Abstract
Aim
Bempedoic acid has shown noteworthy progress in the prevention and management of atherosclerotic cardiovascular disease (ASCVD) in recent years. However, there has been a lack of high-quality evidence regarding the risk reduction of clinical events with bempedoic acid. Therefore, the aim of this article is to conduct a comprehensive evaluation of the impact of bempedoic acid on the incidence of cardiovascular events.
Methods
A systematic review and meta-analysis of randomized controlled trials pertaining to bempedoic acid was carried out. We conducted a systematic search across the Pubmed, Embase, and Cochrane Central Register of Controlled Trials databases to identify relevant studies published from inception to 23 April 2023. A total of four trials comparing the clinical benefit achieved with bempedoic acid versus placebo were included.
Results
Our analysis comprised four trials that encompassed a total of 17,323 patients. In comparison to the placebo, bempedoic acid showed a significant reduction in the risk of major adverse cardiovascular events (MACE) [relative risk (RR), 0.86, 95% confidence interval (CI) 0.87–0.94]. Additionally, bempedoic acid substantially lowered the occurrence of fatal or nonfatal myocardial infarction (RR 0.76, 95% CI 0.66–0.89), hospitalization for unstable angina (RR 0.70, 95% CI 0.55–0.89), and coronary revascularization (RR 0.82, 95% CI 0.73–0.92). There was also a similar reduction in MACE in patients on the maximally tolerated statin therapy.
Conclusion
Bempedoic acid may reduce the risk of cardiovascular events regardless of whether the patient is taking stains or not.
Registration: PROSPERO registration number CRD42023422932.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bempedoic acid has a good clinical efficacy with or without statins, especially in reducing the risk of major adverse cardiovascular events (MACE). |
Bempedoic acid can reduce serum low-density lipoprotein-cholesterol (LDL-C) and high-sensitivity C-reactive protein (hsCRP) with or without statins. |
Bempedoic acid may reduce myalgia in statin-intolerant patients. |
1 Introduction
Cardiovascular disease continues to be the leading cause of mortality globally [1, 2]. Atherosclerosis is the most common process leading to coronary heart disease, peripheral vascular disease, and stroke. Statins have been shown to be efficacious in reducing serum cholesterol levels and mitigating cardiovascular risk, and are considered the fundamental pharmacological component of modern prevention and management of atherosclerotic vascular disease (ASCVD). Accordingly, the 2018 Guideline on the Management of Blood Cholesterol suggests that all patients with diagnosed ASCVD and those at high risk for ASCVD should receive treatment with high-intensity statins [3].
However, the adverse effects of statins, particularly statin-associated muscular symptoms, limit their adequate use [4, 5]. On the one hand, statins adverse reactions result in patients being unable to use statins or limit the ability to receive guideline-recommended doses [6, 7]. On the other hand, statins adverse reactions significantly reduce patient adherence, with studies indicating long-term adherence to statins of < 25% after 5 years [8].
In the future, inflammation reduction and intensive lipid lowering should be considered a complementary approach for patients with ASCVD who are already taking statins. In contrast, new therapeutic approaches may be needed for patients who are unwilling or unable to use statins.
Bempedoic acid is a new therapeutic option for statin-intolerant patients and patients requiring additional low-density lipoprotein-cholesterol (LDL-C) lowering. When used alone or in combination with ezetimibe, bempedoic acid can achieve atherosclerosis treatment through a dual pathway of cholesterol and high-sensitivity C-reactive protein (CRP) lowering [9,10,11,12]. Many previous clinical trials on bempedoic acid have focused on its lipid-lowering effects, although data on major adverse cardiovascular events have been reported in safety evaluations. However, there have been no comprehensive and definitive conclusions regarding cardiovascular event risk reduction. The efficacy of bempedoic acid in reducing the incidence of cardiovascular events in statin-intolerant patients was demonstrated only upon the release of the CLEAR Outcomes trial findings [13].
Therefore, the objective of this meta-analysis was to evaluate the influence of bempedoic acid on cardiovascular outcomes and its safety.
2 Methods
The study was conducted and the results reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. This meta-analysis was registered at PROSPERO on 30 April 2023 (CRD42023422932).
2.1 Data Sources and Search Strategies
Our study involved a comprehensive search of three online databases: PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials. The search was conducted to retrieve relevant data from the inception of these databases up until 23 April 2023. The articles searched were mainly randomized controlled trials (RCTs) on lipid reduction, cardiovascular risk reduction, and the safety of bempedoic acid. Detailed information on the specific implementation of the search strategy is presented in the Supplementary Information (Table 1).
2.2 Eligibility Criteria
Trials meeting the following criteria were included: (1) population: patients with cardiovascular disease or at high risk for cardiovascular disease; (2) intervention: 180 mg bempedoic acid; (3) placebo control; (4) outcome: at least one and more cardiovascular events were reported for each trial’s primary trial endpoint or adverse event, including the five-component major adverse cardiovascular events (MACE), nonfatal or fatal myocardial infarction, hospitalization for unstable angina, or coronary revascularization; (5) randomized controlled trial (RCT) design of the study.
Exclusion criteria: (1) phase 1 and 2 clinical trials; (2) trials that did not report any clinical efficacy outcomes, i.e., did not report the five-component MACE, nonfatal or fatal myocardial infarction, hospitalization for unstable angina, or coronary revascularization; (3) duplicate publications; (4) editorials, reviews, meta-analyses, case reports, and so on; and (5) nonrandomized studies.
2.3 Study Selection and Data Extraction
Two authors conducted a systematic search and independently evaluated the eligibility of all electronic search results based on their titles and abstracts. After agreeing that the citations satisfied eligibility criteria, they screened the complete text of trials that were deemed potentially relevant. In cases of disagreement, consensus was reached through discussion, or if required, a third-party investigator was consulted to make a ruling.
The study characteristics that were extracted for each trial included the authors, year of publication, duration of follow-up, study design, sample size, baseline characteristics of the study population, and outcomes of interest. This extraction process was conducted independently by two reviewers.
2.4 Efficacy and Safety Results
The primary efficacy outcomes of interest for this meta-analysis were the five-component MACE (defined as cardiovascular death, nonfatal stroke, nonfatal myocardial infarction, coronary revascularization, and hospitalization for unstable angina), coronary revascularization, fatal or nonfatal myocardial infarction, and hospitalization for unstable angina; secondary outcomes included death from cardiovascular causes, death from any cause, fatal or nonfatal stroke. Secondly, the efficacy of bempedoic acid on lipid levels and inflammatory indicators was also analyzed, mainly the percentage changes in LDL-C and high-sensitivity CRP indicators. Finally, safety analyses were performed on pooled data from all four clinical trials, including primarily muscle-related adverse events, new or worsening diabetes, gout, and liver enzyme levels. The main muscle-related adverse events were myalgia, muscle weakness, muscle spasm, and pain in the extremity.
2.5 Subgroup Analysis
To analyze the impact of statin background therapy on trial outcomes, we performed a subgroup analysis. Specifically, the four RCT studies were divided into two subgroups (i.e., no or low-dose statin therapy subgroup and maximally tolerated statins subgroup) and analyzed for primary outcomes, secondary outcomes, and safety events.
2.6 Assessment of Risk of Bias
To reflect the overall risk of bias of the studies, we assessed the four eligible RCTs using the Cochrane Risk of Bias Assessment Tool [15]. We evaluated five domains for each study. The evaluation of each domain resulted in categorization as low risk, some concerns, or high risk of bias.
2.7 Statistical Analysis
The statistical analysis of all data was conducted using R (4.2.1). Summary data for dichotomous variables were relative risk (RR) and 95% confidence intervals (Cis). For continuous variables, we used mean and standard deviation (SD) to perform the analysis. Cochran’s Q test was used to analyze treatment effect heterogeneity across trials. Random or fixed-effects models were used to calculate summary statistics depending on the specific analysis. The sensitivity analysis in this paper was done by excluding each study at a time. The assessment of publication bias was not conducted due to the limited number of studies included, which was only four.
3 Results
3.1 Literature Search
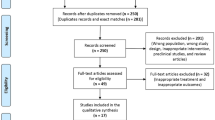
The retrieval of 323 records was accomplished through the three electronic databases. We excluded the 82 duplicate records. Subsequently, articles that failed to satisfy the established criteria for inclusion and exclusion were excluded. The process of selecting studies is shown in Fig. 1. Ultimately, four studies were considered eligible for this meta-analysis.
3.2 Study Characteristics
Table 1 presents the study characteristics of the included RCTs. Overall, 17,323 participants were enrolled in the four trials. Follow-up ranged from 24 weeks for CLEAR Serenity to 40.6 months for CLEAR Outcomes. All four trials were placebo controlled, and the dosage of bempedoic acid was 180 mg/day. The populations included were all patients at high risk for hypercholesterolemia or ASCVD. The CLEAR Outcomes trial had the longest duration and the largest number of participants of the four trials.
Table 2 lists the baseline demographics and clinical characteristics. Patients in the CLEAR Wisdom and CLEAR Harmony trials had higher statin use and were more likely to be male than those in the CLEAR Outcomes and CLEAR Serenity trials. At baseline, patients in the statin-intolerance trial had significantly higher mean LDL-C levels than in the other two trials. In addition, CLEAR Outcomes had a greater proportion of diabetic patients (1.5 times more than the other three trials).
The risk of bias for eligible randomized controlled trials are shown in the Supplementary Information (Fig. 1). These four RCTs were at low risk of bias.
3.3 Cardiovascular Events
We performed subgroup analyses of these four randomized controlled trials according to statin use. The CLEAR Outcomes study (22.9% and 22.5% statin use in the bempedoic acid (BA) group versus placebo group, respectively) and the CLEAR Serenity study (2.92% and 2.78% statin use in the BA group versus placebo group, respectively) were studies of patients who used no or low-dose use of statins, where patients were statin intolerant. The CLEAR Harmony study (99.8% and 100% statin use in BA and placebo groups, respectively) and the CLEAR Wisdom study (90% and 88.8% statin use in BA and placebo groups, respectively) were categorized as the maximal dose of statins.
The results of the study indicated that bempedoic acid reduced the incidence of MACE events in those on low-dose statin use (RR 0.87, 95% CI 0.80–0.95) (Fig. 2). For patients on the maximally tolerated statin, the addition of bempedoic acid also reduced the risk of MACE (Fig. 2). The results did not suggest heterogeneity in the trial.
Analysis of cardiovascular events showed that bempedoic acid significantly reduced fatal or nonfatal myocardial infarction (RR 0.76, 95% CI 0.66–0.89; p < 0.01), coronary revascularization (RR 0.82. 95% CI 0.73–0.92; p < 0.01), and hospitalization for unstable angina (RR 0.70, 95% CI 0.55–0.89; p < 0.01) (Fig. 3).
The results of the subgroup analyses indicated that the clinical cardiovascular benefits of using bempedoic acid were mainly seen in patients using no or low-dose statins at baseline. This means that bempedoic acid monotherapy reduced the risk of fatal or nonfatal myocardial infarction (RR 0.78, 95% CI 0.67–0.92), coronary revascularization (RR 0.83, 95% CI 0.73–0.94), and hospitalization for unstable angina (RR 0.69, 95% CI 0.53–0.89) (Fig. 3). However, for those on the highest dose of statins at baseline, bempedoic acid did not reduce the risk of fatal or nonfatal myocardial infarction (RR 0.56, 95% CI 0.32–1.00), coronary revascularization (RR 0.0.74, 95% CI 0.50–1.10), or hospitalization for unstable angina (RR 0.79, 95% CI 0.42–1.51) (Fig. 3).
In the present meta-analysis, bempedoic acid had no significant therapeutic effect on death from cardiovascular causes, death from any cause, and fatal or nonfatal stroke (Fig. 4).
Neither bempedoic acid alone nor in combination with statins reduced the incidence of death from cardiovascular causes, death from any cause, or fatal or nonfatal stroke (Supplementary Information Fig. 2).
3.4 Percent Change in LDL-C and High-sensitivity CRP
Overall, bempedoic acid significantly decreased LDL-C (Mean Difference (MD) − 19.41%; 95% CI − 20.46 to − 18.35%) and high-sensitivity CRP (MD − 22.55%; 95% CI − 29.39 to − 15.71%). Subgroup analysis showed that the use of bempedoic acid significantly lowered LDL-C (MD − 19.55%; 95% CI − 20.92 to − 18.18%) and high-sensitivity CRP (MD − 24.81%; 95% CI − 27.25 to − 22.36%) levels in the statin-intolerant population (Fig. 5). In patients with hypercholesterolemia or ASCVD on maximally tolerated statin, bempedoic acid resulted in a significant reduction of LDL-C (MD − 17.83%; 95% CI − 22.73 to − 12.92%) and high-sensitivity CRP (MD − 18.19%; 95% CI − 32.47 to − 3.92%) levels (Fig. 5).
3.5 Safety Outcomes
This study showed that bempedoic acid reduced the risk of myalgia in patients with no or low-dose statins (RR 0.83, 95% CI 0.73–0.94) (Fig. 6A). Furthermore, bempedoic acid reduced the risk of new-onset or worsening of diabetes mellitus in patients on the maximum tolerated dose of statins (RR 0.72, 95% CI 0.52–0.99) (Fig. 6B).
Bempedoic acid increased the risk of a number of adverse events. For patients receiving maximal doses of statin, bempedoic acid increased the incidence of pain in the extremity (RR 1.79, 95% CI 1.05–3.04) (Fig. 7A). Bempedoic acid increased the risk of muscle spasms (RR 1.18, 95% CI 1.01–1.39) (Fig. 7B). Regardless of statin use, bempedoic acid was associated with a risk of gout (RR 1.57, 95% CI 1.28–1.93) (Fig. 7C). In patients receiving no or low-dose statins, bempedoic acid decreased elevated hepatic enzyme levels (RR 1.85, 95% CI 1.28–2.68) (Fig. 7D).
Bempedoic acid alone or in combination with statins did not reduce the incidence of muscular weakness, neurocognitive disorders, or creatine kinase level adverse events (Supplementary Information Fig. 3).
3.6 Sensitivity Analysis
We performed sensitivity analyses of the main clinical efficacy and safety outcomes using the “leave-one-out” approach to measure the robustness and reliability of the results. The results of all sensitivity analyses are shown in Figs. 4–6 of the Supplementary Information.
4 Discussion
Four randomized controlled trials of previous studies of bempedoic acid that reported cardiovascular outcomes, including the most recent CLEAR Outcomes study, are included. The primary aim of this meta-analysis was to evaluate the efficacy of bempedoic acid for cardiovascular outcomes in patients with cardiovascular disease or at high risk for cardiovascular disease. Secondly, bempedoic acid safety was also analyzed.
Based on the results of this meta-analysis, bempedoic acid significantly reduced the incidence of MACE, hospitalization for unstable angina, coronary revascularization, and fatal or nonfatal myocardial infarction events compared with placebo. More importantly, bempedoic acid also reduced the incidence of MACE events in patients on the highest tolerated dose of statin. In addition, the combination of bempedoic acid and statin has potential benefits in reducing the incidence of hospitalization for unstable angina, coronary revascularization, and fatal or nonfatal myocardial infarction. However, bempedoic acid did not significantly improve the risk of mortality and fatal or nonfatal stroke. Furthermore, bempedoic acid has been associated with a number of emerging adverse effects along with improved clinical benefits. Compared with placebo, bempedoic acid increased the risk of gout and elevated hepatic enzyme levels. Simultaneously, bempedoic acid enhanced the incidence of pain in the extremities and muscle spasms in patients on high-dose statins.
In contrast to statins, bempedoic acid is a prodrug that undergoes activation in the presence of very-long-chain acyl-CoA synthetase 1 (ASCVL1), and the activated active substance acts upstream of HMG-CoA reductase [16,17,18]. Since the liver is rich in ASCVL1, the elevated hepatic-enzyme level is significantly increased. However, muscle tissue does not express ASCVL1, so bempedoic acid may be superior to statins in avoiding myalgia. Our results also confirm that bempedoic acid reduces the risk of myalgia. Hyperuricemia is the most important risk factor in the development of gout [19, 20].
Bempedoic acid inhibits organic anion transporter protein 2, which may be responsible for its ability to increase uric acid levels [21]. After stopping the drug, the uric acid level will gradually return to the pre-drug level [22]. Because the onset of gout is mainly found in the joints of the lower extremities, it manifests as redness, swelling, and heat pain in the corresponding joints [23]. Therefore, gout and pain in the extremity caused by bempedoic acid can theoretically be avoided by controlling hyperuricemia. Compared with the myalgia associated with statin therapy, gout and pain in the extremities associated with bempedoic acid therapy can be effectively avoided. Previous studies have demonstrated that bempedoic acid inhibits ATP-citrate lyase (ACL) and activates the AMPK signaling pathway in the liver, which may explain the potential benefit of bempedoic acid in reducing new-onset or worsening of diabetes mellitus [24,25,26].
Recently, it was noted that high-sensitivity CRP was significantly more predictive of future cardiovascular events in patients treated with statins than serum LDL-C levels [27]. Therefore, to further reduce the risk of cardiovascular events, drugs that inhibit inflammation may need to be considered in the selection of adjunctive agents other than statins. A variety of anti-inflammatory drugs, including colchicine, tocilizumab, and ziltivekimab, have been or are being actively studied in current atherosclerosis trials. Among them, the CANTOS, COLCOT, and LoDoCo2 trials have shown that targeted anti-inflammatory therapy with either canakinumab or colchicine significantly reduces the incidence of cardiovascular events in patients treated with statins in the absence of any LDL-C reduction [28,29,30]. Our findings indicated that bempedoic acid was able to provide better cardiovascular clinical benefits to patients. This may be due to the fact that the addition of bempedoic acid to statin use significantly reduced high-sensitivity CRP, in addition to further reducing LDL-C [16, 31, 32]. In addition to statins, ezetimibe, and PCSK9, bempedoic acid can achieve good results in lowering both LDL-C and high-sensitivity CRP levels [13, 31, 33,34,35]. However, based on previous studies, we cannot conclude that bempedoic acid is superior to other lipid-lowering agents. Therefore, direct comparative trials are needed in the future to determine whether bempedoic acid is superior to other lipid-lowering agents.
In conclusion, bempedoic acid is a good choice for statin-intolerant patients in reducing the risk of MACE, fatal or nonfatal myocardial infarction, coronary revascularization, and hospitalization for unstable angina. In addition, bempedoic acid significantly reduces myalgia and has potential benefit in new-onset or worsening of diabetes mellitus.
This study is a relatively comprehensive pooled analysis of the clinical benefits of bempedoic acid in combination with statins or as monotherapy on cardiovascular outcome events. Our study has several innovative points. We summarized the side effects added with bempedoic acid treatment. Second, we grouped the trials included in the study according to statin use, which somewhat reduced the interference from statin treatment. Also, the different clinical benefits of maximum tolerated dose statin users when treated with statin alone and in combination with bempedoic acid were understood. This enriches our findings and, to some extent, the credibility of the article. Finally, our analyses of the cardiovascular benefits of bempedoic acid and its effect on high-sensitivity CRP highlight the role of bempedoic acid in reducing cardiovascular risk.
However, there are some shortcomings in this study. First, there were varying degrees of variability in the baseline characteristics of patients in the included randomized controlled trials. Second, the number of patients included in the CLEAR Outcomes trial was too large compared with other trials, which led to it always being able to take up a large weight in the study analysis. Our safety analysis did not include the incidence of cholelithiasis. Finally, there were varying degrees of variation in follow-up across trials, and a small proportion of eligible trials were not included in our study because of imperfect clinical events.
5 Conclusions
Bempedoic acid has a significant clinical benefit in reducing cardiovascular events. However, the adverse effects of bempedoic acid on muscle spasms, liver function, and the incidence of gout also require vigilance. It is hoped that more high-quality studies will be conducted in the future to further refine the clinical benefit of bempedoic acid for statin-tolerant patients regarding cardiovascular events.
References
Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol. 2019 Jun 25;73(24):3234-3237]. J Am Coll Cardiol. 2019;73(24):3168–209.
Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472–83.
Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103.
Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB, Mittleman MA. Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med. 2012;125(2):176–82.
Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–34.
Toth PP, Granowitz C, Hull M, Anderson A, Philip S. Long-term statin persistence is poor among high-risk patients with dyslipidemia: a real-world administrative claims analysis. Lipids Health Dis. 2019;18(1):175.
Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL Cholesterol. N Engl J Med. 2019;380(11):1022–32.
Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial [published correction appears in JAMA. 2020 Jan 21;323(3):282]. JAMA. 2019;322(18):1780–8.
Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7): e011662.
Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203.
Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898.
Keaney JF Jr. Bempedoic acid and the prevention of cardiovascular disease. N Engl J Med. 2023;388(15):1427–30.
Ruscica M, Sirtori CR, Carugo S, Banach M, Corsini A. Bempedoic acid: for whom and when. Curr Atheroscler Rep. 2022;24(10):791–801.
Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7:13457.
Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Krämer BK, et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–8.
Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, et al. A genetic marker of uric acid level, carotid atherosclerosis, and arterial stiffness: a family-based study. Am J Kidney Dis. 2015;65(2):294–302.
Biolo G, Vinci P, Mangogna A, Landolfo M, Schincariol P, Fiotti N, et al. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front Cardiovasc Med. 2022;9:1028355.
Bays HE, Banach M, Catapano AL, Duell PB, Gotto AM Jr, Laufs U, et al. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol. 2020;14(5):649-659.e6.
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–52.
Filippov S, Pinkosky SL, Newton RS. LDL-cholesterol reduction in patients with hypercholesterolemia by modulation of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase. Curr Opin Lipidol. 2014;25(4):309–15.
Pinkosky SL, Filippov S, Srivastava RA, Hanselman JC, Bradshaw CD, Hurley TR, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134–51.
Ruscica M, Banach M, Sahebkar A, Corsini A, Sirtori CR. ETC-1002 (Bempedoic acid) for the management of hyperlipidemia: from preclinical studies to phase 3 trials. Expert Opin Pharmacother. 2019;20(7):791–803.
Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–301.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505.
Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–47.
Di Minno A, Lupoli R, Calcaterra I, Poggio P, Forte F, Spadarella G, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia: systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2020;9(15):e016262.
Alexander JH. Benefits of bempedoic acid - clearer now. N Engl J Med. 2023;388(15):1425–6.
Ruscica M, Tokgözoğlu L, Corsini A, Sirtori CR. PCSK9 inhibition and inflammation: a narrative review. Atherosclerosis. 2019;288:146–55.
Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–82.
Ridker PM, Lei L, Ray KK, Ballantyne CM, Bradwin G, Rifai N. Effects of bempedoic acid on CRP, IL-6, fibrinogen and lipoprotein(a) in patients with residual inflammatory risk: a secondary analysis of the CLEAR harmony trial. J Clin Lipidol. 2023;17(2):297–302.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by the Natural Science Foundation of China (grant no. 81700321), Natural Science Foundation of Shandong Province (grant no. ZR202211060094), and Natural Science Foundation of Shandong Province (grant no. ZR2020MH025).
Conflict of Interest
Ju Zhang, Xiangfeng Guan, Baixue Zhang, Jia Wang, Xiaodong Jin, Yunhe Zhao, and Bo Li declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Authors’ Contributions
JZ, XG, and BZ have participated in the literature search, data curation, data analysis, and drafting of the original manuscript. JW and XJ participated in content guidance and critical revision of the paper for important intellectual content. YZ and BL supervised the work and revised the article critically. The manuscript has not been previously published in its entirety or in part in any language, and all authors have read and approved its submission.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Guan, X., Zhang, B. et al. Bempedoic Acid can Reduce Cardiovascular Events in Combination with Statins or As Monotherapy: A Systematic Review and Meta-analysis. Am J Cardiovasc Drugs 23, 695–708 (2023). https://doi.org/10.1007/s40256-023-00606-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00606-4