Abstract
Background
Crushed formulations of specific antiplatelet agents produce earlier and stronger platelet inhibition. We studied the platelet inhibitory effect of crushed clopidogrel in patients with acute coronary syndrome (ACS) and its relative efficacy compared with integral clopidogrel, crushed and integral ticagrelor.
Objectives
We aimed to compare the platelet inhibitory effect of crushed and integral formulations of clopidogrel and ticagrelor in patients with acute coronary syndrome (ACS).
Methods
Overall, 142 patients with suspected ACS were randomly assigned to receive crushed or integral formulations of clopidogrel or ticagrelor. Platelet inhibition at baseline and 1 and 8 h was assessed using the VerifyNow assay. High on-treatment platelet reactivity (HTPR) ≥ 235 P2Y12 reaction units (PRUs) 1 h after the medication loading dose was also determined.
Results
The PRU and percentage inhibition median (interquartile range) at 1 h for the different formulations were as follows: crushed clopidogrel: 196.50 (155.50, 246.50), 9.36 (− 1.79, 25.10); integral clopidogrel: 189.50 (159.00, 214.00), 2.32 (− 2.67, 19.89); crushed ticagrelor: 59.00 (10.00, 96.00), 75.53 (49.12, 95.18); and integral ticagrelor: 126.50 (50.00, 168.00), 40.56 (25.59, 78.69). There was no significant difference in PRU or percentage platelet inhibition between the crushed and integral formulations of clopidogrel (p = 0.990, p = 0.479); both formulations of ticagrelor were superior to the clopidogrel formulations (p < 0.05). On paired comparison, crushed ticagrelor showed robust early inhibition of platelets compared with the integral formulation (p = 0.03). Crushed clopidogrel exhibited the maximal HTPR of 34.3%, but was < 3% for both formulations of ticagrelor.
Conclusions
The platelet inhibitory effect of crushed clopidogrel is not superior to integral preparation in patients with ACS. Crushed ticagrelor produced maximal platelet inhibition acutely. HTPR rates in ACS are similar and very low with both formulations of ticagrelor, and maximal with crushed clopidogrel.
Clinical Trials Registry of India identifier number CTRI/2020/06/025647.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
The crushed clopidogrel formulation does not provide stronger or early platelet inhibition in patients with heart attacks. |
Resistance to clopidogrel is not very high in Indian patients compared with other ethnicities. |
Crushed ticagrelor produces robust early platelet inhibition compared with integral ticagrelor and clopidogrel formulations in patients with heart attacks, and is more effective in women. |
1 Introduction
Optimal platelet inhibition is paramount in percutaneous coronary intervention (PCI), and even more so when these procedures are necessary in the setting of acute coronary syndrome (ACS). Early and predictable platelet inhibition is mandatory to prevent stent thrombosis and ischemic events, but this should be weighed against the bleeding risk that this agent portends. Platelet reactivity is increased in ACS, and high on-treatment platelet reactivity (HTPR) to certain antiplatelet agents is also commonly encountered in this setting. HTPR has been attributed to the (1) extent of myocardial damage; (2) delayed response due to impaired intestinal absorption of medications in myocardial infarction; (3) thrombotic milieu; and (4) drug interactions [1].
Clopidogrel is still the most widely used P2Y12 inhibitor in developing countries. It is a prodrug and the platelet inhibitory effect in ACS is moderate and variable. Although variable, HTPR to integral clopidogrel occurs in up to 40% of patients depending on the platelet assay [2, 3]. Hence, many societal recommendations based on current evidence suggest ticagrelor or prasugrel for acute and improved platelet inhibition in the ACS setting [4, 5]. In a small pharmacokinetic study, crushed clopidogrel administered through a nasogastric tube in healthy volunteers showed earlier and greater bioavailability acutely compared with the integral tablets [6]. Crushed formulations of ticagrelor and prasugrel have shown earlier and more potent platelet inhibition than the integral formulations [7,8,9]. If the crushed formulation of clopidogrel offers superior platelet inhibition compared with the integral tablet, this could be utilized and adopted widely into practice without incremental cost in countries where treatment cost is borne by the patients (out-of-pocket expenditure) or where ticagrelor or prasugrel are not freely available. In this study, we assessed the platelet inhibitory effect of the crushed and integral formulations of clopidogrel and ticagrelor 1 h after the loading dose, and compared the HTPR with these drug formulations and adverse events in Indian patients. To our knowledge, this is the first study that has compared the crushed formulation of clopidogrel with integral and crushed formulations of ticagrelor.
2 Methods
2.1 Study Setting and Participants
This randomized, double-blind, active comparator, pharmacodynamic study was carried out in a 2800-bed tertiary care teaching hospital in South India from April 2020 through May 2021. The study was approved by the Institutional Review Board and Ethics Committee and was registered with the Clinical Trial Registry of India (CTRI/2020/06/025647).
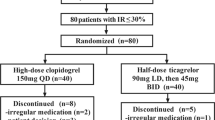
The study was initially designed for patients with ST-elevation myocardial infarction (STEMI) undergoing primary PCI; however, because of the coronavirus disease 2019 (COVID-19) pandemic and operational issues, the institutional practice was changed to a pharmaco-invasive approach for most cases presenting with STEMI. Subsequently, we enrolled all patients with suspected ACS who satisfied the inclusion criteria. Two formulations of each drug, i.e. crushed and integral, were used, resulting in four groups: crushed clopidogrel (CC), integral clopidogrel (IC), crushed ticagrelor (CT), and integral ticagrelor (IT).
A total of 142 patients were randomized and six patients were excluded—three refused sample collection, one had drug intolerance due to recurrent vomiting, and two withdrew from the study after consenting (Fig. 1).
Consecutive patients over 18 years of age with a presumptive diagnosis of ACS, willing to give informed consent and comply with the study requirements, were eligible for recruitment. Patients already taking clopidogrel, ticagrelor, prasugrel, or glycoprotein inhibitors before randomization, or with swallowing difficulty, ongoing bleeding diathesis, platelet count < 80,000 per cu.mm, or allergy to aspirin, clopidogrel or ticagrelor were excluded.
2.2 Randomization and Treatment Allocation
Using the ‘RALLOC’ ado function in STATA IC/16.0 software, four permuted block randomization groups were computer-generated. Patients were blinded to the study drug but not the formulations, and the technician who performed the platelet assay was blinded to the treatment allocation of the drug and the formulation. Patients received a loading dose of clopidogrel (600 mg) or ticagrelor (180 mg) in integral or crushed form, followed by a maintenance dose of 75 mg daily or 90 mg twice daily for the initial 24 h based on the randomization protocol. A loading dose of aspirin 300 mg was also administered. Antiplatelet therapy after the completion of 24 h was left to the treating physician’s discretion. At 1 month of follow-up, 71% of patients were taking clopidogrel, 6% were taking ticagrelor, and 11% were taking prasugrel.
Patients with ACS received low-molecular-weight heparin and guideline-directed anti-ischemic medications. Most of the patients with STEMI were thrombolysed and taken up for rescue or pharmaco-invasive PCI, depending on the clinical indication with appropriate COVID precautions. Patients with ACS other than STEMI underwent early invasive PCI or conservative medical management. All patients undergoing PCI received 70 units/kg of unfractionated heparin. US FDA-approved drug-eluting stents were used in all cases. Intraprocedural or post-procedural use of GPIIbIIIa inhibitors was not prohibited, and the interventionalist decided its use.
2.2.1 Preparation of the Crushed Tablet Suspension
Two ticagrelor (90 mg) or eight clopidogrel tablets (75 mg) were crushed in a mortar for 30 s using a pestle to form a powder; 20 mL of commercially available water for injection was added and stirred gently for 60 s to create a suspension. The content was transferred to a dosing cup and another 15 mL of sterile water was added and mixed using borosilicate stirring rods. A wooden spatula removed the residual powder attached to the pestle. An additional 15 mL of sterile water was added to the mortar to rinse any remaining drug. This was transferred to the dosing cup and the total contents (50 mL) were stirred for another 60 s to ensure that all remaining particles were dispersed uniformly to form an homogenous suspension.
2.3 Blood Sampling for Platelet Function Testing
Samples for platelet function testing were taken at baseline, 0 h (before drug administration), and then at 1 and 8 h following administration of the loading dose. Blood was collected from the antecubital vein into 2 ml vacutainer tubes that contained 3.2% sodium citrate (Greiner Bio-One Vacuette North America, Inc, Monroe, NC, USA) with an anticoagulant to blood ratio of 1:9. The platelet-function test was performed using the VerifyNow cartridge system (Accumetrics Inc., San Diego, CA, USA). The VerifyNow system is a turbidometric-based optical detection system that measures platelet-induced aggregation of fibrinogen-coated microparticles. Results are reported as P2Y12 reaction units (PRU) based on the rate and extent of aggregation, which reflects the amount of P2Y12 receptor-mediated aggregation specific to platelets.
2.3.1 Cut-Off Levels for High On-Treatment Platelet Reactivity and Calculation of Percentage Platelet Inhibition
A cut-off of ≥ 235 was used to define HTPR in our study [10,11,12]. The percentage of platelet inhibition at 1 and 8 h was calculated as ([PRU at 0 h—post treatment PRU]/PRU at 0 h) × 100.
2.4 Study Endpoints
The primary endpoint was platelet inhibition at 1 h following administration of crushed or integral formulations of clopidogrel and ticagrelor, while the secondary endpoints were as follows:
-
1.
In-hospital composite of death (all-cause), myocardial infarction, stroke or transient ischemic attack (TIA), urgent coronary revascularization, or stent thrombosis.
-
2.
Composite of death, myocardial infarction, stroke or TIA, urgent revascularization, or stent thrombosis at 30 days.
-
3.
Prevalence of HTPR with the different formulations of clopidogrel and ticagrelor using the VerifyNow assay within 24 h of the loading dose.
-
4.
Association between HTPR and thrombolysis in myocardial infarction (TIMI) flow in the angiogram.
-
5.
Association between HTPR and baseline risk factors.
The safety endpoint was major and minor bleeding as defined by the Plato criteria [13].
2.5 Sample Size Calculation
A minimum of 30 samples in each group would give us an adequate power of 90% to detect a minimum difference of 10 PRUs in delta change between the groups, considering an alpha of 0.01667 (0.05/3 multiple comparisons). We assumed a standard deviation (SD) of 10 and 15% for the delta change at 1 h and 8 h, respectively, for the calculation. This sample size would also be adequate to detect a minimum of 100 PRUs difference (at 1 h) and 90 PRUs difference (at 8 h) between the groups, with 80% power.
2.6 Statistical Analysis
Data were summarized using mean (SD) or median (interquartile range [IQR]) depending on the normality. The clinical and demographical variables among the four groups were compared using analysis of variance (ANOVA) for continuous variables and Chi-square test for categorical variables. A p-value < 0.05 was considered significant for pairwise comparisons; for multiple comparisons, Bonferroni correction was applied to the p-values. A per-protocol analysis was conducted to compare different drug formulations for efficacy in platelet inhibition and adverse events. The PRU at 1 and 8 h had a skewed distribution; thus, PRUs at all time points were log-transformed and a repeated-measures ANOVA was performed to provide the effect of drug over time. The results were visualized with a median (95% confidence interval [CI]) as a connected chart. The association of HTPR with baseline characteristics at both time points (1 and 8 h) was analyzed using the Chi-square test. The Fisher’s exact test was used to compare in-hospital and 30-day major cardiovascular event (MACE) outcomes, and logistic regression was performed to assess the effect of opioids on HTPR. A p-value < 0.05 was considered significant for all comparisons. All the analyses were performed using STATA IC/16.0.
3 Results
Baseline samples were available for all 136 patients. One-hour PRU values were unavailable for four patients (two for ticagrelor crushed, one for clopidogrel crushed, and one for ticagrelor integral), and 8-h PRU samples were unavailable for six patients (two for ticagrelor crushed, three for clopidogrel integral, and one for ticagrelor whole) due to technical or processing errors.
The time required for preparing the crushed formulation of the medications was 4.68 ± 1.90 min. Overall, there was an additional 3.47-min delay in administering the crushed (13.91 ±3.90) formulation compared with the integral tablets (9.73 ± 3.25) [p < 0.001].
3.1 Baseline Demographics, Risk Factors, Clinical Presentation, Investigations, and Concomitant Medications
All the demographic and baseline characteristics are listed in Table 1; males constituted 72% of the study population. Dyslipidemia was diagnosed less frequently in the crushed clopidogrel group, and tirofiban was used most often in the integral clopidogrel arm. All other parameters were similar in the four groups.
Sixty-five (48%) patients had STEMI, and the remaining were diagnosed with non-STEMI (NSTEMI) or unstable angina. Forty-five patients (69%) with STEMI were thrombolysed, and streptokinase was the lytic agent used in 80% of these cases. Seventy patients (51%) underwent PCI and the remaining patients were either referred for coronary artery bypass surgery or managed conservatively.
3.2 Platelet Inhibition with Different Drugs and Formulations
There was no significant difference in platelet inhibition between the crushed and integral formulations of clopidogrel (p > 0.990 for PRU at 1 and 8 h) and for the percentage change in platelet inhibition (p = 0.479 at 1 h and p > 0.990 at 8 h) (Table 2 and Figs. 2 and 3).
PRUs at different time points in the four treatment groups. Median (95% CI) PRU values are presented. Platelet reactivity is markedly reduced in the ticagrelor groups, with maximum inhibition in the crushed group at 1 h. Both formulations of ticagrelor produced more potent and earlier platelet inhibition than clopidogrel. There was no difference in the PRU between the two clopidogrel formulations at either time point. PRU P2Y12 reactivity units, CI confidence interval
Percentage inhibition of platelets over time according to treatment allocation. Early platelet inhibition (percentage inhibition from baseline) was much higher in the ticagrelor groups compared with clopidogrel at both time points, and there was no significant difference between the two ticagrelor formulations at either time point. The box-whisker plot represents the percentage of platelet inhibition from baseline; the middle line in the box represents the median; the top and bottom lines of the box represent interquartile ranges; the whiskers represent the 5th and 95th percentiles; and the solid black circles represent outliers
The integral and crushed formulations of ticagrelor showed significantly more potent platelet inhibition (lower PRU and higher percentage of inhibition) compared with the integral and crushed preparations of clopidogrel at both time points (p < 0.001 for all comparisons).
On paired comparison of different formulations of ticagrelor, crushed ticagrelor showed robust early platelet inhibition (median difference of 152 PRU units and 76% platelet inhibition from baseline) than integral formulations (median difference of 75 PRUs and 41% platelet inhibition from baseline (p < 0.033 for PRUs and p < 0.027 for percentage inhibition). This difference leveled off at 8 h post-drug administration (Table 2, Figs. 2 and 3).
All four groups had similar in-hospital and 30-day composite outcomes (Table 3). There were numerically more adverse events in the integral ticagrelor group, and bleeding events occurred more frequently in the integral ticagrelor and crushed clopidogrel groups (Table 4)
3.3 High On-Treatment Platelet Reactivity
The crushed clopidogrel treatment group demonstrated the maximum HTPR at 1 and 8 h. On paired comparison of crushed and integral clopidogrel, the difference in HTPR was statistically significant at 1 h (Chi-square statistic 12, p < 0.001).
HTPR values were the lowest and identical for both formulations of ticagrelor at 1 and 8 h. There were no patients with HTPR in either of the ticagrelor groups at 8 h (Table 5). HTPR rates were similar to the entire cohort even when patients who received tirofiban were excluded from the analysis (Online Resource Table 1).
Women exhibited higher HTPR 1 h post loading dose of medications. Integral ticagrelor was not superior to crushed or integral clopidogrel tablets in platelet inhibition at 1 h in women compared with men (Online Resource Table 2). None of the coronary risk factors, the severity of disease at presentation (Killip’s Class), or TIMI flow in the angiogram was associated with HTPR (Table 6 and Online Resource Table 3). Opioid use was not associated with increased HTPR (Online Resource Table 4).
4 Discussion
4.1 Platelet Inhibition with Different Drug Formulations
Contrary to expectation, crushed clopidogrel did not produce earlier or more pronounced platelet inhibition than the integral formulation. There was no difference in platelet inhibition between the two formulations at 1 or 8 h, and thus there is no additional benefit in crushing clopidogrel tablets for earlier platelet inhibition. Only one small study of nine healthy subjects has been described in the literature, where crushed clopidogrel tablets were shown to have better bioavailability, but here, the drug was administered through a nasogastric tube and the platelet reactivity was not measured [6]. The lower efficacy of crushed clopidogrel tablets is probably due to their dissolution characteristics. Clopidogrel dissolves faster in an acidic medium than in a non-acidic medium [14]. The ph of 6.7 in saliva compared with 2.0 in the stomach could explain the poor absorption and blunted platelet inhibitory response compared with the integral formulation. This is the largest pharmacodynamic study that compared crushed and integral clopidogrel in the setting of ACS.
Ticagrelor therapy resulted in earlier and more pronounced platelet inhibition than clopidogrel. This has been previously documented in the Onset/Offset study, which compared ticagrelor and clopidogrel in patients with stable coronary artery disease where platelet inhibition was determined by light transmittance aggregometry (LTA) [15]. The crushed formulation of ticagrelor produced even more potent and early platelet inhibition, similar to another small study that randomized STEMI patients to receive crushed or integral ticagrelor [16]. A systematic review and meta-analysis of five studies comparing crushed and integral ticagrelor (180 mg) demonstrated a pooled mean difference of − 59.2 PRUs in favor of the crushed preparation at 1 h post loading dose [8]. The median difference observed in our study was − 67.5 PRUs. As expected, the superior platelet inhibitory effect of the crushed ticagrelor formulation, as compared with the integral formulation, disappeared in 8 h.
4.2 Difference in Platelet Inhibition in the Indian Population and Other Ethnicities
The mean PRU 8 h following a loading dose of integral ticagrelor (180 mg) was 71 ± 55 and 251 ± 71 for clopidogrel (600 mg), in an East Asian study [17]. The corresponding median (IQR) value in our study for integral ticagrelor was 39.5 (11, 71), and 153 (96, 195) for integral clopidogrel. In predominantly White subjects, a study was undertaken to see the effect of high platelet reactivity on subsequent adverse outcomes in patients undergoing PCI; the PRU, 12 h after a loading dose of clopidogrel 600 mg, was 183 ± 94 [10]. In STEMI patients of European ethnicity whose baseline PRU was similar to our study, the PRU at 1 h after a 180 mg loading dose of integral ticagrelor was 253 (208, 298) Least square estimation (LSE) (95% CI), and 161 (106, 161) LSE (95% CI) with crushed formulations. This was much higher than observed in our study [median (IQR) 126.5 (50, 168) for the integral formulation, and 59 (10, 96) for the crushed formulation]. In the same study, the percentage inhibition of platelets at 1 h was 0.0 (0.0, 20.0) for integral ticagrelor and 48 (18.8, 68.5) for the crushed formulation. In our study, the corresponding values were median (IQR) 40.56 (25.59, 78.69) for integral ticagrelor and 75.53 (49.12, 95.18) for the crushed formulation [16]. Thus, the Indian subjects generally hyper-respond to P2Y12 inhibitors compared with East Asian, Western, or European ethnicities. This could be likely due to genetic factors such as gene polymorphisms in CYP2C19*2 loss-of-function allele, which was present in 33% of the participants in an Indian study but translated to clopidogrel resistance in only 1.4% of the patients [18]. In another Indian study that looked at genetic polymorphisms in patients with ischemic stroke taking clopidogrel, none of the genetic variants CYP2C19*2, *3,*4, CYP2C9*3, CYP2B6, and P2Y12 were found to have a significant association with clopidogrel resistance [19].
4.3 High On-Treatment Platelet Reactivity in the Indian Population Compared with Other Ethnicities
In keeping with the above finding, we found a relatively low prevalence of HTPR to integral clopidogrel after a loading dose of 600 mg, using an HTPR cut-off of ≥ 235 PRUs at 1 h. Crushed clopidogrel demonstrated a significantly higher HTPR of 34% compared with integral clopidogrel and both formulations of ticagrelor. The HTPR rate with integral clopidogrel was 15.63% and 10.34% at 1 and 8 h post-drug administration, respectively, which is much lower than observed in the Western population, where the HTPR rate was 40.8% at 12–24 h after clopidogrel administration [2]. The only other study in Indian patients using the VerifyNow assay reported a non-responder rate of 32% in patients receiving clopidogrel 75 mg twice daily approximately 5 days after PCI [20]. However, this study used a PRU cut-off of ≥ 213 to define clopidogrel resistance. In our study, the HTPR rate was 28%, with a PRU cut-off of ≥ 208 at 1 h following a loading dose of clopidogrel 600 mg. We had previously reported a non-responder rate of 29.2% at 24 h following a 300 mg loading dose of clopidogrel by LTA [21]. Patel et al. reported clopidogrel resistance in 15.4%, by LTA, of patients with ischemic stroke receiving clopidogrel 75 mg for at least 15 days [22]. Both these Indian studies used 10 micromoles of adenosine diphosphate (ADP) as the agonist. Thus, there seems to be a wide range in the clopidogrel responder rate with different platelet assays.
HTPR rates of 12.5% and 70% for crushed and integral formulations of ticagrelor in patients with STEMI were reported in a small European study [16]. In another study comparing ticagrelor with prasugrel in patients with STEMI, the HTPR (defined as ≥ 208 PRUs) was 42.6% for ticagrelor at 2 h post loading of medications [23]. Korean and European studies have previously documented the absence of HTPR with ticagrelor at least 2 h after the loading dose [16, 17]. Ticagrelor exhibited an extremely low HTPR rate of 2.94% 1 h after drug administration, and there were no patients with HTPR at 8 h in our study. Thus, similar to clopidogrel, HTPR rates in ticagrelor were lower in Indian patients than in other ethnicities.
Women exhibited higher HTPR than men 1 h after the loading dose (Table 6, Online Resource Table 2), however this difference disappeared with time. Unlike in men, platelet inhibition with integral ticagrelor was not superior to either formulation of clopidogrel at the earlier time in women. However, crushed ticagrelor formulation retained its potency even in this subgroup of patients. Tavenier et al. also observed and documented the lack of difference in PRU between sexes with the crushed ticagrelor preparation in STEMI patients where the platelet assay was done immediately following primary PCI [24]. Higher on-treatment platelet reactivity in women [25, 26] has been attributed to the greater leucocyte-platelet aggregation formation and protease-activated receptor (PAR)-1-mediated platelet reactivity. The lack of enhanced benefit with ticagrelor or prasugrel in women with high residual on-treatment platelet reactivity was described in another study [27]. Thus, crushed ticagrelor may be advocated in women with ACS to overcome HTPR, especially in urgent PCI. However, the efficacy of this approach must be proven in adequately powered studies with clinical outcomes as endpoints. We did not observe a significant effect in HTPR with opioid use, and HTPR had no bearing on TIMI flow on the coronary angiogram.
5 Adverse Events
There was a trend toward increased adverse effects in the integral ticagrelor arm. These findings were not replicated in the crushed ticagrelor group, demonstrating more aggressive platelet inhibition. Hence, this is likely due to chance and unlikely to be because of the drug, and larger adequately powered studies may be necessary to confirm or refute these findings. One patient developed stent thrombosis; this patient had a baseline PRU of 78, which decreased to 7 following the loading dose of the drug, but reached pretreatment levels at 8 h. Intravascular ultrasound imaging revealed an undersized and underexpanded stent that was optimized by post-dilatation using a larger balloon catheter. This patient made an uneventful recovery and was continued on ticagrelor therapy. Major bleeding was not different between the different drugs and formulations.
6 Limitations
A pharmacokinetic study was not conducted, therefore the bioavailability of the different drugs and formulations could not be ascertained. Patients were only taking the study drug for 24 h, and only a small proportion of the patients remained on the allocated medications. This was a single-center study and we used only a single modality (VerifyNow) to assess platelet function; therefore, our determination of HTPR cannot be extrapolated to platelet function assessed by other tests. Genotyping was not carried out in patients with HTPR, and therefore prevalent polymorphisms could not be identified. Despite these deficiencies, our study is the first to compare the platelet inhibitory effect of different ticagrelor and clopidogrel drug formulations and document the HTPR rates with these medications in Indian patients with ACS.
7 Conclusion
The crushed clopidogrel formulation is not superior to integral clopidogrel for early platelet inhibition and should not be used in ACS. Ticagrelor is more potent than clopidogrel, and crushed ticagrelor produced earlier platelet inhibition than integral ticagrelor. Bleeding events were not higher with ticagrelor. HTPR rates are low for both clopidogrel and ticagrelor in Indian patients compared with other ethnicities. Multicenter studies with clinical endpoints incorporating pharmacokinetic, pharmacodynamic, and genetic polymorphisms must be undertaken in Indian patients to find the optimal dose of ticagrelor, balancing adverse events and clinical efficacy.
References
Tavenier AH, Hermanides RS, Ottervanger JP, Tolsma R, van Beurden A, Slingerland RJ, et al. Impact of opioids on P2Y12 receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial. Eur Heart J Cardiovasc Pharmacother. 2022;8(1):4–12.
Price MJ, Berger PB, Teirstein PS, Tanguay J-F, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097–105.
Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45(2):246–51.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Michelson AD, Frelinger III AL, Braunwald E, Downey WE, Angiolillo DJ, Xenopoulos NP, et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J. 2009;30(14):1753–63.
Zafar MU, Farkouh ME, Fuster V, Chesebro JH. Crushed clopidogrel administered via nasogastric tube has faster and greater absorption than oral whole tablets. J Interv Cardiol. 2009;22(4):385–9.
Rollini F, Franchi F, Angiolillo DJ. Crushed ticagrelor in comatose patients treated with primary percutaneous coronary intervention. EuroIntervention. 2017;12(14):1681–3.
Serenelli M, Pavasini R, Vitali F, Tonet E, Bilotta F, Parodi G, et al. Efficacy and safety of alternative oral administrations of P2Y12-receptor inhibitors: Systematic review and meta-analysis. J Thromb Haemost. 2019;17(6):944–50.
Rollini F, Franchi F, Hu J, Kureti M, Aggarwal N, Durairaj A, et al. Crushed prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary intervention: the CRUSH study. J Am Coll Cardiol. 2016;67(17):1994–2004.
Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29(8):992–1000.
Collet J-P, Cayla G, Cuisset T, Elhadad S, Rangé G, Vicaut E, et al. Randomized comparison of platelet function monitoring to adjust antiplatelet therapy versus standard of care: rationale and design of the assessment with a double randomization of (1) a fixed dose versus a monitoring-guided dose of Aspirin and Clopidogrel after DES implantation, and (2) treatment interruption versus continuation, 1 year after stenting (ARCTIC) study. Am Heart J. 2011;161(1):5-12.e5.
Collet J-P, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100–9.
Becker RC, Bassand JP, Budaj A, Wojdyla DM, James SK, Cornel JH, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011;32(23):2933–44.
Rao KRK, Lakshmi KR. Design, development, and evaluation of clopidogrel bisulfate floating tablets. Int J Pharm Investig. 2014;4(1):19.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–85.
Alexopoulos D, Barampoutis N, Gkizas V, Vogiatzi C, Tsigkas G, Koutsogiannis N, et al. Crushed versus integral tablets of ticagrelor in ST-segment elevation myocardial infarction patients: a randomized pharmacokinetic/pharmacodynamic study. Clin Pharmacokinet. 2016;55(3):359–67.
Park D-W, Lee PH, Jang S, Lim H-S, Kang D-Y, Lee CH, et al. Effect of low-dose versus standard-dose ticagrelor and clopidogrel on platelet inhibition in acute coronary syndromes. J Am Coll Cardiol. 2018;71(14):1594–5.
Sharma AR, Vohra M, Shukla V, Guddattu V, Razak Uk A, Shetty R, et al. Coding SNPs in hsa-miR-1343-3p and hsa-miR-6783-3p target sites of CYP2C19 modulates clopidogrel response in individuals with cardiovascular diseases. Life Sci. 2020;15(245): 117364.
Gairolla J, Ahluwalia J, Khullar M, Kler R, Kishore K, Medhi B, et al. Clopidogrel response in ischemic stroke patients: is polymorphism or gender more important? Results of the CRISP study. J Clin Neurosci. 2020;76:81–6.
Pareed SA, Vijayaraghavan G, Kartha C, Manoj M. Antiplatelet drug resistance in Indians. Ann Clin Cardiol. 2020;2(1):36.
Thomson VS, John B, Pati PK, George OK, George PV, Jose J. Non-responders to clopidogrel therapy among Indian patients undergoing elective/adhoc angioplasty. Indian Heart J. 2008;60(6):543–7.
Patel S, Arya V, Saraf A, Bhargava M, Agrawal C. Aspirin and clopidogrel resistance in Indian patients with ischemic stroke and its associations with gene polymorphisms: a pilot study. Ann Indian Acad Neurol. 2019;22(2):147.
Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, et al. Randomized assessment of ticagrelor versus Prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5(6):797–804.
Tavenier AH, Hermanides RS, Ottervanger JP, Belitser SV, Klungel OH, Appelman Y, et al. Sex differences in platelet reactivity in patients with ST-elevation myocardial infarction: a sub-analysis of the ON-TIME 3 trial. Front Cardiovasc Med. 2021;8: 707814.
Danielak D, Komosa A, Tomczak A, Graczyk-Szuster A, Lesiak M, Glowka F, et al. Determinants of high on-treatment platelet reactivity and agreement between VerifyNow and Multiplate assays. Scand J Clin Lab Investig. 2017;77(3):190–8.
Gremmel T, Kopp CW, Eichelberger B, Koppensteiner R, Panzer S. Sex differences of leukocyte–platelet interactions and on-treatment platelet reactivity in patients with atherosclerosis. Atherosclerosis. 2014;237(2):692–5.
Verdoia M, Pergolini P, Nardin M, Rolla R, Negro F, Gioscia R, et al. Gender differences in platelet reactivity in diabetic patients receiving dual antiplatelet therapy. Cardiovasc Revasc Med. 2020;21(9):1144–9.
Acknowledgements
The authors are indebted to Mr. Surendra Singh, BSc, MLT, for performing the VerifyNow assays, and Mrs. Preethi Chandhini, BSc, for helping with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
A one-time grant was received from Sanofi India Ltd to purchase the VerifyNow assay kits for this study. The company had no role in planning, recruiting, overseeing, or analyzing the study, and no data were shared with the company.
Conflicts of interest
Harsha Teja Perla, Viji S. Thomson, Thomas V. Attumalil, Tulasi Geevar, Anoop George Alex, Rutvi Dave, Sukesh C. Nair, Mahasampath Gowri S, Prem K. Mony, Paul George, and George Joseph declare they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
This study was approved by the Institutional Review Board and Ethics Committee, Christian Medical College and Vellore, India (Minute No.: 12127)
Consent to participate
Informed consent was obtained from all trial participants.
Consent for publication
Not applicable.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Authors' contributions
HTP: study conception, data interpretation, and manuscript writing. VST: design and conception of the study, data interpretation, intellectual content, and manuscript writing. TVA: design, data interpretation, and manuscript writing. TG: study conception, data interpretation, and writing of the manuscript. AGA: data interpretation and writing of the manuscript. RGD: data verification, analysis, and manuscript editing. SCN: intellectual content, revision, and manuscript writing. MG: data verification, analysis, and manuscript preparation. PKM: design of the study, analysis, and manuscript revision. PG: intellectual content, revision, and manuscript editing. GJ: intellectual content, revision, and manuscript writing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perla, H.T., Thomson, V.S., Attumalil, T.V. et al. Randomized, Double-Blind, Active Comparator Pharmacodynamic Study of Platelet Inhibition with Crushed and Integral Formulations of Clopidogrel and Ticagrelor in Acute Coronary Syndrome. Am J Cardiovasc Drugs 23, 455–466 (2023). https://doi.org/10.1007/s40256-023-00591-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00591-8