Abstract
Background
Probiotic, prebiotic, and synbiotic supplementation is becoming more prevalent nowadays. Clinical studies have demonstrated some of the medical benefits of probiotics, prebiotics, and synbiotics within dermatology but an evidence-based review of their effects in adults is needed.
Objective
The aim of this study was to identify evidence for the use of supplementation with probiotics, prebiotics, or synbiotics for the prevention and treatment of dermatological diseases in adults.
Data sources
We conducted a search of the Ovid MEDLINE, Cochrane Central Register of Controlled trials and EMBASE electronic databases from 1 January 1946 to 11 January 2017.
Study selection
Trials examining supplementation in the treatment of dermatological diseases using oral or topical probiotics, synbiotics, and prebiotics in adults over the age of 18 years were selected.
Data extraction
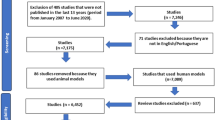
Of 315 articles, 12 met the inclusion criteria.
Data synthesis
Nutritional supplementation with probiotics and prebiotics was shown to improve atopic dermatitis (AD) symptomatology, quality of life, or clinical severity in six of nine studies. One study in psoriasis was shown to improve inflammatory markers, and one study suggested that probiotics could be used as adjunctive therapy in the treatment of acne.
Conclusion
Preliminary studies are optimistic for the use of some strains of probiotics for symptomatic and clinical improvement in AD, and as adjunctive treatment with antibiotics for acne. Further research is necessary to better assess how probiotics and prebiotics may be used within dermatology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Probiotics may be useful in the treatment of adults with atopic dermatitis and acne. |
The mechanism of action remains unclear but is thought to be due to the alteration of the gut microbiome and modulation of the immune system. |
More studies of probiotics and prebiotics for the skin are warranted. |
1 Introduction
Probiotics are live microorganisms that can confer health benefits when administered in adequate doses [1]. The most commonly used microorganisms are Lactobacillus, Bifidobacterium, Enterococcus, Pronionibacterium, and some yeasts such as Saccharomyces boulardii. Their health benefits include the prevention of antibiotic-associated diarrhea, treatment of irritable bowel syndrome, and inflammatory bowel disease [2].
Prebiotics are ingredients and substances that can promote the growth of certain bacteria in the gut. It is believed that an ingredient must possess three key features to be considered a prebiotic. First, it should resist breakdown by mammalian enzymes and gastrointestinal absorption; second, it must be fermented by the microbiotia of the intestine; and, last, it must be able to selectively stimulate the growth and/or activity of the intestinal bacteria, which have been associated with improving human health [3]. Prebiotics usually target the activity of Lactobacillus and Bifidobacterium [4].
Synbiotics are composed of a combination of prebiotics and probiotics. The prebiotic component is thought to assist with the implantation and survival of live microbial dietary supplements. Specifically, the prebiotic component must selectively favor the probiotic organisms in the formulation [5].
There is a growing body of research involving the use of prebiotics, probiotics, and synbiotics in pediatric atopic dermatitis (AD). Research suggests pre- or probiotics may be beneficial in the prevention and amelioration of AD [6], which is thought to be due to the alteration of the intestinal microbiome and modulation of the immune system. Fewer studies exist regarding the use of prebiotics and probiotics in adults with dermatological diseases, and we sought to investigate and review the current clinical evidence [7].
2 Methods
We conducted a search of the Ovid MEDLINE, Cochrane Central Register of Controlled Trials, and EMBASE electronic databases for articles published from 1 January 1946 to 11 January 2017. For Ovid MEDLINE, we used the search terms ‘probiotics’, ‘prebiotics’, ‘synbiotics’, ‘skin diseases’, ‘humans’ and ‘clinical trial. The following Medical Subject Heading (MeSH) terms were combined using the ‘AND’ Boolean operator to find relevant studies in the Cochrane Central Register of Controlled Trials: ‘skin diseases’, ‘probiotics’, ‘prebiotics’, and ‘synbiotics’. Additionally, the following search terms were used in EMBASE for relevant studies: ‘skin’, ‘diseases’, ‘probiotic agent’, ‘prebiotic agent’, ‘synbiotic agent’, ‘clinical trial’, and ‘humans’. Our searches were limited to articles that tested probiotics, prebiotics, or synbiotics among adults only. Furthermore, we searched through the references of the chosen articles for additional studies.
2.1 Inclusion and Exclusion Criteria
We included clinical trials and cohort studies that examined the effect of probiotics, prebiotics, or synbiotics among adults 18 years of age and older with a dermatological condition. Studies that reported the treatment or prevention of dermatological diseases were selected. Publications were excluded if infants, children, or adolescents were included.
2.2 Study Selection and Data Extraction
On the basis of our selection criteria, all authors (MN, NF, ARV, and RKS) independently reviewed all eligible studies and the final 12 full-text articles, and resolved any differences by consensus. For each study, the following information was abstracted and is documented in Tables 1 and 2: (1) skin disease, (2) number of subjects, (3) study design, (4) probiotic intervention, (5) intervention dosage, (6) primary outcome measures, and (7) major results. A total of 420 patients were included in these 12 studies (Fig. 1).
2.3 Quality of Included Studies
Study quality was assessed independently using the Jadad score for analyzing randomized controlled trials (RCTs) [8]; the Jadad score establishes the methodological quality of studies based on a 5-point scale according to reproducibility and appropriateness of randomization, blinding, and the fate of subjects. Using this system, the quality of a trial is categorized as ‘high’ (Jadad score 3–5) or ‘low’ (Jadad score 0–2) [8].
3 Results
3.1 Atopic Dermatitis
Nine studies assessed the impact of probiotics in adults with AD [1], and no studies were identified in adults for the treatment of AD with prebiotics or synbiotics.
3.1.1 Bifidobacterium
One randomized, double-blind, placebo-controlled study using Bifidobacterium animalis (subsp. lactis LKM512) found that subjects in the probiotic group experienced a significant improvement in itch after 8 weeks [9]. This group also experienced an improvement in the dermatology-specific quality-of-life (QoL) questionnaires. The researchers followed the severity of AD utilizing a 5-point scale and found that while probiotic supplementation improved clinical severity compared with baseline, there was no significant difference in severity scores between groups. Researchers found an increase in the anti-nociceptive metabolite kynurenic acid (KYNA) in three patients whose itch improved after the administration of LKM512 [9]. It was postulated that this improvement was due to KYNA production in the intestines; therefore, this could be a potential treatment for AD-associated pruritus [9].
Additionally, a prior 8-week crossover trial found that patients taking B. animalis (subsp. lactis LKM512) experienced moderate improvement in the symptoms of itch and burning when compared with placebo [10]. All patients enrolled had been previously treated with long-term Kampo medicine (the practice of Chinese herbal medicine in Japan), which could make it difficult to differentiate between the effects of Kampo medication and the effects of B. animalis. A statistically significant increase in interferon (IFN)-γ was observed in both groups, however the increase was greater in the probiotic group [10]. The authors of the study did not suggest an explanation for this; however, studies in children have shown an increase in IFNγ after the administration of probiotics. It has been postulated that this is a reflection of an increased T helper (Th)1 response [11], although the significance of this is not clear.
In a study by Yoshida et al. [12], Bifidobacterium breve (strain YY) was administered to adults with AD over an 8-week period. Compared with controls, the probiotic group (n = 16) demonstrated a decline in the SCORAD (SCORing Atopic Dermatitis) index after 8 weeks [12]; however, the only parameter of the SCORAD index that showed a statistically significant decline was the intensity criteria (8.0 ± 2.9–6.8 ± 3.0; p = 0.018). There was a statistically significant reduction in the objective SCORAD (33.7 ± 13.6–23.8 ± 4.0; p = 0.034), which consisted of the extent and intensity parameters but excluded subjective symptoms. Furthermore, there was a statistically significant decline in the QoL ‘Skindex-29-J’ scores (a questionnaire that assesses symptoms, functioning, and emotions in AD patients over the previous 4 weeks [13]) in the probiotic group (p = 0.019) compared with baseline. In the study, subjects were not randomized according to disease severity, and subjects with higher disease severity were found in the probiotic arm of the study. The average total baseline SCORAD scores for the probiotic and placebo groups were 41.0 and 25.7, respectively (p = 0.027). This may have unevenly affected the ability of each treatment group to improve. In the study, stool samples demonstrated the probiotic was able to colonize the gastrointestinal (GI) tract as there was an increase in the colonization rate of the gut microbiome with Bifidobacteria [12].
3.1.2 Lactobacillus
Researchers studied the effects of Lactobacillus salivarius LS01 for 16 weeks in adults with AD [14] and noted a significant reduction in the SCORAD and Dermatology Life Quality Index (DLQI) ratings in the probiotic-treated group (week 0: 27.6 ± 3.4 vs. week 16: 13.1 ± 0.3; p < 0.001). Moreover, there was a statistically significant decline in Th1 and Th2 cytokines compared with baseline in the placebo group only (T0: 28.2 ± 2.5 pg/ml vs. T16: 33.0 ± 3.3). The staphylococcal load in the fecal microbiome in the probiotic-treated group was reduced [14].
One study used Lactobacillus fermentum (ME-3) over a 3-month period in 10 patients with AD compared to another group that did not receive a probiotic [15]. Patients in both groups experienced a non-significant improvement in their SCORAD index. Among the probiotic-treated group, a significant reduction was seen in skin iron levels, diene conjugate (DC) levels, and glutathione redox ratios, which are all markers of oxidative stress. In addition, there was a statistically significant decline in blood markers of oxidative stress, such as oxidatively modified low-density lipoprotein (oxLDL). This demonstrated that AD patients could be at higher oxidative burden, which may be reduced by the administration of a probiotic [15]. The significance of the reduction in oxidative stress is not clear since there was no difference in the improvement of the SCORAD index.
In another study, 34 subjects with AD were administered placebo or probiotic Lactobacillus paracasei K71 fora period of 12 weeks [16]. Skin severity scores (based on eruption intensity and area of involvement developed by the Japanese Dermatological Association [17]) in the probiotic group were decreased from baseline, at week 8 (p < 0.05), and at week 12 (p < 0.01). There was no influence of probiotic use on the QoL or itch scoring. Subsequently, the placebo group had a 1.9-fold greater use in topical therapeutics in the placebo group; however, this difference was not statistically significant [16].
3.1.3 Probiotic Mixtures
Researchers conducted an RCT using a combination of probiotics containing Lactobacillus salivarius LS01 DSM 2275 and Bifidobacterium breve BR03 DSM 16604 [18]. Subjects using probiotics had a significant improvement in clinical scores (SCORAD and DLQI). The researchers also observed a reduction in plasma lipopolysaccharide (LPS) in the probiotic group. Plasma LPS is a marker of inflammation and permeability of the intestinal endothelium. It is thought that altered gut permeability leads to toll-like receptor (TLR)-dependent immune activation. They demonstrated a reduction in CD8/CD38/CD45RO T cell activation in the probiotic group, which is thought to be a marker of immune activation. After treatment with a probiotic, it was found that there was a significant decrease in the staphylococcal load of the feces [18].
In the double-blinded, placebo-controlled, randomized crossover study by Roessler et al. [19], a probiotic drink composed of Streptococcus thermophiles, Lactobacillus paracesi LPC-37, Lactobacillus acidophilus 74-2, and Bifidobaterium animalis subsp. lactis DGCC 420 was administered to adults with and without AD [19]. After 8 weeks of probiotics among patients with AD, a non-significant decrease in the SCORAD was observed (from −15.5 to −20.3%; p = 0.081). Researchers did not observe a statistically significant decline in immunoglobulin (Ig) E levels in AD patients after administration of probiotics [19].
Drago et al. [20] investigated the effect of adding tara gum and Streptococcus thermophilus ST10 DSM 25246 to L. salivarus LS01 DSM 22775 [20]. Tara gum is thought to act as a gelling complex, which would adhere to the intestinal mucus and improve barrier function, thereby improving the activity of L salivarus. Twenty-five AD patients were included and were randomized to receive either a placebo or probiotic. A statistically significant improvement in the SCORAD index was observed after administration of the probiotic (p < 0.0001). Staphylococcus aureus is reportedly found more frequently in the gut microbiome of subjects with AD [21]. Although there was a trend toward a decrease in the S. aureus load in the fecal microflora of the probiotic group, this decrease was not statistically significant (p = 0.08) [20]. Without a follow-up study after the supplementation ceased, it cannot be ascertained whether L. salivarus persisted in the gut after treatment ended.
3.2 Acne
Acne vulgaris is one of the most common chronic dermatological conditions affecting both adolescents and adults [22]. Only one study was found regarding the role of probiotics in the treatment of acne. In a prospective, randomized, open-label study, 45 female patients aged 18–35 years with mild to moderate acne vulgaris were enrolled into three groups: probiotic supplementation, minocycline, and treatment with both probiotics and minocycline. The probiotic was a mixture of Lactobacillus acidophilus (NAS super-strain), Lactobacillus delbrueckii subspecies bulgaricus (LB-51 super-strain), and Bifidobacterium bifidum (Malyoth super-strain). The treatment period lasted a total of 12 weeks and lesion counts were assessed throughout this time. A total of 43 subjects completed the study.
By week 4, all groups had a significant improvement in total lesion count, with no significant differences between groups. Similarly, at the 8-week mark, all groups continued to have a significant improvement in total lesion count (p = 0.001, probiotic only; p < 0.001, remaining groups). The trend continued at week 12 for all three groups. By weeks 8 and 12, the group receiving both probiotics and minocycline had a significantly lower total lesion count compared with those taking probiotics only or minocycline only. All three groups had a significant improvement in non-inflamed lesion count at weeks 4, 8, and 12. At weeks 4, 8, and 12, subjects who were in the probiotic-only group had a greater decrease in non-inflamed lesion count compared with those in the minocycline-only group. Future studies should assess how probiotics affect sebaceous gland function and sebum composition. With regard to the inflamed lesion count, subjects who took either probiotics or minocycline achieved an improvement by week 8, and continued to improve significantly by week 12 compared with baseline. The group that received both probiotics and minocycline experienced a significant reduction as early as week 4, and continued to improve significantly more compared with the other groups.
Thirteen percent of patients in the minocycline-only group experienced vaginal candidiasis. No cases were reported in the group that received both probiotics and minocycline, which suggests that probiotics may help suppress the growth of unwanted microorganisms in the vaginal tract [22]. More studies are needed to evaluate the effects of probiotics on acne treatment, specifically whether it is efficacious as a stand-alone treatment or an adjunctive therapy.
3.3 Wound Healing
Acute wounds and non-healing chronic wounds are a common occurrence in dermatology. Peral et al. evaluated the role of probiotics in wound healing of burn injuries [23]. Eighty patients with second- or third-degree burns were randomized to receive topical wound treatment with either Lactobacillus plantarum (strain not reported) or standard silver sulphadiazine (SD-Ag). Treatment with L. plantarum was found to have similar effects as SD-Ag to promote complete healing of second-degree and early third-degree burn wounds. In late third-degree burn wounds (3–7 days post-burn), L. plantarum application lead to a statistically significant 17% increase in complete healing compared with wounds that had SD-Ag applied. Due to the low number of subjects in each group, researchers were not able to find whether the results were significant. Additional studies with a larger sample size are needed before establishing whether treatment with Lactobacillus plantarum is a beneficial alternative for wound treatment.
3.4 Psoriasis
One study compared the effect of the probiotic Bifidobacterium infantis 35624 on inflammatory markers in three disease states: psoriasis, chronic fatigue syndrome, and ulcerative colitis [24]. Twenty-six patients with psoriasis were recruited and assigned to receive either placebo or probiotic for 8 weeks. Baseline plasma C-reactive protein (CRP), tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels were elevated in psoriasis subjects comparison with healthy controls. CRP declined significantly after 8 weeks of therapy compared with placebo (p = 0.0425) and compared with baseline (p = 0.0161), and TNFα declined significantly after 8 weeks of therapy compared with baseline (p = 0.0269) and placebo (p = 0.0405). Researchers did not observe any changes in IL-6 levels in subjects with psoriasis treated with probiotics for 8 weeks compared with control subjects. When performing a combined analysis of all three inflammatory markers (IL-6, TNFα, and CRP), there was a decrease in the combined analysis in 75% of those receiving probiotics compared with 7% in the group receiving placebo. It is unclear how the combined analysis offers more insight over the individual analyses of IL-6, TNFα, and CRP. Interestingly, these three parameters remained unaffected in the healthy control group after treatment with B. infantis 35624. In this study, it is unclear if the biochemical improvements were accompanied by clinical improvements as no grading of disease severity was performed after baseline. These results suggest that B. infantis 35624 is able to reduce proinflammatory biomarkers in a systemic inflammatory disorder [24]; however, future studies should incorporate clinical grading and assessments.
4 Quality Assessment and Risk of Bias
4.1 Study Qualities from the 12 Studies Analyzed (Tables 1 and 2)
The majority of studies randomized and blinded subjects to treatment; however, not all studies were blinded, leading to a risk of bias. For certain disease states such as acne, psoriasis, and wound healing, only one study was identified (Table 2), therefore, no conclusions can be drawn.
Of the nine studies investigating probiotic use in AD (Table 1), seven used SCORAD as one of their primary outcome measures. Two of the three remaining studies did not use SCORAD, but instead used different severity scoring systems [9, 16]. The remaining study based the clinical endpoint on patient symptomatology only [10]. Of the seven studies that used SCORAD, only four studies found a statistically significant decline in either the overall SCORAD index or one parameter of assessment [12, 14, 18, 20]. Furthermore, of the three studies that did not conduct SCORAD analysis, two studies found a statistically significant difference in QoL measurements [9] or skin severity grading [16], while the last study assessed subjective symptoms only [10].
5 Discussion
On the basis of this systematic review, certain probiotic supplements and mixtures may be helpful in the treatment of AD in adults over the age of 18 years [9, 10, 12, 14, 16, 18, 20]. Many studies included small sample sizes, which affected their generalizability. Overall, there were a limited number of studies regarding the treatment of AD, and even fewer regarding acne, psoriasis, and wound care. The differences in study methodology, dose, type and duration of probiotic, and duration of follow-up may be responsible for the variations in outcomes.
Our systematic review demonstrated there is a limited amount of research into the use of probiotics in adults with dermatological diseases such as AD. Probiotics are thought to benefit the immune system by reducing the adherence of pathogenic bacteria, assisting with the maintenance of tight junctions to reduce gut permeability, helping with the development of gut-associated lymphoid tissue (GALT), stimulating intestinal production of IgA, and downregulating Th2 cytokines through the stimulation of IL-12 and IFNγ [25].
AD has been linked to the ‘hygiene hypothesis’. Early exposure to microbial agents can assist in the maturation of the Th1 cell response. In addition, this reduces the Th2 cell response which contributes to the development of allergic disease [14]. In pregnant women and newborns, probiotics are thought to prevent and treat AD by promoting the differentiation of naive T cells to mature Th1 cells [12]. In adults, the mechanisms are not clear, although there appear to be several possible modes of action. Beyond immune modulation, different gut bacteria populations differentially correlate with the presence of long-chain saturated fatty acids (LCFA) and short-chain fatty acids (SCFA) [26, 27]. LCFA and SCFA appear to interact with the immune system either by LCFA-based stimulation of TLRs [28] or by modulation of regulatory T-cell function [29]. While these hypotheses have been put forth with correlative findings, more mechanistic studies are needed to prospectively assess how probiotics and prebiotics may interface with the immune system and inflammation in ways that would be relevant to the skin.
Probiotics may be a useful adjunct to antibiotics in the treatment of acne [22]. Researchers postulated the improvement in the minocycline plus probiotic arm was due to modulation of the immune system by probiotics, and the combined anti-inflammatory effects of both agents. Larger-scale studies need to be conducted to determine if probiotics have a therapeutic role in the treatment of acne.
Further work needs to be conducted in the area of topical probiotics with regard to wound management. Specifically, more strains need to be evaluated for efficacy and long-term outcomes.
Of the 12 studies included, eight did not report on the incidence of side effects. Of the remaining four studies, one reported tolerable pain on topical application of a probiotic [23], one reported a case of diarrhea [9], and two reported no side effects related to the study agent [14, 16].
Our searches identified one study each for wound healing, acne, and psoriasis, and no studies were identified investigating other dermatological diseases associated with systemic inflammation, such as hidradenitis suppurativa. The studies using probiotics that we identified all utilized different strains (except two studies) and had differing methodology, making a statement regarding their overall efficacy in the treatment of dermatological diseases difficult. No studies regarding the use of prebiotics or synbiotics in adults were identified. Meta-analyses have shown evidence for a significant improvement with synbiotics for the treatment of pediatric AD [30]. A meta-analysis of four studies of probiotics in adults with AD [12, 14, 18, 19] found an improvement in the SCORAD index (−8.26, SD: −13.28, −3.25) [31]; however, this only involved a small number of studies and a small number of patients.
6 Conclusions
More research needs to be conducted into the effects of probiotics, prebiotics, and synbiotics in adults with dermatological diseases before any recommendations can be made. As research into this area grows, these findings will contribute to our knowledge regarding the interaction between the gut and the skin, and may provide a new therapeutic tool to benefit patients with chronic dermatological diseases.
References
Food and Agriculture Organization of the United Nations and the World Health Organization. Probiotics in food: health and nutritional properties and guidelines for evaluation. Food and Agriculture Organization of the United Nations and the World Health Organization; 2001.
Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61(1). doi:10.1002/mnfr.201600240.
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–75.
Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–35.
Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52(12):7577–87.
Foolad N, Armstrong AW. Prebiotics and probiotics: the prevention and reduction in severity of atopic dermatitis in children. Benef Microbes. 2014;5(2):151–60.
Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121(1):116.e11–121.e11.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Matsumoto M, Ebata T, Hirooka J, Hosoya R, Inoue N, Itami S, et al. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2014;13(2):209.e7–216.e7.
Matsumoto M, Aranami A, Ishige A, Watanabe K, Benno Y. LKM512 yogurt consumption improves the intestinal environment and induces the T-helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clin Exp Allergy. 2007;37(3):358–70.
Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, et al. Clinical effects of probiotics are associated with increased interferon-gamma responses in very young children with atopic dermatitis. Clin Exp Allergy. 2005;35(12):1557–64.
Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, Yamada N, et al. Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Med. 2010;53:37–45.
Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30(2):231-6, xiii.
Drago L, Iemoli E, Rodighiero V, Nicola L, Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int J Immunopathol Pharmacol. 2011;24(4):1037–48.
Kaur S, Kullisaar T, Mikelsaar M, Eisen M, Rehema A, Vihalemm T, et al. Successful management of mild atopic dermatitis in adults with probiotics and emollients. Cent Eur J Med. 2008;3(2):215–20.
Moroi M, Uchi S, Nakamura K, Sato S, Shimizu N, Fujii M, et al. Beneficial effect of a diet containing heat-killed Lactobacillus paracasei K71 on adult type atopic dermatitis. J Dermatol. 2011;38(2):131–9.
Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, et al. Guidelines for management of atopic dermatitis. J Dermatol. 2009;36(10):563–77.
Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012;46(Suppl):S33–40.
Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy. 2008;38(1):93–102.
Drago L, Vecchi E, Toscano M, Vassena C, Altomare G, Pigatto P. Treatment of atopic dermatitis eczema with a high concentration of Lactobacillus salivarius LS01 associated with an innovative gelling complex: a pilot study on adults. J Clin Gastroenterol. 2014;48(Suppl 1):S47–51.
Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111(3):587–91.
Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17(2):114–22.
Peral MC, Huaman Martinez MA, Valdez JC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J. 2009;6(1):73–81.
Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4(4):325–39.
McCusker M, Sidbury R. Nutrition and skin: kids are not just little people. Clin Dermatol. 2016;34(6):698–709.
Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40.
Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–9.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73.
Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, Garcia-Romero MT. Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr. 2016;170(3):236–42.
Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;2:217–26.
Acknowledgements
The authors thank Bruce Abbott for his assistance in conducting the systematic search algorithms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this review.
Conflict of interest
Manisha Notay, Negar Foolad, and Alexandra R. Vaughn have no conflicts of interests to declare. Raja K. Sivamani serves as a scientific advisor for Dermveda.
Rights and permissions
About this article
Cite this article
Notay, M., Foolad, N., Vaughn, A.R. et al. Probiotics, Prebiotics, and Synbiotics for the Treatment and Prevention of Adult Dermatological Diseases. Am J Clin Dermatol 18, 721–732 (2017). https://doi.org/10.1007/s40257-017-0300-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-017-0300-2