Abstract
Anti-interleukin (IL)-17 agents have shown excellent therapeutic efficacy in patients with psoriasis and are expected to be expanded to other chronic inflammatory diseases. However, patients receiving anti-IL-17 agents are at an increased risk of developing Candida infection, with some agents reported to increase the incidence in a dose-dependent manner. Interleukin-17 is secreted by the Th17 subset of CD4+ lymphocytes, CD8+ T cells, and innate cells, including natural killer T cells, lymphoid tissue inducer cells, innate lymphoid cells, and γδ-T cells, and plays an important role in antifungal defense. Genetic defects in the IL-17-signaling pathway in both humans and animal models render susceptibility to candidiasis caused by Candida albicans. The purpose of this narrative review is to evaluate the literature on the role of IL-17 in protection against candidiasis, the prevalence of candidiasis in anti-IL-17 agent use, and to offer clinical recommendations on the diagnosis and management of anti-IL-17 medication-associated candidiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Interleukin-17 is a key cytokine in the development of various types of chronic inflammatory disease including psoriasis. |
Anti-interleukin-17 agents produce high clinical response rates in patients with psoriasis; however, they can increase the risk of developing candidiasis. |
This review provides clinical recommendations on the recognition and management of anti-interleukin-17 medication-associated candidiasis. |
1 Introduction
Candida albicans is a commensal fungus in humans, colonizing mucosal surfaces such as the oral cavity, vaginal tract, gastrointestinal tract, and skin. Although normally benign in healthy individuals, C. albicans can cause pathogenic infections such as oropharyngeal candidiasis (OPC) among immunocompromised populations. Immunity to C. albicans is highly dependent on CD4+ T cells, as patients who are human immunodeficiency virus positive often experience recurrent OPC during progression to acquired immune deficiency virus, correlating with T-cell counts [1]. Both innate and adaptive immunity play a role in protection against fungal infections. In humans, adaptive immunity is essential for protection against mucosal candidiasis, indicated by the high susceptibility of human immunodeficiency virus-positive patients to the disease [2].
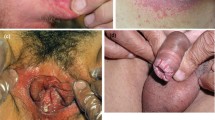
The diagnosis of mucosal candidiasis is confirmed by isolation and identification of Candida spp. in a sterile specimen collected directly from the lesion. For oropharyngeal and vulvovaginal candidiasis, the diagnosis is largely clinical: it is based on recognition of the white plaques that are typically easily scraped off and can be confirmed by a microscopic analysis using potassium hydroxide or fungal culture. As Candida infections in patients with psoriasis often develop in areas that are not readily visible and are often asymptomatic, in order to identify potential mucocutaneous Candida infections, it is recommended to inquire about oral or genital discomfort when examining a patient with psoriasis [3].
Known risk factors for the development of severe candidiasis, including esophageal candidiasis, include antibiotic or corticosteroid use, immunodeficiency syndrome, malignancy, alcohol use, diabetes mellitus, and esophageal motility disorders [4,5,6]. There are some reports suggesting that psoriasis may be associated with oral candidiasis as well [7, 8]. Picciani et al. suggested that the rate of oral candidiasis is higher in patients with psoriasis compared with healthy controls, and psoriasis may be associated with increased disease severity [9]. However, the prevalence of fungal infections among patients with psoriasis is controversial.
Recently, a number of anti-interleukin (IL)-17 medications such as ixekizumab, secukinumab, brodalumab, and bimekizumab have emerged [10] and have led to significant progress in the management of inflammatory diseases including psoriasis. Secukinumab and ixekizumab are anti-IL-17A agents currently approved for the treatment of psoriasis and psoriatic arthritis in the USA [11] and brodalumab is an IL-17 receptor blocker. Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits both IL-17A and IL-17F [12]. As IL-17A plays a pivotal role in host protection against fungal infections, patients treated with these anti-IL-17 agents may develop both vulvovaginal and oropharyngeal candidiasis [13]. Despite multiple reports of candidiasis in patients taking IL-17 inhibitors, the literature describing this complication is sparse. With the rise of anti-IL-17 agents and the potential adverse event of Candida infections, there is a need for further exploration of this topic [14, 15]. A previous systematic review in 2017 showed an overview of the incidence of Candida infections during treatment with IL-17A inhibitors and suggested a practical management guide [14]. The recent advent of bimekizumab has allowed for a rapid and significant improvement in dermatologic and rheumatologic disease activity, but also increased the incidence of Candida infections. Therefore, there is a need for greater clinical guidance on the management of Candida infections in patients taking anti-IL-17 agents. This review aims to provide an update on the role of IL-17 in anti-Candida immunity and candidiasis in the context of anti-IL-17 medication use, and offer some recommendations for the management of oropharyngeal, esophageal, and vulvovaginal candidiasis among these patients.
In this narrative review, a literature search was conducted in PubMed using the keywords “candidiasis and IL-17,” “oropharyngeal candidiasis and IL-17,” and “candida infections in IL-17 inhibitors,” which yielded 101 results. Since the first anti-IL-17 agent was approved in 2015, results from years 2014 to 2021 were extracted for the purposes of this review paper. Original articles were selected from the total articles. Table 1 summarizes the reported incidence of oral, genital, esophageal, and cutaneous candidiasis with anti-IL-17 agents, including secukinumab (IL-17A inhibitor), brodalumab (IL-17 receptor A inhibitor), ixekizumab (IL-17A inhibitor), and bimekizumab (investigational IL-17A/F inhibitor).
2 Anti-IL-17 Therapy and Candidiasis
The incidence of Candida infections varied depending on the anti-IL-17 medication: secukinumab (1.4–13.5%), brodalumab (0.3–7%), ixekizumab (0–3.5%), and bimekizumab (1.9–21.2%) (Table 1). Because of the direct effect of IL-17 on anti-Candida immunity, the incidence of OPC is greater compared with tumor necrosis factor-alpha inhibitors (0.1%) and IL-23 inhibitors (1–2%) [15, 31, 37].
The most common Candida infection site was the oral cavity (secukinumab 0.8–3%, brodalumab 3–7%, bimekizumab 1.9–19.3%). However, ixekizumab trials provided only limited candidiasis site information. Two cases of esophageal candidiasis were reported in patients treated with secukinumab [20, 21] and one each in patients treated with brodalumab [24], ixekizumab [27], and bimekizumab [32]. Genital candidiasis has been reported in patients treated with secukinumab [12, 19, 21] and bimekizumab [12, 36]. Most cases of reported candidiasis in the context of anti-IL-17 medication were mild to moderate and rarely led to termination of biologics. There have been no reports of invasive Candida infections. Severe oral candidiasis has, however, been reported in patients treated with ixekizumab and bimekizumab [29,30,31].
3 Dosage and Risks
In a study comparing the efficacy and safety of two IL-17 inhibitors, bimekizumab had greater skin clearance in psoriasis than secukinumab, but was associated with an increased incidence of candidiasis (bimekizumab; 21.2% and secukinumab; 4.6%) [12]. The most commonly used dose of bimekizumab was 320 mg every 4 weeks (q4w). The incidence of Candida infection in patients receiving this dosing regimen ranged from 6.4 to 21.2% [12, 30, 32,33,34,35]. In several studies, bimekizumab has been reported to increase the incidence of candidiasis in a dose-dependent manner [32, 33]. However, Ritchlin et al. reported that 1/43 (2%) and 2/41 (5%) patients in the bimekizumab 160-mg q4w group and the 320-mg initial dose followed by the 160-mg q4w group, respectively, developed Candida infection, whereas no patients in the 320-mg group developed candidiasis [34]. There have been many reports showing a dose-dependent increase in candidiasis incidence with secukinumab as well [12, 16, 17, 19, 20]. The duration of administration varied from trial to trial, but the most common dosing schedule was weekly administration until week 4, and then q4w [12, 16, 17, 19, 21]. The incidence of candidiasis by secukinumab dose was 2.5–8% in the 300-mg group, 1–7% in the 150-mg group, and 1–2% in the 75-mg group. For brodalumab, two studies mentioned the incidence of Candida infection by dose, and both showed a higher incidence in the 210-mg group than in the 140-mg group (210 mg; 1.3–2.3%, 140 mg group; 0.5–1.3%) [22, 25]. Although there is a little evidence for ixekizumab, Combe et al. showed that the incidence of candidiasis was higher in the group receiving ixekizumab 80 mg every 2 weeks (3.6%) than in the group receiving it q4w (1.7%) [27]. Further evidence on the risk of candidiasis at different doses is needed.
4 Treatment Response and Recurrence
Most cases of Candida infections were mild or moderate and resolved with antifungal therapy. Only two studies reported Candida infection that led to study discontinuation; of the patients with psoriasis treated with bimekizumab, three patients who developed oral candidiasis and one patient who developed esophageal candidiasis discontinued treatment because of infection [31, 32]. The head-to-head trial of bimekizumab vs secukinumab had resolution of oral candidiasis with antifungal treatment in 85% of cases [12]. This study also showed that among patients with psoriasis who developed oral candidiasis, 43.1% of bimekizumab-treated patients and 36.4% of secukinumab-treated patients reported more than one instance of the infection [12], suggesting that patients treated with these agents could be at risk of developing treatment-resistant Candida infection. In a phase IIb, randomized controlled trial of bimekizumab, two cases of oral candidiasis, one case of OPC, and one case of genital candidiasis resulted in a short interruption of treatment and required systemic antifungal therapy [34]. In all other trials, all cases of candidiasis as an adverse event were reported to respond to treatment and be cured during the trial period.
5 Pathogenesis
The oral mucosa is a vital physical barrier limiting pathogen invasion. In OPC, candidalysin, a fungal toxin, is secreted by hyphae and damages the oral mucosal epithelium, triggering production of IL-17 from innate and adaptive lymphocytes via IL-1-dependent signals [37]. Interleukin-17 then activates essential antifungal responses, including myeloid and lymphoid chemoattractants and antimicrobial peptides, particularly β defensin-3 [38]. The IL-17 cytokine family consists of six members (IL-17A through IL-17F) and five receptors (IL-17RA through IL-17RE), among which IL-17A and IL-17F are the best-characterized cytokines [11]. In mouse models, IL-17A and IL-17F have shown to contribute to clearance of the fungal pathogen [31]. In humans, chronic mucocutaneous candidiasis is associated with aberrations in the IL-17/Th17 pathway, such as loss-of-function mutations in IL17RA, ACT1, and IL17F genes, and gain-of-function mutations in the STAT1 gene [31]. Therefore, it is expected that the clinical use of IL-17 inhibitors will confer increased susceptibility to mucosal candidiasis because of interference with antifungal immunity. Although there is limited information on the role of cytokines in skin, proinflammatory cytokines including IL-17 are significantly upregulated during the initial phase of cutaneous infection and correlated with rapid elimination of C. albicans [39, 40].
The improved clinical efficacy of bimekizumab when compared with secukinumab was also associated with an increased incidence of candidiasis [12]. This is likely explained by the combined inhibition of IL-17A and IL-17F, which contributes to protection against Candida infections of the oral mucosa [41,42,43,44,45,46,47]. It is also possible that bimekizumab has a higher incidence of Candida infection because of its stronger IL-17A inhibition as bimekizumab has a higher IL-17A affinity than secukinumab [48]. The incidence of candidiasis was higher with bimekizumab in a comparison of the two agents [12]. However, ixekizumab has a lower incidence of Candida infection despite having the same affinity as bimekizumab, suggesting that the reason for the higher risk of candidiasis with bimekizumab is owing to the inhibition of IL-17F in addition to IL-17A. Brodalumab has also been reported to have a relatively high incidence of Candida infection [26], which may be due to its broad inhibition of IL-17 signaling by blocking the receptor.
6 Treatment
The treatment for mild-to-moderate OPC is usually topical, consisting of clotrimazole or miconazole for 7–14 days [38]. Topical application to manage OPC minimizes drug interactions and adverse effects associated with systemic antifungal agents [15]. Alternatively, nystatin is recommended for mild OPC (nystatin suspension [100,000 U/mL] 4–6 mL four times daily, or one to two nystatin pastilles [200,000 U each] four times daily, for 7–14 days [38].
For moderate-to-severe infection, oral fluconazole (100–200 mg daily for 7–14 days) is recommended as first-line systemic therapy [14]. If patients have recurrent infection, additional treatment with oral fluconazole 100 mg 3 times weekly is possible [38]. If infection persists despite systemic treatment, reconfirmation or susceptibility testing may be considered; there has been increasing concern of fluconazole resistance in OPC [49].
Common adverse events associated with fluconazole use are largely gastrointestinal (nausea, vomiting, abdominal pain, and diarrhea). Other adverse events include hepatotoxicity, QT prolongation, cholestasis, and leukopenia, among others. Caution should be exercised when administering fluconazole to individuals with known prolonged QT interval or those co-administering medications metabolized via the cytochrome P450 enzyme [50].
Systemic antifungal therapy is always required for the management of esophageal candidiasis. Oral fluconazole, 200–400 mg (3–6 mg/kg) daily, for 14–21 days is recommended for the treatment of esophageal candidiasis. Intravenous fluconazole 400 mg (6 mg/kg) daily or echinocandins (micafungin 150 mg daily, caspofungin 70-mg loading dose followed by 50 mg daily, or anidulafungin 200 mg daily) are recommended for patients who cannot tolerate oral therapy [38].
For the treatment of vulvovaginal candidiasis, topical antifungal agents (e.g., clotrimazole cream, miconazole vaginal suppository or cream, terconazole vaginal suppository or cream, butoconazole cream, and tioconazole ointment) are recommended. Alternatively, a single dose of oral fluconazole 150 mg can be considered [51]. For severe acute vulvovaginal candidiasis, oral fluconazole 150 mg, given every 72 hours for a total of two or three doses can be used [52].
7 Management Recommendations
This paper is based on the results of published clinical trials and, as the number of patients taking anti-IL-17 agents increases in the future, the prevalence of Candida infections may also increase. It is important to mention that most patients in the trial responded to antifungal therapy and continued their treatment with anti-IL-17 agents.
After reviewing the literature for successful therapies for mucosal Candida infections, recommendations for evaluation and treatment of anti-IL-17 medication-associated candidiasis are summarized in Fig. 1. When administering the anti-IL-17 agents, it is recommended that good oral care and blood glucose control be accomplished prior to drug administration to prevent the development of Candida infections [5, 6]. If OPC is suspected in patients receiving anti-IL-17 agents, dermatologists should perform a microscopic examination or fungal culture. If the patient shows symptoms for esophageal candidiasis, a referral to gastroenterology for an endoscopic diagnosis, treatment recommendations, and monitoring should be considered [4]. When patients treated with anti-IL-17 agents have the symptoms of suspected vulvovaginal candidiasis, dermatologists should treat with topical antifungal drugs or oral fluconazole [38], and a referral to gynecology can be considered. If clinical symptoms are characteristic, treatment should be initiated as soon as possible (prior to test results) in order to alleviate symptoms and prevent the spread of infection [38]. It is important to routinely screen patients receiving anti-IL-17 therapy as patients may not be able to recognize the symptoms of mucosal candidiasis [3].
Anti-interleukin (IL)-17 medication-associated Candida infections management algorithm. *Nystatin suspension (100,000 U/mL) 4–6 mL four times daily, or one to two nystatin pastilles (200,000 U each) four times daily, for 7–14 days. **For infections resistant to fluconazole, itraconazole (200 mg once daily) or posaconazole (400 mg twice daily for 3 days then 400 mg daily for up to 28 days) is recommended [38]. Voriconazole (200 mg twice daily) and amphotericin B deoxycholate (100 mg/mL 4 times daily) can also be used for the fluconazole-resistant cases. Intravenous echinocandins or intravenous amphotericin B deoxycholate could be other alternatives for the treatment for refractory infections [38, 52]. OPC oropharyngeal candidiasis
8 Conclusions
Based on the current literature review of IL-17 antagonists, the use of anti-IL-17 agents is associated with candidiasis dependent on the agent used; however, there were no reports of invasive infections. However, serious mucosal Candida infections have been reported in patients treated with secukinumab, ixekizumab, and bimekizumab [20, 29,30,31].
Mucosal candidiasis is one of the most common adverse effects of anti-IL-17 medications. Interleukin-17 is a cytokine that plays a key role in fungal defense in the human body, and it has been shown that genetic abnormalities in IL-17 and its receptors can cause chronic cutaneous mucosal candidiasis. Therefore, it is important for clinicians to be aware of the symptoms and the management options for mucosal candidiasis, including when to refer to gastroenterology or gynecology.
Anti-IL-17 medication not only improves skin manifestations (e.g., psoriasis), but also cardiovascular inflammation, metabolic factors, and psoriatic arthritis (including peripheral arthritis, enthesitis, dactylitis, and axial involvement) [53]. Anti-IL-17 therapy may be expanded to various diseases in the future. As patients receiving anti-IL-17 agents are part of a patient population with difficult-to-control chronic inflammatory diseases, discontinuing these agents altogether is not a desirable choice.
Fortunately, according to the literature reviewed in this study, discontinuation of, or increased spacing doses for, anti-IL-17 agents has rarely been needed to control mucosal candidiasis. With early and accurate diagnosis, and appropriate treatment, patients are more likely to be able to continue their anti-IL-17 medications.
References
Dongari-Bagtzoglou A, Fidel PL Jr. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84(11):966–77.
Salt E, Wiggins AT, Rayens MK, Huaman MA, Mannino D, Schwieterman P, et al. Risk factors for targeted fungal and mycobacterial infections in patients taking tumor necrosis factor inhibitors. Arthritis Rheum. 2016;68(3):597–603.
Armstrong AW, Bukhalo M, Blauvelt A. A clinician’s guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am J Clin Dermatol. 2016;17(4):329–36.
Hoversten P, Otaki F, Katzka DA. Course of esophageal candidiasis and outcomes of patients at a single center. Clin Gastroenterol Hepatol. 2019;17(1):200-2.e1.
Hoversten P, Kamboj AK, Katzka DA. Infections of the esophagus: an update on risk factors, diagnosis, and management. Dis Esophagus. 2018;31(12):doy094
Akpan A. Oral candidiasis. Postgrad Med J. 2002;78(922):455–9.
Pietrzak A, Grywalska E, Socha M, Roliński J, Franciszkiewicz-Pietrzak K, Rudnicka L, et al. Prevalence and possible role of Candida species in patients with psoriasis: a systematic review and meta-analysis. Mediat Inflamm. 2018;2018:9602362.
Chadeganipour M, Shadzi S, Mohammadi R. Fungal infections among psoriatic patients: etiologic agents, comorbidities, and vulnerable population. Autoimm Dis. 2021;2021:1174748.
Picciani BL, Michalski-Santos B, Carneiro S, Sampaio AL, Avelleira JC, Azulay DR, et al. Oral candidiasis in patients with psoriasis: correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. J Am Acad Dermatol. 2013;68(6):986–91.
He C, Xue C, Zhu G, Kang P. Efficacy and safety of interleukin-17 inhibitors in the treatment of chronic rheumatic diseases: a combined and updated meta-analysis. J Clin Pharm Ther. 2021;46(4):895–906.
Ritchlin CT, Krueger JG. New therapies for psoriasis and psoriatic arthritis. Curr Opin Rheumatol. 2016;28(3):204–10.
Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52.
Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311.
Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177(1):47–62.
Fidel PL Jr. Candida-host interactions in HIV disease: implications for oropharyngeal candidiasis. Adv Dent Res. 2011;23(1):45–9.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis: results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(1):27-36.e1.
Thaçi D, Humeniuk J, Frambach Y, Bissonnette R, Goodman JJ, Shevade S, et al. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol. 2015;173(3):777–87.
Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–93.
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–39.
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–46.
Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–86.
Papp K, Leonardi C, Menter A, Thompson EH, Milmont CE, Kricorian G, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71(6):1183-90.e3.
Yamasaki K, Nakagawa H, Kubo Y, Ootaki K. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176(3):741–51.
Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28.
Yamaguchi Y, Takatsu N, Ootaki K, Nakagawa H. Long-term safety of brodalumab in Japanese patients with plaque psoriasis: an open-label extension study. J Dermatol. 2020;47(6):569–77.
Combe B, Rahman P, Kameda H, Cañete JD, Gallo G, Agada N, et al. Safety results of ixekizumab with 1822.2 patient-years of exposure: an integrated analysis of 3 clinical trials in adult patients with psoriatic arthritis. Arthritis Res Ther. 2020;22(1):14.
van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392(10163):2441–51.
Langley RG, Kimball AB, Nak H, Xu W, Pangallo B, Osuntokun OO, et al. Long-term safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol. 2019;33(2):333–9.
Warren RB, Blauvelt A, Bagel J, Papp KA, Yamauchi P, Armstrong A, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–41.
Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–98.
Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study. J Am Acad Dermatol. 2020;83(5):1367–74.
van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2020;79(5):595–604.
Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395(10222):427–40.
Papp KA, Merola JF, Gottlieb AB, Griffiths CEM, Cross N, Peterson L, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277-86.e10.
Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–32.
Monin L, Gaffen SL. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb Perspect Biol. 2018;10(4): a028522.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-50.
Sawada Y, Setoyama A, Sakuragi Y, Saito-Sasaki N, Yoshioka H, Nakamura M. The role of IL-17-producing cells in cutaneous fungal infections. Int J Mol Sci. 2021;22(11):5794.
Campois TG, Zucoloto AZ, de Almeida Araujo EJ, Svidizinski TIE, Almeida RS, da Silva Quirino GF, et al. Immunological and histopathological characterization of cutaneous candidiasis. J Med Microbiol. 2015;64(8):810–7.
Thompson GR 3rd, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, et al. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):488–95.
Bedair AA, Darwazeh AM, Al-Aboosi MM. Oral Candida colonization and candidiasis in patients with psoriasis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):610–5.
Ruhnke M, Rickerts V, Cornely OA, Buchheidt D, Glöckner A, Heinz W, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54(4):279–310.
Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(Suppl. 1):34–40.
Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol. 2017;2(17):eaam8834.
Aggor FEY, Break TJ, Trevejo-Nuñez G, Whibley N, Coleman BM, Bailey RD, et al. Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci Immunol. 2020;5(48):eaba0570.
Glatt S, Jemec GBE, Forman S, Sayed C, Schmieder G, Weisman J, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157(11):1279–88.
Adams R, Maroof A, Baker T, Lawson ADG, Oliver R, Paveley R, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
Messer SA, Moet GJ, Kirby JT, Jones RN. Activity of contemporary antifungal agents, including the novel echinocandin anidulafungin, tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: report from the SENTRY Antimicrobial Surveillance Program (2006 to 2007). J Clin Microbiol. 2009;47(6):1942–6.
Amichai B, Grunwald MH. Adverse drug reactions of the new oral antifungal agents: terbinafine, fluconazole, and itraconazole. Int J Dermatol. 1998;37(6):410–5.
Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG. 2002;109(1):85–95.
Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203–11.
von Stebut E, Boehncke WH, Ghoreschi K, Gori T, Kaya Z, Thaci D, et al. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol. 2019;10:3096.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the preparation of this article.
Conflict of interest
Mika Yamanaka-Takaichi, Soha Ghanian, David A, Katzka, and Rochelle R. Torgerson have no conflicts of interest to disclose. Afsaneh Alavi is a consultant for AbbVie, BI, Janssen, InflaRx, Novartis, and UCB and an investigator for BI, Processa, and Castle Creek.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
MYT and SG performed the literature review and DAK, RRT, and AA edited the manuscript.
Rights and permissions
About this article
Cite this article
Yamanaka-Takaichi, M., Ghanian, S., Katzka, D.A. et al. Candida Infection Associated with Anti-IL-17 Medication: A Systematic Analysis and Review of the Literature. Am J Clin Dermatol 23, 469–480 (2022). https://doi.org/10.1007/s40257-022-00686-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00686-z