Abstract
Background
Although angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have been recommended for patients with heart failure, their clinical and prognostic impact in the very acute phase of acute heart failure (AHF) is unclear, mainly because data on their safety and efficacy are lacking.
Methods
This study was a post hoc analysis of the REALITY-AHF trial. Patients with AHF who did not take an ACEI or ARB at admission were enrolled. Patients who received these medications within 48 h of admission were categorized as the ACEI/ARB group, and all other patients were categorized as the no ACEI/ARB group. The primary endpoint was a composite of all-cause death and heart failure readmission within 1 year of admission.
Results
Of the 1682 patients in the REALITY-AHF cohort, 900 were enrolled in this study, and 288 (32%) were included in the ACEI/ARB group. After propensity score matching, 152 pairs were evaluated, and no significant difference was found for in-hospital mortality, worsening renal function, or length of hospital stay. The ACEI/ARB group had significantly higher event-free survival (hazard ratio 0.51; 95% confidence interval 0.32–0.82; p = 0.006).
Conclusions
Early initiation of ACEIs/ARBs within 48 h of admission for hospitalized patients with AHF was not associated with adverse events and correlated with improved outcomes at 1 year from admission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The safety and efficacy of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) in the very acute phase of acute heart failure (AHF) is unclear. |
Early initiation of ACEIs/ARBs correlated with improved outcomes at 1 year in patients with AHF. |
Early initiation of ACEIs/ARBs was not associated with adverse events, including worsening renal function. |
1 Introduction
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have traditionally been the cornerstones of heart failure treatment, especially in patients with reduced ejection fraction [1,2,3]. However, most of the studies that have shown beneficial effects tested the drugs in chronic heart failure; very little is known about the effect on outcomes of starting ACEIs/ARBs in the very acute phase of acute heart failure (AHF) in patients not receiving these medications at admission. It could be that early treatment with ACEIs/ARBs can be beneficial in terms of possibly improving hemodynamic status given that activation of the renin–angiotensin–aldosterone system is one of the key mechanisms of AHF, and some small studies in severe heart failure have shown hemodynamically beneficial effects from early administration of intravenous captopril [4, 5]. On the other hand, this hypothesis has not been well-supported in the myocardial infarction population, and concerns have arisen regarding hypotension and renal dysfunction, which have not been directly evaluated in an AHF population [6, 7]. Therefore, we investigated the safety and clinical implications of starting ACEIs/ARBs in the very acute phase of AHF.
2 Methods
2.1 Study Design and Patients
This is a post hoc analysis of the REALITY-AHF (Registry Focused on Very Early Presentation and Treatment in Emergency Department of Acute Heart Failure) trial, the study design and results of which have been published elsewhere [8,9,10,11,12]. Briefly, 1682 consecutive patients aged ≥ 20 years diagnosed with AHF through the emergency departments of 20 participating hospitals in Japan were registered and followed-up. The primary objective of the REALITY-AHF trial was to investigate the prognostic impact of time to treatment in the acute phase of AHF. AHF was diagnosed using the Framingham criteria [13]. This study was conducted according to the Declaration of Helsinki and Japanese Ethical Guideline for Medical and Health Research involving Human Subjects. The study protocol was approved by the ethics committee of each participating hospital, and study information was registered in the publicly available University Hospital Information Network (unique identifier: UMIN000014105) before the first patient was enrolled. We prospectively followed-up patients at least every 3 months after discharge up to December 2016 to obtain prognostic data. For those without follow-up at each participating hospital, prognostic data were collected via telephone interviews. Readmission event was defined as heart failure readmission only if it fulfilled the criteria for heart failure readmission described in the American College of Cardiology/American Heart Association Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials [14].
The present analysis focused on the clinical and prognostic impact of the very early introduction of ACEIs/ARBs within 48 h of hospital presentation in patients with AHF who were not receiving ACEIs/ARBs before admission. We divided the study cohort in two different ways. First, patients were divided into two groups according to whether they were treated with ACEIs/ARBs within 48 h of hospital presentation (ACEI/ARB group) or not (no ACEI/ARB group) (two-group comparison). To further assess whether early (within 48 h of admission) treatment with ACEIs/ARBs was associated with better prognosis than late (> 48 h of admission) treatment, we divided the entire cohort into either early ACEI/ARB, late ACEI/ARB, or no ACEI/ARB groups (three-group comparison) and compared them in terms of prognosis.
Estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [15]. The primary endpoint was a composite of all-cause death and heart failure readmission within 1 year of admission, and the secondary endpoint was safety outcomes, including worsening renal function (WRF) within 5 days after admission, defined as absolute increase in serum creatinine > 0.3 mg/dl and > 25% from baseline, length of hospital stay, and in-hospital mortality [16]. Trajectories of blood pressure were also compared between groups.
2.2 Statistical Methods
Data are expressed as mean ± standard deviation (SD) for normally distributed variables and as median (interquartile range [IQR]) for non-normally distributed data. Categorical data are expressed as numbers (%). When necessary, variables were transformed for further analyses. Between-group differences were evaluated using the Student’s t test or the Mann–Whitney U test for continuous variables, and Chi squared or Fisher’s exact tests for categorical variables. Cumulative incidence curves for the composite endpoint were calculated using Kaplan–Meier estimates and analyzed with the log-rank test. Cox regression analyses were performed to evaluate the association between the very early introduction of ACEIs/ARBs and the primary endpoint. To evaluate the difference in the longitudinal trajectory of blood pressure over time between groups with and without ACEI/ARB treatment within 48 h while accounting for the within-individual correlation of repeated blood pressure measurement, we used a linear mixed-effect model. A subject factor was included as a random effect, and time was modeled linearly.
To control confounding as much as possible, propensity score matching was performed. The propensity score was estimated based on a logistic model constructed with the following variables: age; sex; systolic blood pressure; history of hypertension, diabetes mellitus and coronary artery disease; left ventricular ejection fraction (LVEF); prescription of diuretics and β-blocker at admission; sinus rhythm on electrocardiogram at admission; white blood cell counts; hemoglobin; glucose levels; blood urea nitrogen; eGFR; and B-type natriuretic peptide (BNP), which were measured at admission. Propensity score matching was performed for the ACEI/ARB and no ACEI/ARB groups (two-group comparison) with one-to-one caliper matching using a caliper width equal to 20% of the SD of the logit of the calculated propensity score [17]. To assess the performance of the matching, standardized mean differences were calculated for all baseline variables, and a difference < 0.1 was considered negligible (i.e., the two groups were well-balanced) [18]. The variables used in the Cox regression model (chosen based on previous literature) as adjustment variables were age, sex, systolic blood pressure, weight, New York Heart Association class, history of heart failure, history of chronic obstructive lung disease, LVEF, β-blocker prescription, and creatinine, blood urea nitrogen, serum sodium, and BNP levels.
A two-sided p value < 0.05 was considered statistically significant. Statistical analyses were performed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
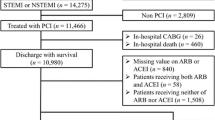
Of the 1682 patients in the REALITY-AHF cohort, 900 patients were naïve to ACEIs/ARBs at the time of admission, and 288 (32%) patients were treated with ACEIs/ARBs within 48 h of hospital arrival (Fig. 1 in the Electronic Supplementary Material [ESM]).
First, we performed a two-group comparison, and the baseline characteristics of both groups before and after propensity score matching are shown in Table 1. Before matching, patients in the ACEI/ARB group were younger, were predominantly male, had higher blood pressure, were more likely to have de novo heart failure, and were less likely to have been previously treated with heart failure drugs. Regarding biomarker profiles, their white blood cell count, hemoglobin, and glucose levels were higher, BNP levels were lower, and renal function was better. In the propensity score analysis, 304 patients were matched based on the propensity score, and these differences in patient background were well-balanced in the matched cohort, and standardized mean differences between the two groups were < 0.1 for all variables (Fig. 2 in the ESM). Of note, the use of intravenous drugs that had the potential to affect blood pressure (i.e., catecholamine and vasodilators) was also well-balanced between the groups.
During the study period, we observed 49 in-hospital deaths, 148 WRF within 5 days, and 326 all-cause deaths or heart failure readmission within 1 year of admission. The median length of hospital stay was 16 days (interquartile range [IQR] 10–25 days).
No difference in incidence of WRF within 5 days and length of hospital stay was observed between the groups, whereas in-hospital mortality was significantly higher in the no ACEI/ARB group before matching (Table 2). However, this difference was not retained in the matched cohort, and there was no significant between-group difference with respect to in-hospital mortality, incidence of WRF, and length of hospital stay.
Figure 1 shows the trajectories of blood pressure levels between the ACEI/ARB and no ACEI/ARB groups in the matched cohort. With the linear mixed-effects model, we found no significant between-group difference in either systolic or diastolic blood pressure (p = 0.210 and 0.447, respectively).
Regarding 1-year outcomes, there was a significant between-group difference in the Kaplan–Meier analysis (log-rank: p < 0.001) before matching, and this finding was retained even in the matched cohort (log-rank: p = 0.005) (Fig. 2). Cox regression analysis showed that the ACEI/ARB group was associated with better event-free survival, both before (hazard ratio [HR] 0.43; 95% confidence interval [CI] 0.33–0.57, p < 0.001) and after (HR 0.51; 95% CI 0.32–0.82; p = 0.006) propensity score matching.
We also checked the interaction between heart failure phenotypes, defined by ejection fraction, and ACEI/ARB treatment within 48 h of admission on prognosis and found no statistically significant impact (p for interaction = 0.393).
Next, we performed a three-group comparison by dividing the entire cohort into three groups: early ACEI/ARB, late ACEI/ARB, and no ACEI/ARB. There were several differences in baseline characteristics between the early ACEI/ARB and late ACEI/ARB groups. The early ACEI/ARB group was associated with high blood pressure, fewer patients with a history of heart failure, more patients with a history of hypertension, and fewer patients with coronary artery disease than was the late ACEI/ARB group (Table 3). Fewer patients in the early ACEI/ARB group were receiving heart failure medications at the time of admission and had preserved renal function than those in the late ACEI/ARB group. Kaplan–Meier analysis indicated a significant difference in the 1-year outcomes among the three groups (Fig. 3). In univariate Cox regression analysis using the early ACEI/ARB group as a reference, only the no ACEI/ARB group (HR 3.11; 95% CI 2.21–4.37; p < 0.001) but not the late ACEI/ARB group (HR 1.31; 95% CI 0.84–2.03; p = 0.233) was associated with significantly worse prognosis. However, both the no ACEI/ARB group (HR 2.54; 95% CI 1.50–4.31; p < 0.001) and the late ACEI/ARB group (HR 1.99; 95% CI 1.11–3.56; p = 0.020) were associated with a worse prognosis than was the early ACEI/ARB group after adjustment for covariates in the multivariable Cox regression analysis.
4 Discussion
In the present study, we investigated the clinical and prognostic impacts of initiating ACEI/ARB therapy in the very acute phase of AHF. We found that initiating treatment with ACEIs/ARBs within 48 h was not associated with short-term adverse outcomes, including WRF. Moreover, it was associated with improvement in 1-year prognosis. To the best of our knowledge, this is the first study to show the clinical and prognostic implications of ACEI/ARB treatment in the very acute phase of AHF in patients naïve to these drugs.
ACEIs/ARBs have been one of the cornerstones of heart failure treatment; however, the optimal timing of starting ACEI/ARB in hospitalized patients with AHF not receiving ACEI/ARB treatment at the time of admission is unclear [1,2,3, 19]. Masoudi et al. [20] retrospectively investigated medications prescribed at discharge in 17,456 patients with heart failure with reduced ejection fraction (HFrEF) who were hospitalized for AHF, and demonstrated that patients receiving ACEIs at discharge were associated with better 1-year survival. Sanam et al. [21] conducted a similar retrospective study using propensity score matching analysis and showed lower 30-day and 1-year mortality after discharge in hospitalized patients with AHF receiving ACEIs/ARBs at the time of discharge. Although these studies clarified the importance of starting ACEI/ARB treatment before discharge in patients hospitalized with AHF, especially those with reduced LVEF, when ACEI/ARB treatment should be started after admission remained unclear. The current American guidelines provide no specific recommendations regarding this question, and the current European guidelines state that “every attempt should be made to initiate ACEI/ARB after hemodynamic stabilization” [22, 23]. Likewise, no obvious statement has been made for the use of ACEIs/ARBs in the context of AHF.
The major concerns about starting ACEI/ARB treatment in the acute phase are hypotension and WRF, which might lead to subsequent poor prognosis [24, 25]. In healthy subjects, the kidneys can usually maintain renal perfusion pressure with its auto-regulatory response even when systemic blood pressure changes considerably. The key mechanism of this regulatory system is balancing the constriction of afferent and efferent arteriole, and angiotensin II is one of the vasopressor substances to these arterioles [26]. However, this compensatory system is blunted in patients with heart failure, and a decrease in systemic blood pressure can be directly associated with a reduction in renal blood flow and subsequent decline in glomerular filtration rate (GFR) [27]. Indeed, some studies have demonstrated an association between hypotension in the acute phase and WRF in patients with AHF [28, 29]. Moreover, blockage of angiotensin II by ACEIs/ARBs in the acute phase could accelerate decreasing perfusion pressure, resulting in declining GFR. However, our study showed that starting ACEI/ARB treatment within 48 h of arrival in the emergency department was not associated with WRF within 5 days after admission. However, this finding should be interpreted cautiously, as the number of subjects reduced substantially after propensity score matching. Moreover, the analysis may not be adequately powered to detect the association between ACEI/ARB use in the acute phase and short-term adverse events.
Another unexpected result is that we did not find between-group differences in the trajectories of blood pressure changes in 48 h of admission. This is contrary to our expectations; however, one possible explanation is that hypotension induced by introducing ACEI/ARB treatment is known to be more likely in patients with lower intravascular volume [30, 31]. Patients with AHF who need to be hospitalized are usually likely to have volume overload, and this might make the difference in blood pressure changes discernible. Another reason might be the small doses of ACEIs/ARBs used in the acute phase; however, we could not analyze this possibility as we did not obtain any information on the ACEI/ARB dose prescribed in our registry. In any case, it could be that the unexpected lack of difference in blood pressure changes led to the lack of difference in WRF incidence between groups. However, this finding and hypothesis both need to be evaluated in a randomized controlled trial.
Our study results demonstrated that the early prescription of ACEIs/ARBs is associated with improved 1-year prognosis after admission. It could be that early initiation of these medications avoided situations in which clinicians simply forgot to prescribe these medications. However, it is notable that patients who initiated ACEIs/ARBs within 48 h of admission were associated with a better prognosis than were those who started ACEIs/ARBs at > 48 h after admission. This result implies that the timing of starting ACEIs/ARBs may affect the prognosis of patients with AHF. Another finding that should be acknowledged is that the prognostic impact of early prescription of ACEIs/ARBs was consistent irrespective of whether the patient had heart failure with preserved ejection fraction (HFpEF) or HFrEF, although previous large trials demonstrated that the prognostic benefit of ACEI/ARB treatment was more evident in patients with HFrEF than in those with HFpEF [32, 33]. This might be because too few patients were enrolled to enable us to assess the statistical significance of the interaction. One possibility is that the mechanism of the prognostically beneficial effects of ACEIs/ARBs can differ between chronic heart failure and AHF. Given that no study has focused on the prognostic role of ACEIs/ARBs prescribed in the acute phase in AHF, further study is warranted.
Several limitations should be acknowledged. This analysis was not predefined, post hoc analysis of the REALITY-AHF study in which the association between time to AHF treatments and clinical outcomes in patients with AHF were investigated. Even though we tried to eliminate bias as much as possible by using propensity score matching analysis, patients who were treated with ACEIs/ARBs in the acute phase might have been more stable and the heart failure more mild than those without, and there may have been unmeasured confounding factors that were not taken into account. We evaluated only patients with AHF who had not taken ACEIs/ARBs before admission, and whether patients with AHF who had already taken ACEIs/ARBs should keep taking these medications in the very acute phase was unclear. Moreover, we did not collect data on exactly when ACEIs/ARBs were prescribed, only whether the patient received ACEIs/ARBs within 48 h.
5 Conclusion
Administration of ACEIs/ARBs within 48 h of admission for hospitalized patients with AHF who are not already receiving these drugs may be safe and correlate with improved outcomes at 1 year.
References
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35.
SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302.
Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1992;327:669–77.
Gheorghiade M, Hall V, Lakier JB, Goldstein S. Comparative hemodynamic and neurohormonal effects of intravenous captopril and digoxin and their combinations in patients with severe heart failure. J Am Coll Cardiol. 1989;13:134–42.
Annane D, Bellissant E, Pussard E, Asmar R, Lacombe F, Lanata E, et al. Placebo-controlled, randomized, double-blind study of intravenous enalaprilat efficacy and safety in acute cardiogenic pulmonary edema. Circulation. 1996;94:1316–24.
Sigurdsson A, Swedberg K. Left ventricular remodelling, neurohormonal activation and early treatment with enalapril (CONSENSUS II) following myocardial infarction. Eur Heart J. 1994;15(Suppl B):14–9 (discussion 26–30).
Latini R, Tognoni G, Maggioni AP, Baigent C, Braunwald E, Chen ZM, et al. Clinical effects of early angiotensin-converting enzyme inhibitor treatment for acute myocardial infarction are similar in the presence and absence of aspirin: systematic overview of individual data from 96,712 randomized patients. Angiotensin-converting. J Am Coll Cardiol. 2000;35:1801–7.
Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, et al. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:3042–51.
Kondo T, Okumura T, Matsue Y, Shiraishi A, Kagiyama N, Yamaguchi T, et al. Specialty-related differences in the acute-phase treatment and prognosis in patients with acute heart failure—insights from REALITY-AHF. Circ J. 2018;83:174–81.
Kuroda S, Damman K, Ter Maaten JM, Voors AA, Okumura T, Kida K, et al. Very early diuretic response after admission for acute heart failure. J Card Fail. 2019;25:12–9.
Yamaguchi T, Kitai T, Miyamoto T, Kagiyama N, Okumura T, Kida K, et al. Effect of optimizing guideline-directed medical therapy before discharge on mortality and heart failure readmission in patients hospitalized with heart failure with reduced ejection fraction. Am J Cardiol. 2018;121:969–74.
Kagiyama N, Kitai T, Hayashida A, Yamaguchi T, Okumura T, Kida K, et al. Prognostic value of BNP reduction during hospitalization in patients with acute heart failure. J Card Fail. 2019;1:1. https://doi.org/10.1016/j.cardfail.2019.04.004.
Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15.
Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. Circulation. 2015;132:302–61.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–95.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314:1637.
SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–91.
Masoudi FA, Rathore SS, Wang Y, Havranek EP, Curtis JP, Foody JM, et al. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724–31.
Sanam K, Bhatia V, Bajaj NS, Gaba S, Morgan CJ, Fonarow GC, et al. Renin-angiotensin system inhibition and lower 30-day all-cause readmission in medicare beneficiaries with heart failure. Am J Med. 2016;129:1067–73.
WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18:891–975.
Cleland JG, Dargie HJ, Ball SG, Gillen G, Hodsman GP, Morton JJ, et al. Effects of enalapril in heart failure: a double blind study of effects on exercise performance, renal function, hormones, and metabolic state. Br Heart J. 1985;54:305–12.
Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, et al. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J. 2013;77:687–96.
Yuan BH, Robinette JB, Conger JD. Effect of angiotensin II and norepinephrine on isolated rat afferent and efferent arterioles. Am J Physiol Physiol. 1990;258:F741–50.
Schroten NF, Damman K, Hemmelder MH, Voors AA, Navis G, Gaillard CAJM, et al. Effect of additive renin inhibition with aliskiren on renal blood flow in patients with Chronic Heart Failure and Renal Dysfunction (Additive Renin Inhibition with Aliskiren on renal blood flow and Neurohormonal Activation in patients with Chronic Heart Failure and Renal Dysfunction). Am Heart J. 2015;169(693–701):e3.
Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G, et al. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre-RELAX-AHF. Eur J Heart Fail. 2011;13:961–7.
Cotter G, Metra M, Davison BA, Jondeau G, Cleland JGF, Bourge RC, et al. Systolic blood pressure reduction during the first 24 h in acute heart failure admission: friend or foe? Eur J Heart Fail. 2018;20:317–22.
Hodsman GP, Isles CG, Murray GD, Usherwood TP, Webb DJ, Robertson JI. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286:832–4.
Panzenbeck MJ, Loughnan CL, Madwed JB, Winquist RJ, Fogal SE. Captopril-induced hypotension is inhibited by the bradykinin blocker HOE-140 in Na(+)-depleted marmosets. Am J Physiol. 1995;269:H1221–8.
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81.
Zhang Q, Chen Y, Liu Q, Shan Q. Effects of renin–angiotensin–aldosterone system inhibitors on mortality, hospitalization, and diastolic function in patients with HFpEF. Herz. 2016;41:76–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partly supported by a Grant-in-Aid for Early-Career Scientists (Grant no. 18K15862) and The Cardiovascular Research Fund, Tokyo, Japan.
Conflict of interest
Dr. Yuya Matsue has received an honorarium and grants from Otsuka Pharmaceutical Co. Kenji Yoshioka, Tetsuo Yamaguchi, Takeshi Kitai, Nobuyuki Kagiyama, Takahiro Okumura, Keisuke Kida, Shogo Oishi, Eiichi Akiyama, Satoshi Suzuki, Masayoshi Yamamoto, Shunsuke Kuroda, Akihiko Matsumura, and Kenzo Hirao have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshioka, K., Matsue, Y., Yamaguchi, T. et al. Safety and Prognostic Impact of Early Treatment with Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers in Patients with Acute Heart Failure. Am J Cardiovasc Drugs 19, 597–605 (2019). https://doi.org/10.1007/s40256-019-00355-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-019-00355-3