Abstract
Background
The role of aspirin in cardiovascular primary prevention remains controversial. Moreover, evidence for the potential benefits of aspirin in patients with high cardiovascular risk remains limited.
Objective
The aim of this study was to explore the role of low-dose aspirin in primary prevention.
Methods
The PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov databases were searched for randomized clinical trials (RCTs) from the date of inception to August 2021. The efficacy outcomes were major adverse cardiovascular events (MACE), myocardial infarction (MI), ischemic stroke (IS), all-cause mortality, and cardiovascular mortality, whereas safety outcomes were major bleeding, intracranial hemorrhage, and gastrointestinal (GI) bleeding. Subgroup analyses were based on different cardiovascular risks and diabetes statuses. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using the fixed- and random-effects models, and trial sequential analysis (TSA) was conducted to determine the robustness of the results.
Results
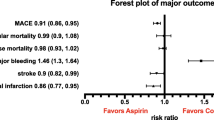
A total of 10 RCTs fulfilled the inclusion criteria. The use of aspirin was associated with a significant reduction in the risk of MACE (RR 0.89, 95% CI 0.84–0.93), MI (RR 0.86, 95% CI 0.78–0.95), and IS (RR 0.84, 95% CI 0.76–0.93); however, aspirin also increased the risk of safety outcomes, i.e. major bleeding (RR 1.42, 95% CI 1.26–1.60), intracranial hemorrhage (RR 1.33, 95% CI 1.11–1.59), and GI bleeding (RR 1.91, 95% CI 1.44–2.54). Subgroup analyses revealed that in the absence of a statistically significant interaction, a trend toward a net benefit of lower incidence of cardiovascular events (number needed to treat of MACE: high risk: 682 vs. low risk: 2191) and lesser risk of bleeding events (number needed to harm of major bleeding: high risk: 983 vs. low risk: 819) was seen in the subgroup of high cardiovascular risk. Meanwhile, the greater MACE reduction was also detected in the high-risk group of diabetes or nondiabetes patients. Furthermore, a post hoc subgroup analysis indicated a significant rate reduction in patients aged ≤ 70 years but not in patients aged > 70 years. TSA confirmed the benefit of aspirin for MACE up to a relative risk reduction of 10%.
Conclusion
The current study demonstrated that the cardiovascular benefits of low-dose aspirin were equally balanced by major bleeding events. In addition, the potential beneficial effects might be seen in the population ≤ 70 years of age with high cardiovascular risk and no increased risk of bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The cardiovascular benefits of low-dose aspirin were equally balanced by bleeding risks. |
The potential beneficiaries were likely those ≤ 70 years of age at high risk of cardiovascular disease and low risk of bleeding. |
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of global mortality [1, 2], much of which is attributable to poorly treated ASCVD risk factors. As well as a healthy lifestyle throughout life, medication management is also important in prevention strategies [3]. For decades, aspirin has been widely administered for ASCVD prevention. Although the benefit of aspirin for secondary prevention of ASCVD is better established [4, 5], aspirin use in primary prevention remains controversial. Regarding three randomized controlled trials (RCTs) published in 2018 (Aspirin to Reduce Risk of Initial Vascular Events [ARRIVE], A Study of Cardiovascular Events in Diabetes [ASCEND], and Aspirin in Reducing Events in the Elderly [ASPREE]) [6,7,8,9], more recent meta-analyses [10,11,12,13,14,15] have indicated that aspirin’s absolute benefits were largely counterbalanced by the bleeding hazard, and the routine use of aspirin for primary prevention therefore needs to be reconsidered. However, these analyses included several trials that enrolled patients with known atherosclerosis and peripheral vascular disease [16, 17], which may have led to selection bias and affected the meta-analysis results. The 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on the primary prevention of cardiovascular disease recommended that low-dose aspirin (75–100 mg/day) might be considered among patients aged 40–70 years who are at higher cardiovascular risk and no increased bleeding risk (Level IIb) [3]. Concerning populations with different cardiovascular risks, there is less consistency in the magnitude of low-dose aspirin use for cardiovascular endpoints across various meta-analyses. As reported in a more recent meta-analysis, a reduction in major adverse cardiovascular events (MACE) was only observed in diabetes patients with moderate–high cardiovascular risk [18]. In contrast, no difference was found in other meta-analyses [10, 15, 19, 20], in which the aspirin dosage ranged from low dose (75–100 mg/day) to high dose (325–650 mg/day), which was unrepresentative in current clinical practice. In consequence, whether risk-stratified subgroups benefit from low-dose aspirin use remains unknown.
A recently published trial of The International Polycap Study 3 (TIPS-3) [21] showed that low-dose aspirin did not lead to a lower incidence of cardiovascular events than placebo. Consequently, we aimed to perform an updated meta-analysis focused on low-dose aspirin use for primary prevention in patients who had no prior history of ASCVD, and to explore whether the effect of this intervention varied according to different cardiovascular risks.
2 Material and Methods
2.1 Search Strategies
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations were regarded as a guideline to perform our meta-analysis [22]. We searched the PubMed, EMBASE, Cochrane Library, and Clinical Trials.gov databases from inception to the end of August 2021 using the following medical subject heading (MeSH) and free-text terms: ‘aspirin’ or ‘salicylic acid’ or ‘salicylates’ and ‘primary prevention’ and ‘cardiovascular disease’ in ‘PICOS’ principle. Publication type was limited to RCTs. In addition, a manual search was performed by searching references of former meta-analyses and relevant studies that were not identified in our electronic search. No language restrictions were imposed.
2.2 Study Selection
Two reviewers (MMW and ZJL) independently screened the records, while a third reviewer (DXG) made the final decision when disagreements occurred. Inclusion criteria were (1) RCTs that included at least 1000 patients; (2) participants without a prior history of ASCVD (including peripheral arterial disease, coronary artery disease, prior myocardial infarction [MI], prior stroke, or transient ischemic attack); (3) low-dose aspirin was defined as a daily aspirin regimen (75–100 mg/day) regardless of the drug names, administration routines, or as an adjunct to other forms of primary prevention treatment; and (4) outcomes were reported in the composite of MACE, MI, ischemic stroke (IS), all-cause mortality, cardiovascular mortality, major bleeding, intracranial hemorrhage, and gastrointestinal (GI) bleeding. Exclusion criteria were (1) non-RCTs; (2) comparing aspirin with other positive drugs as control treatment; (3) abstract-only studies; and (4) clinical trials with unrelated outcomes. A decision on the final inclusion of studies was obtained by discussion.
2.3 Data Extraction
The following information was closely screened and independently extracted by two reviewers (MMW and ZJL) to a standardized collection form (Tables S1–S4 in electronic supplementary material [ESM] 1) that we had previously created. Data were collected from the included studies as follows: basic characteristics of the included patients, clinical information about the intervention/control arms, essential outcome data, and study design. When essential data were not reported, we communicated with the original author of the study to obtain the desired data. Furthermore, missing data were also collected in ClinicalTrials.gov when the NCT number was available. A third reviewer (DXG) then cross-checked the data for any errors during data extraction.
2.4 Definition of Outcomes
The efficacy outcomes for this meta-analysis included MACE, MI, IS, all-cause mortality, and cardiovascular mortality, while the safety outcomes included major bleeding, intracranial hemorrhage, and GI bleeding.
The definition of MACE was a composite of nonfatal stroke, nonfatal MI, and cardiovascular mortality. If the trials did not report MACE as an outcome according to this definition, an expanded MACE endpoint included nonfatal MI, nonfatal stroke (excluding confirmed intracranial hemorrhage) or transient ischemic attack, or death from any vascular cause (excluding confirmed intracranial hemorrhage) [7]. Moreover, the outcome of MI included both fatal and nonfatal events. Other outcomes were all defined as per the study’s definition, and endpoint data were extracted with the aim of maintaining the consistency of definitions.
2.5 Quality Assessment
Two reviewers (MMW and ZJL) independently evaluated the quality of each selected study using the Cochrane risk-of-bias tool across five domains (sequence generation, allocation concealment, blinding, detection bias, attrition bias, and reporting bias). Furthermore, publication bias was suggested by visual inspection of the funnel plots, and the Egger’s test was used to identify the asymmetry of funnel plots for publication bias.
2.6 Statistical Analysis
Statistical analysis was performed using RevMan software (Review Manager [RevMan] version 5.3, The Cochrane Collaboration, Copenhagen, Denmark) and Stata 12.0 software (Statacorp LLC, College Station, TX, USA). When data from three or more studies were available, outcomes were pooled using risk ratios (RRs) [Mantel–Haenszel method for the fixed-effect model, and the DerSimonian and Laird method for the random-effects model) for dichotomous variables. Mann–Whitney U tests were used to conduct statistical analyses, and a two-sided p value < 0.05 was considered statistically significant. Heterogeneity across the different trials and between subgroups was assessed using Cochran’s Q test, and a p value <0.05 was considered statistically significant. Furthermore, the I2 statistic was used to calculate the degree of heterogeneity between the included studies. I2 > 50% was considered to represent significant heterogeneity; however, if the I2 value was not significant (I2 < 50%), a fixed-effect model was additionally calculated [23]. Potential publication bias was estimated using funnel plots and Egger’s test with at least 10 studies; a p value <0.05 was considered a significant publication bias.
To further illustrate these outcome estimations, the absolute risk reduction (ARR) or absolute risk increase (ARI) and number needed to treat (NNT) or number needed to harm (NNH) were also analyzed. This was performed as follows: event incidence rates were divided by their respective mean follow-up periods and multiplied by 100 to obtain the incidence rate per 100 patient-years. Of these, the ARR or ARI were calculated by subtraction, and the NNT or NNH was subsequently derived by dividing 1 by the calculated ARR or ARI [14] Furthermore, a net clinical benefit was also calculated using the difference between the NNT of MACE and the NNH of major bleeding when available.
2.7 Subgroup and Sensitivity Analyses
A prespecified subgroup analysis for the efficacy and safety outcomes was performed according to the different cardiovascular risks of enrolled patients in each trial. This was calculated using the 10-year estimated MACE rate in the placebo arm based on the method reported in previous meta-analyses [10, 24, 25], and was calculated by multiplying the annualized event rate for MACE in the control group by 10 years. For grading the different cardiovascular risks, the computed value of the 10-year estimated MACE rate < 10% was defined as low risk, while the other populations were defined as high risk [26, 27]. We also conducted an exploratory subgroup analysis to investigate the effect of aspirin use in patients with or without diabetes.
The following sensitivity analyses were performed: (1) the influence of each study was assessed by testing whether deleting each in turn would have significantly changed the pooled results of the meta-analysis; and (2) the influence of studies that enrolled patients with asymptomatic peripheral or aortic atherosclerotic disease was assessed through the inclusion of these trials [16, 17].
2.8 Trial Sequential Analysis
Meta-analyses are regarded as an interim analysis on the way towards obtaining the required information size (RIS) [28]. However, intervention effects are often spuriously overestimated (type I errors) or underestimated (type II errors) because of too few participants or clinical diversity regarding patients, interventions, outcomes, etc. [29, 30]. Trial sequential analysis (TSA) is an approach that provides the RIS to help reduce these errors and increase the robustness of the meta-analyses [31]. The RIS in the meta-analysis is defined as the number of events or patients from the included studies necessary to accept or reject the statistical hypothesis [32]. TSA was conducted for the efficacy outcomes using TSA software (version 0.9 beta; http://www.ctu.dk/tsa). We chose a 10% relative risk reduction (RRR) according to the TSA manual and former meta-analyses used [20, 25], the proportion in the control group of the cumulative meta-analysis (CMA), a 5% (α < 0.05; two-sided) risk of a type 1 error, and 80% statistical power to calculate the RIS and the cumulative Z-curve’s eventual breach of relevant trial sequential monitoring boundaries.
3 Results
3.1 Study Selection
We identified 1943 studies using our search strategy, of which 193 duplicate studies were removed. After title and abstract screening, 103 potentially relevant studies were identified. After reviewing the full text, 10 studies [6,7,8,9, 21, 33,34,35,36,37,38] met our inclusion criteria. A flow chart showing the study selection is presented in Fig. 1.
3.2 Study Characteristics
The characteristics of the included studies and participants are listed in Tables 1, 2 and 3. Among the total sample, 67,704 patients were randomized to low-dose aspirin, and 67,853 patients were randomized to a control group. The mean follow-up was 6.14 years (range 3.6–10.3). The comparator treatment was placebo in seven studies [6,7,8,9, 21, 35, 37, 38] and no aspirin in three studies [33, 34, 36]. Among the included studies, the results of the ASPREE trial were reported in two reports, and as a result, we used two references [8, 9]. Males were exclusively enrolled in one study [37] and females were exclusively enrolled in another [35]. Overall, 84,024 participants (62%) were female. Two studies exclusively enrolled participants with diabetes [7, 33], with 30,408 participants (22.4%) having diabetes. Two studies enrolled older participants, with a mean age of >70 years [8, 9, 34]; the remaining participants were ≤ 70 years of age. The median 10-year estimated MACE rate was 9.2% (range 2.6–15.9%). According to the 10-year estimated MACE rate, five studies [7, 21, 34, 37, 38] were in high cardiovascular risk and five studies [6, 8, 9, 34,35,36] were in low risk.
3.3 Risk of Bias
The risk-of-bias assessment results are shown in Fig. S1 in ESM 1. With the exception of one study [35], nine studies [6,7,8,9, 21, 33, 34, 36,37,38] described the random sequence generation (e.g., a computer-generated random list, a computer-generated randomization table) and were regarded as low risk of bias. Four studies [21, 33, 34, 37] stated the allocation concealment process, and six studies [6,7,8,9, 35, 36, 38] were considered as unclear risk of bias because we were unclear whether the envelopes were concealed. For blinding of participants, personnel, and outcome assessment, three open-label studies [33, 34, 36] were regarded as high risk of bias. In the ‘incomplete outcome data’ domain, all studies were based on the intention-to-treat principle and were regarded as low risk of bias. In the ‘selective reporting’ domain, all studies were deemed to have a low risk of bias, and in the ‘other biases’ domain, all studies were deemed to have a low risk, except for one study [36] as a result of early termination. With regard to publication bias, the funnel plot distributions of the data points for outcomes with at least 10 studies (MACE and all-cause mortality) were generally symmetric and thus showed no obvious signs of systematic differences among studies (Fig. S2 in ESM 1). The Egger’s test did not detect any significant publication bias (MACE, p = 0.22; all-cause mortality, p = 0.46).
3.4 Efficacy Outcomes
3.4.1 Major Adverse Cardiovascular Events
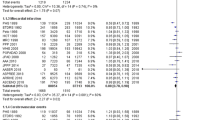
For MACE, 10 studies [6,7,8,9, 21, 33,34,35,36,37,38] reported a total of 5484 events (2575 with aspirin and 2909 with no aspirin). Compared with no aspirin, the use of aspirin was associated with a significant decrease in MACE (RR 0.89, 95% CI 0.84–0.93; ARR 0.079%, NNT 1269), with low heterogeneity (p = 0.90, I2 = 0%) (Fig. 2a; Table 4).
3.4.2 Myocardial Infarction and Ischemic Stroke
Nine studies [6, 8, 9, 21, 33,34,35,36,37,38] provided data on the incidence of MI, and six trials [7,8,9, 33,34,35, 37] provided data on the incidence of IS. Compared with no aspirin, the use of aspirin was associated with a significant decrease in MI (RR 0.86, 95% CI 0.78–0.95; ARR 0.033%, NNT 3045), with low heterogeneity (p = 0.14, I2 = 35%). The reduction was also observed in IS (RR 0.84, 95% CI 0.76–0.93; ARR 0.044%, NNT 2268), with low heterogeneity (p = 0.79, I2 = 0%) (Fig. 2b, c; Table 4).
3.4.3 Mortality
All-cause mortality was reported in 10 studies [6,7,8,9, 21, 33,34,35,36,37,38] and cardiovascular mortality was reported in nine studies [6,7,8,9, 21, 34,35,36,37,38]. The use of aspirin did not lead to a significant reduction in all-cause mortality (RR 0.98, 95% CI 0.93–1.02) or cardiovascular mortality (RR 0.91, 95% CI 0.82–1.00), with low heterogeneity for both outcomes (all-cause mortality: p = 0.33, I2 = 13%; cardiovascular mortality: p = 0.82, I2 = 0%) (Fig. 3).
3.5 Safety Outcomes
For the outcome of major bleeding, six studies [7,8,9, 21, 34, 37, 38] reported a total of 1547 events (902 with aspirin and 645 with no aspirin). The use of aspirin was associated with an increased rate of major bleeding (RR 1.42, 95% CI 1.26–1.60; ARI 0.110%, NNT 904) and with low heterogeneity (p = 0.30, I2 = 18%). Data reported intracranial hemorrhage in eight studies [6,7,8,9, 34,35,36,37,38] and GI bleeding in nine studies [6, 8, 9, 21, 33,34,35,36,37,38]. For intracranial hemorrhage, aspirin use was associated with a higher risk of intracranial hemorrhage (RR 1.33, 95% CI 1.11–1.59; ARI 0.018%, NNT 5620) compared with no aspirin and with low heterogeneity (p = 0.67, I2 = 0%). GI bleeding (RR 1.91, 95% CI 1.44–2.54; ARI 0.113%, NNH 884) was also more common with aspirin use but with high heterogeneity (p < 0.00001, I2 = 81%) [Fig. 4, Table 4]. In the multivariable meta-regression analysis, which explored the potential sources of heterogeneity on the outcome of GI bleeding, it was revealed that study design (open-label vs. double-blind) could be used to explain partial heterogeneity in the pooled RR (Table 5). Pooled statistics for the efficacy and safety outcomes are summarized in Fig. 5.
Summary statistics of the effect of low-dose aspirin on efficacy and safety outcomes. a Overall population; b subgroup by cardiovascular risks; c subgroup by diabetes status; d subgroup of MACE by diabetes status and cardiovascular risks. MACE major adverse cardiovascular events, MI myocardial infarction, IS ischemic stroke, GI gastrointestinal, RR risk ratio, CI confidence interval
3.6 Sensitivity Analyses and Subgroup Analyses
We conducted a subgroup analysis to investigate whether the effects of aspirin on primary prevention of ASCVD differed according to baseline cardiovascular risk. High cardiovascular risk with a 10-year MACE rate of more than 10% was observed in five studies [7, 21, 33, 37, 38], and low cardiovascular risk was observed in five studies [6, 8, 9, 34,35,36]. The results showed that the high-risk subgroup yielded a higher reduction in the outcome of MACE (high risk: RR 0.87, 95% CI 0.81–0.93, ARR 0.147%, NNT 682, vs. low risk: RR 0.91, 95% CI 0.84–0.98, ARR 0.046%, NNT 2191; test for subgroup differences, p = 0.39). For MI, the protective effect of aspirin was only significant in the high-risk subgroup (high risk: RR 0.76, 95% CI 0.64–0.91, vs. low risk: RR 0.91, 95% CI 0.81–1.02; test for subgroup differences, p = 0.11); however, the opposite effect was observed in the outcome of IS (high risk: RR 0.85, 95% CI 0.72–1.01, vs. low risk: RR 0.83, 95% CI 0.73–0.94; test for subgroup differences, p = 0.76). Furthermore, it also indicated that the high-risk group obtained more beneficial trend than the low-risk group in all-cause mortality (high risk: RR 0.94, 95% CI 0.87–1.01, vs. low risk: RR 1.01, 95% CI 0.94–1.08; test for subgroup differences, p = 0.14). For safety outcomes, there was no increase in the intracranial hemorrhage rate for the high-risk group, but an increase was reported in the low-risk group (high risk: RR 1.16, 95% CI 0.83–1.63, vs. low risk: RR 1.40, 95% CI 1.13–1.73; test for subgroup differences, p = 0.36). Briefly, the low cardiovascular risk group yielded a higher rate of bleeding compared with the high-risk group, although the p-values for the test for subgroup differences in these results were higher than 0.05. Therefore, these two subgroups did not reach statistical significance (Tables 4, 6, Figs. S3 and S4 in ESM 1).
We further conducted a subgroup analysis to explore the difference between diabetes and non-diabetes. Data for the diabetes or nondiabetes subgroups were reported in seven studies [8, 9, 21, 34,35,36,37,38]; diabetes were exclusively reported in two studies [7, 33] and nondiabetes were reported in one study [6]. Both subgroups showed a decreased risk of MACE, which was consistent with the overall population analysis (diabetes: RR 0.88, 95% CI 0.81–0.95, vs. nondiabetes: RR 0.88, 95% CI 0.82–0.95). There was no significant difference for other outcomes in either group. We later divided the subgroups according to cardiovascular risk on the outcome of MACE. The same trend was detected with the overall population. Lacking statistical difference between groups, a positive effect was more significant in the high-risk group, whether in the diabetic population or not (Pinteraction > 0.05) (Tables 4, 6, Fig. S5 in ESM 1). Pooled statistics for the subgroup analyses stratified by cardiovascular risk or diabetes status are summarized in Fig. 5.
Sensitivity analysis of outcomes with a significant difference was conducted, with the findings showing that the results were consistent with the full analysis after excluding each individual study, except for intracranial hemorrhage. One trial heavily contributed toward the overall effect due to its high incidence of intracranial hemorrhage, which was mostly attributable to the mean age of the enrolled population (> 70 years). We later performed a post hoc subgroup analysis to explore this factor (Table S5 and Fig. S6 in ESM 1). Sensitivity analysis was also conducted to assess the impact of excluding the Prevention of Progression of Arterial Disease and Diabetes (POPADAD) [16] and Aspirin for Asymptomatic Atherosclerosis (AAA) trials [17]. There was no significant difference in any of the outcomes when these two trials were included in the analysis (Table S6 and Fig. S7 in ESM 1).
A post hoc subgroup analysis was further conducted according to mean age. Data for participants with a mean age > 70 years were reported in two studies [8, 9], while other participants were all ≤ 70 years of age. Numerically, aspirin-induced cardiovascular benefits were only observed in the younger age subgroup compared with the older age subgroup; however, aspirin-induced bleeding risks were more common in the older age subgroup. In addition, the p value for the test for subgroup differences of all-cause mortality was < 0.05, which indicated that statistical difference between the two subgroups was present in this outcome (Table 6, Figs. S8 and S9 in ESM 1).
3.7 Trial Sequential Analysis
In TSA, we observed that the cumulative Z-curve exceeded both the conventional and TSA monitoring boundaries for outcomes of MACE and IS. Similarly, for all-cause mortality, the cumulative Z-curve crossed neither the traditional boundary nor the trial sequential monitoring boundary, but did cross the futility boundary. The pooled sample size of both exceeded the calculated optimum sample size, indicating that conclusions on the above-mentioned outcomes were robust and were hardly modified as a result of additional related trials. Inversely, for the other efficacy outcomes (MI, cardiovascular mortality), both the TSA monitoring boundary and the futility boundary had not been crossed, indicating that the results were unreliable and that more studies should be included (Fig. 6).
Trial sequential analysis of efficacy endpoints using the fixed-effect model meta-analysis, based on an anticipated intervention effect of 10% relative risk reduction, a control event incidence with alpha 5%, and power 80%. MACE major adverse cardiovascular events, MI myocardial infarction, IS ischemic stroke
4 Discussion
In this meta-analysis, 10 studies that enrolled a total of 135,557 participants demonstrated that the use of low-dose aspirin for the primary prevention of ASCVD was associated with a decreased incidence of MACE, MI, and IS, but increased the incidence of major bleeding. Aspirin use had no association with mortality rates. In absolute terms, approximately 1269 patients would need to be treated to prevent one MACE, and approximately 904 patients would need to be treated to cause one major bleeding. Subgroup analyses revealed a greater benefit would be seen in those patients with high cardiovascular risk, regardless of whether or not they had diabetes. In absolute terms, the analyses showed the net benefit would favor the use of aspirin in high-risk patients with a lower NNT than NNH for the efficacy and safety outcomes (MACE vs. major bleeding: 682 vs. 983), respectively. In a post hoc subgroup analysis, a significant all-cause mortality reduction was observed in patients ≤ 70 years of age.
Compared with aspirin use in the secondary prevention of ASCVD, aspirin use for primary prevention has been widely debated. This uncertainty has been reflected in contradictory guideline recommendations [3, 27, 39]. According to the new draft guidelines, the US Preventive Services Task Force (USPSTF) recommended against low-dose aspirin for the primary prevention of ASCVD in all adults aged ≥ 60 years [40, 41]. Similarly, the 2021 European Society of Cardiology (ESC) guidelines report weak evidence to support aspirin use in primary prevention; further studies are needed to confirm the net benefit for patients ≤ 70 years of age with high cardiovascular risk [27]. On the other hand, the ACC/AHA guideline [3] only endorses aspirin use for patients aged 40–70 years with a higher cardiovascular risk, and without a risk of bleeding, for primary prevention. Moreover, the 2022 American Diabetes Association (ADA) guideline also recommended the same option for aspirin use (75–162 mg/day) in diabetes [42]. Thus, given current trial and guideline evidence, aspirin would no longer be recommended for all primary prevention patients, and likely only for a minority of these patients. Furthermore, due to the prevalence of aspirin use for primary prevention, the phenomenon of aspirin misuse is very common in those who might not obtain benefit but increase the bleeding risk [43,44,45]. Furthermore, ASCVD risk factors are often poorly treated, even in patients considered to be at high cardiovascular risk [46, 47]. Thus, there is an urgent need to find the population who would benefit most and who warrant increased focus on discontinuation of inappropriate aspirin use. In our study, subgroup analyses were performed based on cardiovascular risks and mean age; mean age was < 70 years in the high cardiovascular risk group. Thus, the results from subgroup analyses were in line with what would be expected, i.e. that participants with high cardiovascular risk and who were aged ≤ 70 years had the potential to obtain more cardiovascular advantages from aspirin use, and less bleeding risks. In addition, the data of diabetes mellitus and non-diabetes mellitus patients were also extracted to explore the differences between the two groups. The results showed that both groups obtained a decreased risk of MACE and no significant differences existed between them. When those were distinguished according to cardiovascular risk, the same trend was also detective that the high-risk group obtained a more beneficial effect in these two groups. However, interaction between the subgroups did now show a statistically significant difference. A similar result was also found in a large observational study, i.e. that aspirin treatment reduced the risk of ST-segment elevation MI in the higher cardiovascular risk group rather than in the lower-risk group [48]
The recently published TIPS-3 trial [21] was an RCT with a 2 × 2 × 2 factorial design; participants with elevated cardiovascular risk were enrolled to determine the efficacy and safety of polypill (containing statins and antihypertensive drugs) with or without aspirin, compared with matching placebo. The study suggested that compared with placebo, there was no significant decrease in cardiovascular outcomes in the low-dose aspirin or polypill groups; however, there was a significant decrease in the aspirin plus polypill group. Therefore, we inferred that aspirin combined with statins or antihypertensive medications would lead to important benefits for primary cardiovascular disease prevention. This was also confirmed by a recently published individual participant data meta-analysis [49] indicating that a fixed-dose combination therapy, including low-dose aspirin, statins, and antihypertensive drugs (either delivered as a polypill or as separate drugs), was effective in preventing major cardiovascular events. This view was contrary to some recent trials [6, 7] that have called into question the use of aspirin in primary prevention. The potential explanation was that the observed event rate was considerably less than anticipated in these trials, and the benefits were less significant in patients at low to middle cardiovascular risk. Furthermore, the observed event rates were often lower than expected, likely due to better CVD risk factor management and contemporary treatments such as use of statins. This caused the beneficial effect of aspirin to appear to be weakened in preventing cardiovascular events. This issue has previously been examined by the Antithrombotic Trialists’ (ATT) collaborators, pointing out that in most of the older trials, aspirin reduced both MI and IS risks in patients who did not receive statin therapy [50].
In the era of precision medicine, a tailored strategy is required to maximize benefit and minimize harm for individuals who use aspirin for the primary prevention of cardiovascular disease. Discussion from two experts on aspirin use showed that it is important to obtain the net benefit through simultaneously considering the cardiovascular risk-modifying factors and bleeding risk factors in real practice [51]. Cardiovascular risk should not only consider the risk-decreasing factors of contemporary ASCVD risk factor management such as statins, which would be expected to result in a 30% relative reduction in ASCVD risk [52], but also take into account risk-enhancing factors that were not included in risk prediction tools. In addition, certain special populations present heterogeneity of aspirin treatment efficacy. An observational study [48] advised that when making individual treatment decisions in diabetes, clinicians and patients should not only consider the 10-year risk of CV disease but also the number and quality of CV risk factors because of the benefit in patients with hypertension and hypercholesterolemia, but not in smokers. Furthermore, a cohort study [53] found low-dose aspirin would be of no benefit for patients with chronic kidney disease, and even increased the risk of cardiovascular events in patients with low bodyweight. In addition, due to the high bleeding risk associated with aspirin use, guidelines do not recommend aspirin for the primary prevention of ASCVD among adults of any age who are at increased risk of bleeding.
Considering upper GI bleeding, which is the most common complication in patients under antiplatelet therapy [54, 55], proton pump inhibitors (PPIs) might limit the risk of major GI bleeding and enhance the benefit–risk ratio toward intended populations [56]. Under the circumstances, to reduce the occurrence of GI ulcers and increase adherence rates, Aralez Pharmaceuticals, Inc. has created Yosprala, a coordinated delivery tablet combining omeprazole and enteric-coated (EC) aspirin into one tablet [57]. In addition, the innovation point of aspirin formulations was raised to avoid its GI adverse effects, such as nano-liposomal encapsulation of aspirin [58], and the innovative oral or sublingual formulation of aspirin micronized and co-grinded with collagen [59], which could increase the net clinical benefit by reducing its cytotoxic effect on the GI tract.
Compared with former meta-analyses, our meta-analysis has several strengths. First, previous meta-analyses [10, 15, 19, 20] investigated the effect of aspirin in patients with different cardiovascular risks based on studies reporting that aspirin dosage ranged from low dose (75–100 mg/day) to high dose (325–650 mg/day). Higher aspirin doses do not achieve greater antiplatelet inhibition than lower doses, but simply increase the risk of adverse effects [60]. Therefore, we only included studies in which aspirin was utilized in a low-dose therapy, which is commonly recommended for primary prevention in current guidelines.
Furthermore, a more recently published meta-analysis, which only focused on the diabetic population [18], showed more beneficial effect of low-dose aspirin for primary prevention in moderate/high risk. As a consequence, our meta-analysis was the first study to comprehensively evaluate the role of low-dose aspirin in the overall population stratified by baseline cardiovascular risk, and supports the current guidelines.
Second, with regard to the moderate-to-high heterogeneity of the beneficial effect on the outcome of MI in former meta-analyses [11, 20, 25, 61]. We utilized stricter inclusion and exclusion criteria by including studies in which aspirin was utilized as a low-dose therapy, and excluding two trials that were previously included in prior meta-analyses; these two trials [16, 17] evaluated aspirin in patients with asymptomatic peripheral arterial disease, which violated our primary prevention criteria. As a result, we examined the source of heterogeneity on the outcome of MI.
Third, cardiovascular risk was estimated by calculating the number of events in the control arm, which was more accurate than the currently used tool for the assessment of benefits and risks. Furthermore, our study used p-values for the difference to verify statistical differences in subgroups [62]. However, the inconsistent view proposed by a recently published article [63] indicated that p values for interaction should not be provided in the forest plots due to problems of multiplicity and the limiting value for inference. As a consequence, the potential benefit population revealed by subgroup analyses should be interpreted with caution.
Fourth, TSA was mainly conducted to focus on the efficacy outcomes to consider the information size and the effect size, and was therefore more conservative and more accurate when reaching a conclusion [64]. TSA suggested ample evidence for a 10% RRR on the outcomes of MACE and IS, and provided futility for further trials to evaluate the outcome of all-cause mortality. However, for outcomes of MI and cardiovascular mortality, the results were not robust and might be modified with additional related trials; further studies on these topics may be needed. As the borderline statistical significance in the pooled RR of cardiovascular mortality which p-value was near to 0.05. This might be due to the short follow-up (a mean follow-up of more than 10 years only existed in two trials) in most included studies. Further studies with more extended follow-up are needed to evaluate the potential effect.
Our meta-analysis also has several limitations. First, there was considerable variation in the definition of bleeding outcomes. As a consequence, we performed our analysis using the random-effects model. Confidence intervals for the average intervention effect would be wider, and relevant claims of statistical significance would be more conservative, even though there was no significant heterogeneity on the outcome of major bleeding and intracranial hemorrhage. Furthermore, the definition of GI bleeding events in some trials was not further detailed, which led to high heterogeneity. We attempted to overcome this limitation by conducting sensitivity and meta-regression analyses to explore the reason for such heterogeneity. The subgroup and sensitivity analyses indicated that the result was robust.
Second, the Hypertension Optimal Treatment (HOT) trial [38] included approximately 5% of patients with a prior history of cardiovascular disease. Given the small proportion of these patients in the trial, we decided to include them in the analysis and performed a sensitivity analysis by excluding this trial. In addition, the sensitivity analysis excluding HOT did not alter the overall findings.
Third, we could not conduct the subgroups according to the risk of bleeding and the effects of concomitant use of PPIs on safety outcomes, since related data were not reported in most of the trials. The development of a bleeding risk calculator is needed to support clinicians’ assessment of risk versus benefit.
Finally, due to the data being reported in very few studies, we could not conduct a subgroup analysis of diabetes status for each outcome that was investigated in the overall population. Moreover, due to lack of patient-level data, we could not perform subgroup analyses for other baseline characteristics that might benefit from aspirin. Further studies are needed to confirm the influence of these factors.
5 Conclusion
Our meta-analysis elucidated that low-dose aspirin therapy played a role in reducing the rate of MACE, MI, and IS; however, it increased the risk of major bleeding, intracranial hemorrhage, and GI bleeding. In addition, subgroup analyses suggested that benefits with a lack of statistical significance were observed in patients ≤ 70 years of age with a high cardiovascular risk for the overall population, regardless of whether or not those patients had diabetes. Further studies are needed to confirm the effects of aspirin in these populations.
References
Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–94.
Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12.
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–646.
Jones WS, Mulder H, Wruck LM, et al. Comparative effectiveness of aspirin dosing in cardiovascular disease. N Engl J Med. 2021;384:1981–90.
Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036–46.
Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–39.
Mcneil JJ, Nelson MR, Woods RL, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med. 2018;379:1519–28.
Mcneil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–18.
Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events a systematic review and meta-analysis. JAMA. 2019;321:277–87.
Xie W, Luo Y, Liang X, et al. The efficacy and safety of aspirin as the primary prevention of cardiovascular disease: an updated meta-analysis. Ther Clin Risk Manag. 2019;15:1129–40.
Shah R, Khan B, Latham SB, et al. A meta-analysis of aspirin for the primary prevention of cardiovascular diseases in the context of contemporary preventive strategies. Am J Med. 2019;132:1295-1304.e3.
Lin M-H, Lee C-H, Lin C, et al. Low-dose aspirin for the primary prevention of cardiovascular disease in diabetic individuals: a meta-analysis of randomized control trials and trial sequential analysis. J Clin Med. 2019;8:609.
Gelbenegger G, Postula M, Pecen L, et al. Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Med. 2019;17:198.
Nudy M, Cooper J, Ghahramani M, et al. Aspirin for primary atherosclerotic cardiovascular disease prevention as baseline risk increases a meta-regression analysis. Am J Med. 2020;133:1056–64.
Belch J, Maccuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337: a1840.
Fowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–8.
Masson W, Barbagelata L, Lavalle-Cobo A, et al. Low-doses aspirin in the primary prevention of cardiovascular disease in patients with diabetes: meta-analysis stratified by baseline cardiovascular risk. Diabetes Metab Syndr. 2022;16: 102391.
Masson G, Lobo M, Masson W, et al. Aspirin in primary prevention. Meta-analysis stratified by baseline cardiovascular risk. Arch Cardiol Mex. 2020;90:293–9.
Zhao B, Wu Q, Wang L, et al. Pros and cons of aspirin for the primary prevention of cardiovascular events: a secondary study of trial sequential analysis. Front Pharmacol. 2020;11: 592116.
Yusuf S, Joseph P, Dans A, et al. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med. 2021;384:216–28.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the us preventive services task force. Ann Intern Med. 2016;164:804–13.
Mahmoud AN, Gad MM, Elgendy AY, et al. Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur Heart J. 2019;40:607–17.
Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337.
Kulinskaya E, Wood J. Trial sequential methods for meta-analysis. Res Synth Methods. 2014;5:212–20.
Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61:763–9.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39.
Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75.
Kang H. Trial sequential analysis: novel approach for meta-analysis. Anesth Pain Med. 2021;16:138–50.
Saito Y, Okada S, Ogawa H, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation. 2017;135:659–70.
Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510–20.
Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304.
Roncaglioni MC. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95.
Meade TW, Wilkes HC, Kelleher CC, et al. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351:233–41.
Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62.
Chinese Society of Cardiology of Chinese Medical Association, et al. Chinese guideline on the primary prevention of cardiovascular diseases [in Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:1000–38.
Anonymous. Aspirin use to prevent cardiovascular disease: preventive medication. Available at: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication#fullrecommendationstart.;375
Mahase E. US taskforce advises against low dose aspirin for primary prevention of cardiovascular disease. BMJ. 2021;375: n2521.
Draznin B, Aroda VR, Bakris G, et al. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45:S144-s174.
Chen Y, Yin C, Li Q, et al. Misuse of aspirin and associated factors for the primary prevention of cardiovascular disease. Front Cardiovasc Med. 2021;8: 720113.
Christensen MB, Jimenez-Solem E, Ernst MT, et al. Low-dose aspirin for primary and secondary prevention of cardiovascular events in Denmark 1998–2018. Sci Rep. 2021;11:13603.
Hira RS, Gosch KL. Potential impact of the 2019 ACC/AHA guidelines on the primary prevention of cardiovascular disease recommendations on the inappropriate routine use of aspirin and aspirin use without a recommended indication for primary prevention of cardiovascular disease in cardiology practices: insights from the NCDR PINNACLE Registry. Circ Cardiovasc Qual Outcomes. 2022;15(3): e007979.
Kotseva K, De Backer G, De Bacquer D, et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: a report from the European Society of Cardiology EURObservational Research Programme EUROASPIRE V survey in 16 European countries. Eur J Prev Cardiol. 2021;28(4):370–9.
Maiello M, Cecere A, Zito A, et al. Low-dose aspirin for primary prevention of cardiovascular events in postmenopausal women with type-2 diabetes: the prescriptive approach in the real world. Int J Prev Med. 2021;12:140.
Bugiardini R, Pavasović S, Yoon J, et al. Aspirin for primary prevention of ST segment elevation myocardial infarction in persons with diabetes and multiple risk factors. EClinicalMedicine. 2020;27: 100548.
Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet. 2021;398:1133–46.
Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60.
Burns RB, Pignone M, Michos ED. Would you recommend aspirin to this patient for primary prevention of atherosclerotic cardiovascular disease? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2021;174:1439–46.
Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services task force. JAMA. 2016;316:2008–24.
Oh YJ, Kim AJ, Ro H, et al. Low-dose aspirin was associated with an increased risk of cardiovascular events in patients with chronic kidney disease patients and low bodyweight: results from KNOW-CKD study. Sci Rep. 2021;11:6691.
Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. 2000;321:1183–7.
Palmer RH. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13:2023–4.
Siller-Matula JM, Delle-Karth G. Addition of omeprazole to dual antiplatelet therapy with clopidogrel plus aspirin lowers the risk of upper gastrointestinal bleeding. Evid Based Med. 2011;16:144–5.
Ingram CA, Giang G, Mccrory K, et al. Yosprala: coordinated delivery of a proton pump inhibitor and aspirin. J Pharm Technol. 2020;36:78–83.
Khodayar S, Bardania H, Shojaosadati SA, et al. Optimization and characterization of aspirin encapsulated nano-liposomes. Iran J Pharm Res. 2018;17:11–22.
Mollace R, Gliozzi M, Macrì R. Efficacy and safety of novel aspirin formulations: a randomized, double-blind, placebo-controlled study. Pharmaceutics. 2022;14:187.
Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387–99.
Christiansen M, Grove EL, Hvas A-M. Primary prevention of cardiovascular events with aspirin: toward more harm than benefit—a systematic review and meta-analysis. Semin Thromb Hemost. 2019;45:478–89.
Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219.
Harrington D, D’agostino RB Sr, Gatsonis C, et al. New guidelines for statistical reporting in the journal. N Engl J Med. 2019;381:285–6.
Bangalore S, Kumar S, Wetterslev J, et al. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011;342: d2234.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
As this was a systematic review of meta-analyses, no ethical approval was required.
Funding
The authors received no support from any organizations for the submitted work.
Conflict of interest
Mingming Wang, Haijie Yu, Zuojing Li, Daxin Gong, and Xiaoxi Liu have no financial or proprietary interests in any material discussed in this article.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors
Consent to participate
Not applicable (meta-analysis).
Consent for publication
Not applicable.
Code availability
Not applicable.
Data availability
The authors confirm that the data supporting the findings of this study are available in the article and its online supplementary material.
Author contributions
MW and HY were engaged in the design of the study, interpretation of the data, statistical analyses, and drafting of the manuscript. ZL and DG were responsible for data extraction, statistical analyses, interpretation of the data, and administrative and technical support. MW and XL were responsible for the conception and critical revision of the manuscript. All authors read and approved the final version of manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, M., Yu, H., Li, Z. et al. Benefits and Risks Associated with Low-Dose Aspirin Use for the Primary Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Control Trials and Trial Sequential Analysis. Am J Cardiovasc Drugs 22, 657–675 (2022). https://doi.org/10.1007/s40256-022-00537-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00537-6