Abstract
Background
The evidence for adding small-molecule drugs to an ongoing biologic treatment is sparse, but combination therapies appear to be advantageous in appropriately selected patients with psoriasis. To our knowledge, efficacy and safety of combination therapy with apremilast and biologics has not previously been reviewed.
Materials and Methods
A literature search was performed on Medline (PubMed), Embase, Web of Science, and the Cochrane Library. Inclusion criteria were a diagnosis of psoriasis, age ≥ 18 years, concomitant treatment with apremilast and a specified biologic agent, and available safety and/or efficacy results. All papers written in English and published from database inception to August 2021 were included. No limit was set regarding study size.
Results
The literature search yielded 447 citations. Of these, 19 studies published from 2015 to 2020 were included in the review. All papers referred to retrospective studies, comprising case reports (n = 9), case series (n = 8), or cohort studies (n = 2). A total of 172 patients with psoriasis were identified. Clinical subtypes included plaque psoriasis (n = 164), palmoplantar pustulosis (n = 7), and acute pustular psoriasis (n = 1). The observation period ranged from 3 weeks to 24 months. Geographical origin of studies was North America (n = 11), Europe (n = 4), and Asia (n = 4). In general, apremilast-biologic combination therapy was reported to be safe; across papers, one serious adverse event was registered (hospitalization due to weight loss). Adverse events (AEs) were otherwise mostly mild and gastrointestinal. No differences in AEs were observed in studies comparing apremilast mono- and combination therapy. In several papers, sufficient information about AEs was not reported or could not be extracted. Clinical response to combination treatment was evaluated at various time points, and only few studies used validated scores. In the remaining papers, efficacy data were descriptive and/or in photographic form, or not available. In total, two patients discontinued therapy due to lack of efficacy.
Conclusion
Evidence for combined treatment with apremilast and biologics is limited and restricted to retrospective studies of various quality. Based on available data, apremilast may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Systemic combination therapy seems to be advantageous in selected patients with psoriasis. |
Efficacy and safety of adding apremilast to a biologic treatment has not previously been reviewed. |
Available data comprise retrospective case reports (n = 9), case series (n = 8) and cohort studies (n = 2), with a total of 172 patients. |
Design, size, quality, and heterogenicity of studies are major limitations. |
Apremilast may constitute an efficacious and safe add-on treatment to biologics, but properly conducted clinical investigations are warranted. |
1 Introduction
Although monotherapy with biologic agents is highly effective in psoriasis [1], some patients never obtain complete response or experience secondary failure over time [2]. In these cases, adding a small-molecule drug to the biologic therapy may be considered. High-quality evidence for this approach is sparse but according to recommendations from the Medical Board of the National Psoriasis Foundation, combination therapies may have advantages in appropriately selected patients [3].
In 2014, apremilast was the first small-molecule drug in almost 20 years to be approved by the US Food and Drug Administration (FDA) for management of moderate to severe plaque psoriasis. At launch, apremilast gained much attention due to its’ oral administration and favorable safety profile, but lower efficacy compared to similarly prized biologics has led to a modest use of apremilast in many countries [4].
The patent of apremilast is due to expire within the coming years. Generics tend to cost less than brand-name medicines, and multiple generic drugs are often approved for the same product, leading to further cost reductions. This leaves apremilast with the potential to be a cost-effective, add-on treatment in psoriasis. To our knowledge, the efficacy and safety of combination therapy with apremilast and biologics for psoriasis have not previously been reviewed.
2 Material and Methods
2.1 Literature Search
The study protocol was registered at PROSPERO (ID: CRD42021269266) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
A literature search was performed on Medline (PubMed), Embase, Web of Science, and the Cochrane Library using the words: psoriasis, palmoplantar pustulosis, psoriatic, apremilast, biologic, adalimumab, brodalumab, certolizumab, etanercept, guselkumab, infliximab, ixekizumab, risankizumab, secukinumab, tildrakizumab, ustekinumab, combination, combining, add-on, and double-therapy (Table 1).
2.2 Inclusion and Exclusion Criteria
Inclusion criteria were a diagnosis of psoriasis, age ≥ 18 years, concomitant treatment with apremilast and a specified biologic agent, and available safety and/or efficacy results. All original papers written in English published from database inception to August 2021 were included. Publications from identical study populations were included if treatments or observation periods differed. No limit was set regarding study size.
2.3 Data Extraction and Quality Assessment
Two authors (MG and FA) independently screened records based on titles and abstracts. Duplicates were removed before retrieving full-text articles. Any disagreement between reviewers was resolved by conferring with a third reviewer (AE). After inclusion of eligible studies, one author (MG) reviewed reference lists to identify additional studies missing in the initial literature search.
Data extraction from included publications was performed in a standardized manner. Level of evidence was determined according to the Journal of the American Medical Association (JAMA) ‘Grading Recommendations and Evidence Quality’ guidelines: grade A, systematic review/meta-analysis, randomized controlled trial, or all-or-none observational study; grade B, systematic review/meta-analysis of lower-quality clinical trials or studies with limitations and inconsistent findings, lower-quality clinical trial, cohort study or case-control study; grade C, consensus guidelines, usual practice, expert opinion, or case series [5].
3 Results
3.1 Literature Search
The literature search yielded 447 citations. After screening titles/abstracts and removing duplicates, 87 articles underwent full-text review. Of these, 68 were excluded, as inclusion criteria were not fulfilled. In total, 19 studies published from 2015 to 2020 were included in the review (Table 2) [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The literature selection is documented in the PRISMA flow diagram (Fig. 1).
3.2 Heterogenicity of Studies
All included papers were retrospective with low–moderate level of evidence (grade B–C). Study designs were case reports (n = 9) [9, 10, 12,13,14, 18, 19, 22, 23], case series (n = 8) [6,7,8, 11, 15,16,17, 20, 21, 24, 25], or cohort studies (n = 2) [15, 16]. The largest publication included 89 patients, of which only 60 could be included in the review, as other apremilast combination treatments were also covered in the data material [16]. In one case report, the same patient underwent two different combination therapies consecutively [9].
Across studies, the observation period ranged from 3 weeks to 24 months. Two of the included papers comprised the same group of patients evaluated at different time points [15, 16]. Geographical origin of studies was North America (n = 11), Europe (n = 4), and Asia (n = 4).
A total of 172 patients with psoriasis were identified. Clinical subtypes included plaque psoriasis (n = 164 patients), palmoplantar pustulosis (n = 7), and acute pustular psoriasis (n = 1). Concomitant psoriatic arthritis (PsA) was reported in 31 patients.
3.3 Combination Therapies
In addition to apremilast, patients were co-treated with tumor necrosis factor (TNF)-α inhibitors (adalimumab (n = 38), etanercept (n = 31), infliximab (n = 26), golimumab (n = 3), and certolizumab pegol (n = 2)), interleukin (IL)12/23 inhibitors (ustekinumab (n = 51)), or IL-17A inhibitors (secukinumab (n = 11), ixekizumab (n = 7), and brodalumab (n = 3).
In all but one case [9] apremilast was added to the biologic treatment and not vice versa. Besides apremilast and a biologic drug, some patients also received methotrexate (n = 21), methotrexate and prednisolone (n = 2), or acitretin (n = 3); none were treated with more than one biologic therapy at the same time.
3.4 Safety
In general, apremilast/biologic combination therapy was reported safe by authors. Adverse events (AEs) comprised gastrointestinal symptoms (n = 29), weight loss (n = 8), depressed mood (n = 1), and folliculitis (n = 1). However, in several studies, sufficient information about AEs was either not provided or could not be extracted. That also applies for the studies comparing apremilast mono- and combination therapy, which included other types of apremilast combination therapies, for example with methotrexate. In both articles, no safety differences were found between patients receiving apremilast mono- and combination therapy [15, 16].
Across papers, one serious AE was registered, as one patient underwent hospitalization due to significant weight loss during treatment [16]. Information about the drug related to this specific event could not be extracted, because the data material also comprised other apremilast combination treatments. The weight loss did not lead to therapy discontinuation.
3.5 Efficacy
In all but one article [12], combination therapy was concluded to be effective by authors. Clinical responses were evaluated at various time points, and only few studies used validated clinical scores such as the psoriasis area and severity index (PASI) or affected body surface area (BSA). In some of these papers, however, baseline values were missing. In the remaining studies, efficacy data were descriptive and/or in photographic form, or not possible to assess due to study design or data presentation.
3.6 Therapy Discontinuation
In most papers, apremilast-biologic combination treatment was stated ongoing. Four patients discontinued therapy due to lack of efficacy [9, 12] (n = 2) or side effects [17] (n = 2). In one article, 14 of 81 patients discontinued prior to 12 weeks of therapy, but non-biologic therapies were included in these data [6].
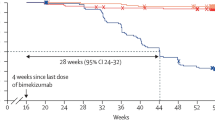
The papers by Ighani et al. referred to the same study population[15, 16]. The primary publication presented week 0–16 data, but did not provide information about discontinuations before follow-up [16]. That also accounted for the long-term assessment covering weeks 16–52 [15]. When comparing patients receiving combination therapy with apremilast and a biologic drug in the two articles, the total number dropped from 60 to 18 patients at weeks 16 and 52, respectively.
4 Discussion
The literature on combined apremilast-biologic therapy is limited and consists solely of retrospective studies, primarily case reports and series, which makes it difficult to evaluate efficacy and safety. However, based on available data from a total of 172 patients, combining apremilast with biologic therapies appears to be effective and overall well tolerated in patients with psoriasis. In accordance with apremilast phase 3 trials, reported side effects to combination therapy are mostly mild and affecting the gastrointestinal system [26].
Limitations of the existing data relate to design, size, quality, and heterogenicity of studies. There are no prospective or randomized trials, available studies are generally small with short-term follow-up, and relevant clinical information, for example psoriasis subtype, standardized severity scoring, use of concomitant topical treatment, and information about side effects, are missing from many publications. Also, in several of the included articles, it is impossible to extract demographics, efficacy, or safety data related to apremilast-biologic treatment specifically, as patients with other inflammatory conditions or non-biologic treatments have been included in the material.
Publication bias and lack of drop-out data constitutes a major uncertainty when evaluating the current literature. This is illustrated by the Ighani papers [15, 16], where at least 42 of 60 patients must be suspected to have discontinued apremilast-biologic coadministration within the first year of therapy. Along this line is a publication by Abignano et al. [27], which had to be excluded from the review, as it was not possible to identify the proportion of patients with plaque psoriasis in concomitant apremilast-biologic therapy. However, 28 of 71 patients with PsA (39%) required discontinuation due to lack of efficacy and/or side effects. The mean treatment period was 130 days. Of note, two patients developed depression, of whom one had suicidal ideation.
For some patients with psoriasis, biologic monotherapy is insufficient to obtain or maintain disease control [2]. While increasing dosage is an option, this may raise concerns and risk of side effects. On the other hand, switching to another biologic drug is known to be associated with less efficacy and drug survival impairment [28]. With both higher dosages and lower-line biologic therapies, increased expenses may apply. An alternative treatment approach is, therefore, to add a small-molecule drug to the current biologic therapy. This strategy has been practiced for years, most notably with methotrexate, although high-quality evidence is limited [3].
It remains unclear why combination therapy with apremilast and a biologic drug may amplify clinical response in patients with psoriasis, but it likely relates to different modes of action. Being a cyclic nucleotide phosphodiesterase (PDE)4 inhibitor, apremilast works intracellularly by increasing cyclic adenosine monophosphate (cAMP), and thereby has broader anti-inflammatory properties than biologic agents [29]. Compared to healthy skin, PDE4 activity is increased in psoriatic lesions [30], and PDE4 inhibition seems to down-regulate essential cytokines in psoriasis pathogenesis [31]. The loss of exclusivity of apremilast is due within the coming years, and although generic drugs do not necessarily imply lower costs, they tend to be significantly cheaper than brand-name medications according to the FDA. Interestingly, while apremilast for years has been positioned as the only oral PDE4 inhibitor in dermatology, clinical phase 2 trials are currently running on, for example, roflumilast and orismilast for psoriasis (NCT05190419 and NCT04549870).
In conclusion, evidence for combined treatment with biologics and apremilast is limited and restricted to retrospective studies of various quality. Based on available data, apremilast may constitute an efficacious and safe add-on treatment to biologic therapy in patients with psoriasis, but properly conducted clinical investigations are needed. Currently, no prospective trials on apremilast and biologic combination treatment are registered at ClinicalTrials.gov (assessed 24 April 2022).
References
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–72. https://doi.org/10.1016/j.jaad.2018.11.057.
Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–19. https://doi.org/10.1111/bjd.16102.
Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432–8. https://doi.org/10.1001/jamadermatol.2014.3456.
Loos AM, Liu S, Segel C, et al. Comparative effectiveness of targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2018;79(135–44): e7. https://doi.org/10.1016/j.jaad.2018.02.027.
Robinson JK, Dellavalle RP, Bigby M, Callen JP. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol. 2008;144:97–9. https://doi.org/10.1001/archdermatol.2007.28.
AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313–6. https://doi.org/10.1177/1203475416631328.
Alomran A, Zancanaro P, Prussick L, et al. Apremilast in combination with an interleukin 17A inhibitor in the treatment of recalcitrant palmoplantar psoriasis: a chart review. J Psoriasis Psoriatic Arthritis. 2018;3:122–5.
Aragón-Miguel R, González IM, González-Valle O, Aragón-Díez A. Combination therapy of apremilast and biologic agent as a step-up strategy option of psoriasis and psoriatic arthritis. J Am Acad Dermatol. 83(6 supplement) (abstract) 2020.
Armesto S, Gonzalez Vela C, Sanchez J, et al. Treating multidrug-resistant psoriasis with brodalumab, apremilast, methotrexate and prednisone combination therapy in the COVID-19 pandemic. Dermatol Ther. 2020;33: e14464. https://doi.org/10.1111/dth.14464.
Danesh MJ, Beroukhim K, Nguyen C, et al. Apremilast and adalimumab: a novel combination therapy for recalcitrant psoriasis. Dermatol Online J. 2015;21. https://doi.org/10.5070/D3216027825.
De A, Das S, Dhoot D, Sarda A. Apremilast coadministered with Secukinumab for safe and effective control of psoriasis with resultant reduction of maintenance dose of the biologic. Indian J Dermatol. 2019;64:239–41. https://doi.org/10.4103/ijd.IJD_548_18.
Galluzzo M, D’Adamio S, Campione E, et al. Treating a multidrug-resistant psoriatic HLA-C*18:01 allele carrier with combination Ustekinumab Apremilast therapy. Mol Diagn Ther. 2018;22:717–21. https://doi.org/10.1007/s40291-018-0354-8.
Georgakopoulos JR, Ighani A, Yeung J. Short- and long-term management of an acute pustular psoriasis flare: a case report. J Cutan Med Surg. 2017;21:452–6. https://doi.org/10.1177/1203475417712499.
Hadi A, Lebwohl M. Secukinumab and apremilast combination therapy for recalcitrant psoriasis. J Psoriasis Psoriatic Arthritis. 2017;2:59–61.
Ighani A, Georgakopoulos JR, Shear NH, et al. Maintenance of therapeutic response after 1 year of apremilast combination therapy compared with monotherapy for the treatment of plaque psoriasis: a multicenter, retrospective study. J Am Acad Dermatol. 2018;79:953–6. https://doi.org/10.1016/j.jaad.2018.04.043.
Ighani A, Georgakopoulos JR, Walsh S, et al. A comparison of apremilast monotherapy and combination therapy for plaque psoriasis in clinical practice: a Canadian multicenter retrospective study. J Am Acad Dermatol. 2018;78:623–6. https://doi.org/10.1016/j.jaad.2017.09.060.
Kishimoto M, Komine M, Hioki T, et al. Real-world use of apremilast for patients with psoriasis in Japan. J Dermatol. 2018;45:1345–8. https://doi.org/10.1111/1346-8138.14617.
Masuda K, Kanehisa F, Katoh N. Combination therapy of apremilast and biological product in a patient with psoriasis. In: Acta dermato-venereologica conference 5th world psoriasis and psoriatic arthritis conference, p 392018.
Mayba J, Gooderham M. Treatment of palmoplantar pustulosis with the combination of ustekinumab and apremilast: a case report. Arc Cas Rep CMed. 2016;2:128.
Metyas S, Tomassian C, Messiah R, et al. Combination therapy of apremilast and biologic agent as a safe option of psoriatic arthritis and psoriasis. Curr Rheumatol Rev. 2019;15:234–7. https://doi.org/10.2174/1573397115666181130094455.
Mikhailitchenko AL, Crowley EL, Gooderham M. Eight-patient case series of palmoplantar pustulosis treated successfully with apremilast. J Psoriasis Psoriatic Arthritis. 2019;4:7–10. https://doi.org/10.1177/24755303.
Nisar MK. Combining secukinumab and apremilast to successfully treat refractory psoriatic skin and joint disease: A novel approach. Eur J Rheumatol. 2019;6:60–1. https://doi.org/10.5152/eurjrheum.2018.17188.
Rothstein BE, McQuade B, Greb JE, et al. Apremilast and secukinumab combined therapy in a patient with recalcitrant plaque psoriasis. J Drugs Dermatol. 2016;15:648–9.
Sacchelli L, Patrizi A, Loi C, Bardazzi F. Combination therapy of apremilast and secukinumab in patients with moderate-to-severe, recalcitrant plaque psoriasis. Clin Exp Dermatol. 2019;44:e243–4. https://doi.org/10.1111/ced.14000.
Takamura S, Sugai S, Taguchi R, Teraki Y. Combination therapy of apremilast and biologics in patients with psoriasis showing biologic fatigue. J Dermatol. 2020;47:290–4. https://doi.org/10.1111/1346-8138.15193.
Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/=156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77(310–7): e1. https://doi.org/10.1016/j.jaad.2017.01.052.
Abignano G, Fadl N, Merashli M, et al. Apremilast for the treatment of active psoriatic arthritis: a single-centre real-life experience. Rheumatology (Oxford). 2018;57:578–80. https://doi.org/10.1093/rheumatology/kex454.
Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br J Dermatol. 2020;183:294–302. https://doi.org/10.1111/bjd.18981.
Peng T, Qi B, He J, et al. Advances in the development of phosphodiesterase-4 inhibitors. J Med Chem. 2020;63:10594–617. https://doi.org/10.1021/acs.jmedchem.9b02170.
Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753–63. https://doi.org/10.1016/j.cellsig.2016.01.007.
Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. https://doi.org/10.3389/fphar.2018.01048.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
M. Gyldenløve: None. F. Alinaghi: None. C. Zachariae: Investigator for AbbVie, Sanofi, Janssen Cilag, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Novartis, Regeneron, and LEO Pharma. Paid speaker for Eli Lilly, Novartis, CSL, and LEO Pharma. Consultant and/or advisory board member for AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, Takeda, Amgen, and CSL. L. Skov: Received research funding from Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the LEO Foundation, and the Kgl Hofbundtmager Aage Bang Foundation. Honoraria as consultant and/or speaker for AbbVie, Eli Lilly, Novartis, Pfizer, LEO Pharma, Janssen Cilag, UCB, Almirall, Bristol-Myers Squibb, and Sanofi. Investigator for AbbVie, Pfizer, Sanofi, Janssen Cilag, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Novartis, Regeneron, Galderma, and LEO Pharma. A. Egeberg: Received research funding from Pfizer, Eli Lilly, Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation. Honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Zuellig Pharma Ltd., Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Union Therapeutics, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals.
Ethics approval, informed consent, and code availability
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions (credit taxonomy)
Conceptualization (MG), methodology (MG and FA), literature search and data extraction (MG, FA, and AE), resources (FA, CZ, and LS), writing—original draft (MG), writing—review and editing (FA, AE, LS, and CZ).
Rights and permissions
About this article
Cite this article
Gyldenløve, M., Alinaghi, F., Zachariae, C. et al. Combination Therapy with Apremilast and Biologics for Psoriasis: A Systematic Review. Am J Clin Dermatol 23, 605–613 (2022). https://doi.org/10.1007/s40257-022-00703-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00703-1