Shahri N, Williams W, Cannady E. AB0492 evaluation of potential drug–drug interactions with baricitinib. Ann Rheum Dis. 2015;74:1063–1063.
Schlösser AR, Shareef M, Olydam J, Nijsten TEC, Hijnen DJ. Tralokinumab treatment for patients with moderate-to-severe atopic dermatitis in daily practice. Clin Exp Dermatol. 2023;48:510–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
Megan Lam, Richard W. Kim, and Aaron M. Drucker conducted the literature search and drafted the manuscript. All authors critically revised and approved the final manuscript.
Funding
No funding was received for conducting this study.
Availability of Data and Material
This study did not use any primary data not available from other sources.
Conflicts of Interest
Dr. Drucker has received compensation from the British Journal of Dermatology (reviewer and section editor), American Academy of Dermatology (guidelines writer), National Eczema Association (grant reviewer), Canadian Dermatology Today (manuscript writer), and Canadian Agency for Drugs and Technologies in Health (consultant). Dr. Simpson reports personal fees from Advances in Cosmetic Medical Derm Hawaii LLC, AbbVie, Amgen, AOBiome LLC, Arcutis Biotherapeutics, Arena Pharmaceuticals, Aslan Pharma, Boehringer Ingelheim USA, Inc., Boston Consulting Group, Bristol Myers Squibb—BMS, Collective Acumen LLC (CA), CorEvitas, Dermira, Eli Lilly, Evelo Biosciences, Evidera, ExcerptaMedica, FIDE, Forte Bio RX, Galderma, GlaxoSmithKline, Incyte, Janssen, Johnson & Johnson, Kyowa Kirin Pharmaceutical Development, Leo Pharm, Medscape LLC, Merck, MauiDerm, MLG Operating, MJH holding, Pfizer, Physicians World LLC, PRImE, Regeneron, Revolutionizing Atopic Dermatitis Inc, Roivant, Sanofi-Genzyme, Trevi therapeutics, Valeant, Vindico Medical education, and WebMD. Dr. Drucker has received research grants to his institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes for Health Research, US National Institutes of Health, and Physicians Services Incorporated Foundation. Dr. Abuabara has received research grants to her institution from the National Eczema Association, the US National Institutes of Health, the LEO Foundation, Pfizer, and Cosmetique Internacional SNC. She has received compensation from TARGET RWE. Dr. Simpson reports grants (or serves as principal investigator role) from AbbVie, Acrotech Biopharma Inc, Amgen, Arcutis, Aslan, Castle Biosciences, CorEvitas, Dermavant, Dermira, Eli Lilly, Incyte, Kymab, Kyowa Kirin, National Jewish Health, Leo, Pfizer, Regeneron, Sanofi, and Target RWE. These potential conflicts of interest have been reviewed and managed by Oregon Health and Science University. Mr. Kim and Dr. Lam have no relevant financial or non-financial interests to disclose.
Ethics Approval
This article does not contain animal or human subjects that require approval by an ethics board.
Patient Consent to Participate
This article does not discuss any individuals or materials that would require consent for participation.
Patient Consent to Publish
This article does not discuss any individuals or materials that would require consent for publication.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, R.W., Lam, M., Abuabara, K. et al. Targeted Systemic Therapies for Adults with Atopic Dermatitis: Selecting from Biologics and JAK Inhibitors. Am J Clin Dermatol 25, 179–193 (2024). https://doi.org/10.1007/s40257-023-00837-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-023-00837-w