Abstract
Sebelipase alfa (Kanuma®, Kanuma™), the first commercially available recombinant human lysosomal acid lipase (LAL), is approved in various countries worldwide, including those of the EU, the USA and Japan, as a long-term enzyme replacement therapy for patients diagnosed with LAL deficiency (LAL-D), an ultra-rare, autosomal recessive, progressive metabolic liver disease. In an ongoing study in nine infants presenting with early-onset LAL-D (Wolman disease), open-label treatment with sebelipase alfa significantly improved 1-year survival compared with historical controls. A substantial mortality benefit was maintained at 2 years of age, as was a reduction in disease-related activity. In an ongoing study of 66 children and adults with late-onset LAL-D (cholesteryl ester storage disease), 20 weeks’ double-blind treatment with sebelipase alfa significantly reduced multiple disease-related hepatic and lipid abnormalities compared with placebo. Sustained improvements in markers of liver damage and dyslipidaemia were seen after 76 weeks’ open-label treatment in an extension of this trial and, similarly, after 2 years’ open-label treatment in an extension of another study in nine adults with late-onset LAL-D. Sebelipase alfa therapy has thus far been generally well tolerated, with signs and symptoms consistent with anaphylaxis being the most serious adverse reactions experienced by patients receiving the drug in clinical trials. Due to the rarity of the disease, these studies have enrolled a limited number of patients. Nonetheless, the available data indicate that sebelipase alfa is an effective disease-specific therapy for individuals with LAL-D who have historically been managed using supportive therapies (e.g. cholesterol reduction, hematopoietic stem cell transplantation, and liver transplantation).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First recombinant human LAL |
Administered as an intravenous infusion once weekly or once every other week, depending on age |
Addresses underlying cause of LAL-D, correcting disease-related liver and lipid abnormalities |
Improved survival in infants with early-onset disease |
Some hypersensitivity reactions occurred in clinical trials; however, patients were not routinely premedicated |
1 Introduction
Lysosomal acid lipase (LAL) deficiency (LAL-D) is an ultra-rare, autosomal recessive, progressive metabolic liver disease caused by mutations in the LAL gene (LIPA) that markedly reduce LAL activity [1–3]. LAL is responsible for hydrolysing cholesteryl esters and triglycerides within low-density lipoprotein (LDL) cholesterol particles into free cholesterol and free fatty acids; LAL-D leads to lysosomal accumulation of cholesteryl esters and, to a lesser extent, triglycerides, predominantly in the liver, blood vessel walls and other organs [1–3].
LAL-D presents as a clinical continuum, with two major phenotypes: the early-onset variant (historically known as Wolman disease) and the late-onset variant (historically known as cholesteryl ester storage disease) [1–3]. Early-onset LAL-D is the result of a complete or near complete absence of LAL activity and occurs in infants (estimated incidence 1 in 500,000 live births [3]). It is the most rapidly progressive form of the disease and is characterized by massive hepatosplenomegaly, malabsorption (due to diarrhoea and vomiting), growth retardation, liver failure and, in approximately half of all cases, adrenal calcification, with patients rarely surviving beyond 6 months of age [1–3].
In contrast, later-onset LAL-D is the result of a partial loss of LAL activity and presents post-infancy (estimated incidence between 1 in 40,000 and 1 in 400,000 individuals [2, 4]). It has a more variable clinical course than early-onset LAL-D, with some patients remaining asymptomatic (or asymptomatic until adulthood) [1–3]. Typical clinical features of LAL-D in children and adults include elevated serum transaminases, hepatomegaly, microvesicular hepatosteatosis on biopsy and dyslipidaemia, with progressive liver damage (i.e. fibrosis leading to cirrhosis and, eventually, liver failure) and accelerated atherosclerosis (secondary to the chronic hyperlipidaemia) contributing to premature demise in some patients [1–3]. In part because many of the typical clinical manifestations of late-onset LALD are shared with other more prevalent cardiovascular, liver and metabolic diseases (e.g. familial hypercholesterolaemia) [1, 2]), this condition is currently both under-recognised and under-diagnosed [1–3]. A screening algorithm for LAL-D has been proposed and discussed in detail elsewhere [2]; diagnostic suspicion can be confirmed by demonstration of markedly deficient LAL activity or mutations in the LIPA gene [1, 2].
Historically, in the absence of any disease-specific treatments, the management of LAL-D has focused on supportive therapies to reduce the burden of disease complications [1–3]. Such interventions have included liver transplantation for liver failure, hematopoietic stem cell transplantation (HSCT) in infants with early-onset LAL-D, and the use of lipid-lowering drugs [e.g. HMG-coA reductase inhibitors (statins)] to control hypercholesterolaemia in children and adults with late-onset LAL-D. Although liver transplantation has been effective in providing relief from liver failure, extrahepatic organ involvement has resulted in significant disease burden and, in some patients, premature demise. HSCT has been performed in a few infants. However, this approach has been associated with serious complications; most attempts have resulted in early death. Statins, alone or in combination with other lipid-lowering agents, have reduced LDL cholesterol levels in many patients, but have little, if any, effect on liver abnormalities and progressive damage [1–3]. As these therapies do not successfully address the multi-system nature of the disease and are associated with poor treatment outcomes, there is a need for novel interventions that target the underlying defect in LAL-D [2].
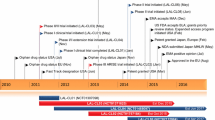
Against this background, the potential of enzyme replacement therapy (ERT) as a strategy to treat LAL-D has long been recognized, although attempts to develop an effective agent have hitherto proved unsuccessful [5, 6]. Sebelipase alfa (Kanuma®, Kanuma™) is the first commercially available recombinant human LAL (rhLAL); it has been approved for long-term ERT in patients of all ages with LAL-D in various countries worldwide, including those of the EU [7], the USA [8] and Japan [9]. This narrative review summarizes the pharmacological properties of sebelipase alfa and discusses therapeutic efficacy and tolerability data pertaining to its use in infants, children and adults diagnosed with LAL-D.
2 Pharmacodynamic Properties of Sebelipase Alfa
Sebelipase alfa, a rhLAL produced in and purified from egg whites of transgenic hens, has the same amino acid sequence as the native human enzyme [5, 10]. It acts to replace the deficient LAL enzyme, catalysing the lysosomal hydrolysis of cholesteryl esters and triglycerides to free cholesterol, glycerol and free fatty acids, reducing lysosomal lipid accumulation and thereby improving the liver abnormalities and dyslipidaemia associated with LAL-D [5, 10].
Sebelipase alfa has N-linked glycans with terminal N-acetylglucosamine and mannose structures, as well as mannose-6-phosphate moieties [11]. Cellular uptake of fluorescently-labelled sebelipase alfa into macrophages and fibroblasts via mannose and mannose-6-phosphate receptors, respectively, has been demonstrated in vitro [11]; localization to lysosomes has been confirmed using confocal microscopy [11]. Normalization of LAL enzymatic activity was demonstrated in fibroblasts from a patient with LAL-D [11].
Studies using a preclinical (rodent) model of LAL-D showed that intravenously administered sebelipase alfa corrected abnormalities associated with this condition, as evidenced by reductions in liver lipid content [12–14], size [12, 13] and histopathology [12, 13, 15, 16], reductions in spleen and jejunum size [13], reductions in serum transaminase levels [12], and increases in body weight [13].
In a phase I/II clinical trial in nine adult patients with LAL-D (LAL-CL01) [6], intravenous sebelipase alfa (four once-weekly infusions of 0.35, 1 or 3 mg/kg) was associated with significant (p ≤ 0.05) mean reductions from baseline to day 28 in levels of alanine aminotransferase (ALT; 41%) and aspartate aminotransferase (AST; 32%). Consistent with the hypothesis that the drug can rapidly mobilize abnormal lysosomal lipids from affected tissues, mean serum levels of total cholesterol, triglycerides and LDL cholesterol increased by 70, 69 and 87%, respectively, from baseline to day 28 [6]. However, this increase was transient; mean serum levels of these lipid parameters subsequently improved [lower LDL cholesterol, total cholesterol and triglycerides, higher high-density lipoprotein (HDL) cholesterol] from original baseline levels in LAL-CL04, an extension to LAL-CL01 [6] (Sect. 4.2).
3 Pharmacokinetic Properties of Sebelipase Alfa
The pharmacokinetics of intravenous sebelipase alfa appear to be non-linear, with greater than dose-proportional increases in exposure observed between between 1 and 3 mg/kg doses in adults (based on a noncompartmental analysis of data from the LAL-CL01/LAL-CL04 studies; Sects. 2 and 4.2) and between 0.35 and 3 mg/kg doses in infants aged <6 months (based on data from the LAL-CL03 study; Sect. 4.1) [7]. No accumulation was seen with sebelipase alfa dosages of 1 mg/kg once weekly or once every other week, or with 3 mg/kg once weekly [7].
Mean pharmacokinetic parameters for sebelipase alfa in adults and children with LAL-D have been estimated using a population pharmacokinetic analysis of 65 patients who received the drug at a dosage of 1 mg/kg once every other week in the LAL-CL02 study (Sect. 4.2) [7]. According to this model, the area under the sebelipase alfa plasma concentration-time curve at steady-state was 0.9–1.1, 1.4–1.5 and 1.9–2.0 μg h/mL in patients aged 4–11, 12–17 and ≥18 years, respectively (n = 24, 23 and 18). Maximum plasma concentrations of sebelipase alfa (0.5–0.6, 0.7–0.8 and 1.0–1.1 μg/mL in patients aged 4–11, 12–17 and ≥18 years, respectively) were reached in approximately 1.1–1.4 h. The central volume of distribution (V c ) ranged from 3.3–5.5 L, clearance ranged from approximately 29–38 L/h and the elimination half-life was 0.1 h. Neither Vc nor clearance were significantly influenced by age, body weight or sex [7].
Similar to that in adults and children, the elimination half-life of sebelipase alfa ranged from 0.1–0.2 h in infants (n = 4) who received the drug at a dosage of 3 mg/kg once weekly in the LAL-CL03 study [7].
Since sebelipase alfa is a recombinant human protein, it is expected to be degraded through protein hydrolysis [7]. It is also expected to be an unlikely candidate for cytochrome P450 or other drug–drug interactions [7].
4 Therapeutic Efficacy of Sebelipase Alfa
4.1 Early-Onset Lysosomal Acid Lipase Deficiency (LAL-D)
The efficacy of sebelipase alfa in infants with early-onset LAL-D was evaluated in an open-label, single-arm, multinational, phase 2/3 study (LAL-CL03; hereafter referred to as VITAL) [17]. To date, results from this ongoing study have been published in preliminary form (as abstracts [18–20]) and/or reported on a clinical trials registry [17], with some trial design details available from product labelling (in the EU [7] and USA [8]).
VITAL enrolled nine infants (five males and four females) with LAL-D (confirmed by reduced enzyme activity or by genetic testing) and growth failure or other evidence of rapidly progressive disease within the first 6 months of life. The median age at study entry was 3.0 months (range 1.1–5.8 months) [17]. All participants had significant liver dysfunction, as evidenced by elevated levels of ALT (median 145 U/L) and AST (median 125 U/L) at baseline [7, 8]; eight had early growth failure [20].
Patients received sebelipase alfa 0.2 mg/kg (n = 1) or 0.35 mg/kg (n = 8) once weekly for the first 2 weeks and then 1 mg/kg once weekly. Due to suboptimal clinical response, all (surviving) patients underwent dose escalation to 3 mg/kg once weekly between 4 and 88 (median 11) weeks after starting treatment at 1 mg/kg once weekly; one patient underwent dose escalation to 5 mg/kg once weekly at week 88 [7, 8].
Sebelipase alfa therapy appeared to be associated with substantial improvements in survival and clinically meaningful improvements in multiple disease activity parameters to beyond 1 [18, 19] and 2 [20] years of age in infants with early-onset LAL-D. The primary endpoint of survival at 12 months of age was met by six (67%) of the nine infants at the time of the data cut-off (June 2014) [18]. In comparison, the 12-month survival rate was 0% in a historical cohort of 21 untreated infants presenting with LAL-D who had similar clinical characteristics [21]. The median age at death among the three infants who did not meet the primary endpoint was 2.9 months (range 2.8–4.3 months). A fourth infant also died subsequent to meeting the primary endpoint (at age 15 months) [7]. None of these deaths were considered to be related to the drug (Sect. 5).
The remaining five patients have continued to receive sebelipase alfa [19]; as of the July 2015 data cut-off, all five had survived to beyond 2 years of age (range 2 years 5 months to 4 years 7 months), with a mean time in the trial of 33.8 months [20]. Consistent with findings from earlier data cut-off dates [18, 19], the surviving individuals had improvements from baseline in disease activity parameters, including ALT (median decrease of 45.6%), AST (median decrease of 39.4%), haemoglobin (median increase of 29.8%), and albumin (median increase of 11.8%) levels [20]. They also experienced reductions in hepatosplenomegaly (quantitative data not available) and improvements in weight gain (median weight percentile increased from 3.6% at baseline to 35.1% at data cut-off). Furthermore, 4 of the 5 patients scored ‘normal’ on their most recent Denver II developmental screening test; the fifth patient scored ‘suspect’ [20].
4.2 Late-Onset LAL-D
The efficacy of sebelipase alfa in children and adults with late-onset LAL-D was evaluated in a randomized, double-blind, placebo-controlled, multinational, phase 3 study (LAL-CL02; hereafter referred to as ARISE) [22]. Results from this ongoing study have been published in full [22] or in preliminary form (as abstracts [23–26]). The long-term efficacy of sebelipase alfa is also being assessed in LAL-CL04, an ongoing, open-label, multicenter extension of the LAL-CL01 study in adults with LAL-D [6] (Sect. 2).
In ARISE, 66 patients (33 males and 33 females) aged ≥4 years with LAL-D (confirmed by reduced enzyme activity) and an ALT level ≥1.5 × the upper limit of normal (ULN) [34 U/L for females aged 4–69 and males aged 4–9 years; 43 U/L for males aged 10–69 years] received sebelipase alfa 1 mg/kg (n = 36) or placebo (n = 30) once every other week for 20 weeks (11 infusions) [22]. The median age at randomization was 13 years (range 4–58 years); median ALT and AST levels were 90 (range 52–212) U/L and 74.5 (range 41–173) U/L in the sebelipase alfa group, and 86.5 (range 50–237) U/L and 71.0 (range 39–220) U/L in the placebo group. Of the patients who had liver biopsies at baseline (n = 32), all had fibrosis and approximately one-third (31%) had cirrhosis [22]. One-half (50%) of sebelipase alfa recipients and two-thirds (67%) of placebo recipients had an LDL cholesterol level ≥190 mg/dL; ≈40% of patients were receiving ≥1 lipid-lowering medication [22].
Sebelipase alfa therapy resulted in a reduction in multiple disease-related hepatic and lipid abnormalities in children and adults with LAL-D [22]. At the end of the 20-week double-blind period, sebelipase alfa recipients had significantly (p < 0.001) greater mean reductions from baseline in ALT (58 vs. 7 U/L) and AST (42 vs. 6 U/L) levels than placebo recipients, and a significantly (p ≤ 0.03) higher proportion of sebelipase alfa recipients achieved normalization of ALT levels (the primary endpoint) and normalization of AST levels (a key secondary endpoint) [22] [Fig. 1]. Decreases in aminotransferase levels were accompanied by a relative reduction in hepatic fat content (as measured by magnetic resonance imaging) of 32% in the sebelipase alfa group versus 4% in the placebo group (p < 0.001). Among patients with paired liver biopsies at baseline and week 20, 10 (63%) of 16 treated with sebelipase alfa compared with 4 (40%) of 10 treated with placebo had an improvement (i.e. ≥5% reduction) in microvesicular steatosis; however, the between-group difference was not statistically significant [22]. The decrease in liver volume in sebelipase alfa-treated patients was almost fourfold greater than that in placebo-treated patients (10.3 vs. 2.7%). However, because this secondary endpoint immediately followed reduction in microvesicular steatosis in the prespecified hierarchy, the between-group difference could not be interpreted as being statistically significant [22].
Efficacy of 20 weeks’ sebelipase alfa therapy in terms of normalizing high levels of serum transaminases in the ARISE study in children and adults with lysosomal acid lipase deficiency [22]. One patient in the placebo group had a normal aspartate aminotransferase level at baseline
Regarding serum lipid levels (all secondary endpoints), sebelipase alfa recipients had significantly greater mean reductions from baseline in LDL cholesterol (28 vs. 6%; p < 0.001), non-HDL cholesterol (28 vs. 7%; p < 0.001) and triglyceride (26 vs. 11%; p = 0.04) levels than placebo recipients, and a significantly greater mean increase from baseline in the HDL cholesterol level (20 vs. 0%; p < 0.001). Of note, decreases in the LDL cholesterol level were seen in sebelipase alfa recipients, irrespective of whether or not they were receiving ≥1 lipid-lowering medication at baseline (mean reductions from baseline of 37 and 23%, respectively) [22]. In addition to LDL cholesterol, a number of other key atherogenic biomarkers, including apolipoprotein A1, apolipoprotein B, and LDL particle number, were significantly (p ≤ 0.022 vs. placebo) improved from baseline with sebelipase alfa, regardless of concomitant lipid-lowering medication usage [23].
Sixty-five patients entered a subsequent open-label extension phase (up to 130 weeks) in which they initially received sebelipase alfa 1 mg/kg once every other week, with dose escalation permitted, based on clinical response [7, 22, 24–26]. Unless stated otherwise, the preliminary data reported herein are findings for all patients following 52 [24] or 76 [25] weeks’ exposure to the drug; baseline refers to the last reading before sebelipase alfa inception, i.e. at the beginning and the end of the 20-week double-blind period for patients originally randomized to sebelipase alfa and placebo, respectively.
During the open-label phase, patients originally randomized to sebelipase alfa maintained the improvements in disease-related hepatic and lipid abnormalities they achieved during the double-blind period, whereas those originally randomized to placebo had marked and sustained improvements that mirrored those seen in the sebelipase alfa group during the double-blind period [24, 25]. After 52 weeks’ exposure to sebelipase alfa, 47 and 56% of patients achieved ALT and AST normalization, respectively; 73 and 85% of patients reached an ALT and AST level ≤1.5 × ULN, respectively [24]. These outcomes were at least maintained after 76 weeks’ exposure to sebelipase alfa, with 52 and 65% of patients achieving ALT and AST normalization, respectively, and 87 and 95% of patients reaching an ALT and AST level ≤1.5 × ULN, respectively [24]. Mean LDL cholesterol, non-HDL cholesterol and triglyceride levels decreased from baseline by 28, 27, and 17%, respectively after 76 weeks’ treatment; the mean HDL cholesterol level increased from baseline by 23% [25].
Other observations from the open-label phase included reductions from baseline in hepatic fat content and liver volume of 25 and 13%, respectively, after 52 weeks’ exposure to sebelipase alfa [24]. In addition, liver fibrosis was assessed using the Ishak staging system at the week 52 visit, representing 52 weeks’ sebelipase alfa exposure for patients originally randomized to the drug and 30 weeks’ sebelipase alfa exposure for patients originally randomized to placebo [26]. Twenty patients had paired liver biopsies at baseline and week 52; eight had cirrhosis at baseline. Among the 12 patients with 52 weeks’ exposure to sebelipase alfa, eight had a reduction in fibrosis (six and two with a ≥2- and 1-stage reduction, respectively). In comparison, 4 of the 8 patients with 30 weeks’ exposure to sebelipase alfa had a 1-stage reduction in fibrosis. Additional data from 10 of the 20 patients indicated that two had a 1-stage reduction in fibrosis after 20 weeks’ exposure to sebelipase alfa. As such, reductions in fibrosis tended to increase with increasing sebelipase alfa exposure. One of the six patients with a ≥2-stage reduction and 5 of the 8 patients with a 1-stage reduction had cirrhosis at baseline [26].
Upon enrolment in LAL-CL04, eight patients aged 18–65 years with confirmed LAL-D and either hepatomegaly on physical examination or elevated transaminases (1.5 to 3 × ULN) who received four once-weekly infusions of sebelipase alfa 0.35, 1 or 3 mg/kg in LAL-CL01 received four further once-weekly infusions at the same dose before transitioning to every-other-week infusions of 1 mg/kg (if they received 0.35 or 1 mg/kg in LAL-CL01) or 3 mg/kg (if they received 3 mg/kg in LAL-CL01) [6, 27]. The median time between the last dose of sebelipase alfa in LAL-CL01 and the first dose in LAL-CL04 was 18 weeks (range 9–28 weeks) [27]. Results after 52 [27], 78 [28], 90 [29] and 104 [30] weeks’ follow-up in this long-term extension study have been published in full [27] or in preliminary form (as abstracts [28–30]).
Patients who re-initiated sebelipase alfa therapy in LAL-CL04 had rapid reductions in transaminase levels (similar to those seen in LAL-CL01), which were sustained during long-term dosing and were accompanied by improvement in the serum lipid profile [27–30]. At week 52, mean ALT and AST levels were normal, with mean reductions from baseline in LAL-CL01 of 58 and 40%, respectively (both p = 0.016). LDL cholesterol, total cholesterol and triglyceride levels were also decreased relative to baseline in LAL-CL01 (by 60, 39 and 36%, respectively; all p < 0.05), whereas the HDL cholesterol level had increased relative to baseline in LAL-CL01 (by 29%; p = 0.016) [27]. The corresponding changes in these disease activity parameters at subsequent visits, including at week 104, were of similar magnitude and, in general, were statistically significant [28–30].
5 Tolerability of Sebelipase Alfa
Intravenous sebelipase alfa therapy has so far been generally well tolerated in ongoing clinical trials [10, 31], with the proviso that the number of patients exposed to the drug, especially infants, remains limited.
Two pooled safety analyses of infants, children and adults participating in three clinical trials [VITAL, ARISE, and LAL-CL08 (n = 106) [7, 8, 10]; LAL-CL04, ARISE, and LAL-CL06 (n = 105) [31]] are available; one of these analyses is available as an abstract [31]. Treatment-emergent adverse events (TEAEs), the majority of which were mild to moderate in severity and not considered to be related to the study drug, were reported by 84 [10] and 93% [31] of sebelipase alfa recipients. Treatment-related adverse events (TRAEs) were reported by 29% of sebelipase alfa recipients [10]; infusion-associated reactions (IARs; defined as any TEAE that occurred during or within 4 h of completion of the infusion that was considered to be related to the study drug) occurred in 15% of sebelipase alfa-treated patients [10, 31]. Serious TEAEs occurred in 10 [31] and 18% [10] of patients receiving sebelipase alfa; the majority of these events were not considered to be related to the study drug [10]. To date, no patient receiving sebelipase alfa in a clinical trial has discontinued treatment due to a drug-related adverse event and no drug-related deaths have occurred [19, 20, 31].
The most serious adverse reactions in sebelipase alfa recipients were signs and symptoms consistent with anaphylaxis; these occurred in 3 (3%) of 105 patients in clinical trials, including 1 (7%) of 14 infants and 2 (2%) of 92 children and adults [7, 8]. Anaphylaxis occurred as early as the sixth infusion and as late as 1 year after treatment initiation [7, 8]. Overall, 21 (20%) sebelipase alfa-treated patients, including 9 (64%) of 14 infants and 12 (13%) of 92 children and adults, experienced signs and symptoms either consistent with or that may be related to a hypersensitivity reaction [7, 8]. The majority of reactions occurred during or within 4 h of the completion of the infusion. Of note, patients were not routinely pre-medicated prior to receiving sebelipase alfa in these studies [7, 8].
In VITAL, the most common adverse reactions (i.e. reported by ≥3 of the 9 infants receiving sebelipase alfa) were: diarrhoea and vomiting (six patients each); fever and rhinitis (five patients each); anaemia (four patients); and cough, nasopharyngitis and urticaria (three patients each) [8]. One infant experienced a serious TRAE (an IAR of tachycardia, pallor, chills and pyrexia) that resolved [18–20]. The four deaths that have occurred to date in this trial are deemed to be related to underlying disease (three) or complications of an abdominal paracentesis (one) [18, 19].
Thirty-one (86%) of 36 sebelipase alfa recipients and 28 (93%) of 30 placebo recipients experienced at least one adverse event during the 20-week double-blind period of ARISE [22]. The most common adverse events (i.e. reported by at least three sebelipase alfa recipients) were: headache (10 patients); pyrexia (seven patients); diarrhoea, oropharyngeal pain and upper respiratory tract infection (six patients each); epistaxis and nasopharyngitis (four patients each); and abdominal pain, asthenia, constipation, cough, nausea and vomiting (three patients each). The frequency and overall distribution of adverse events among sebelipase alfa recipients was similar to that among placebo recipients. Five (14%) sebelipase alfa-treated patients compared with six (20%) placebo-treated patients reported TRAEs; however, IARs were uncommon, occurring in two (6%) and four (13%) sebelipase alfa and placebo recipients. Serious TEAEs occurred in two (6%) sebelipase alfa recipients compared with one (3%) placebo recipient; one sebelipase alfa-treated patient experienced a serious TRAE (an atypical IAR) that resulted in dosing being paused [22].
The safety profile of sebelipase alfa during the open-label period of this trial was consistent with that of the drug in the double-blind period [22, 24, 25]. During 76 weeks’ exposure to the drug, TEAEs (most commonly headache, nasopharyngitis, cough, and pyrexia) were mostly mild to moderate in severity; no patient discontinued treatment due to a TEAE. Four patients experienced serious TEAEs, including one serious TRAE (an IAR) [25].
Anti-drug antibodies (ADAs) have been observed in patients treated with sebelipase alfa in clinical trials [7, 8, 20, 22]. ADAs usually developed within the first 2–3 months of exposure [7, 8] and seemed to occur more frequently in infants than in children or adults [7], although they were mostly transient [7, 8] and did not have any effect on safety (or efficacy) variables [22]. ADAs developed during treatment with sebelipase alfa in 4 (57%) of 7 evaluable infants in VITAL [20], 1 (20%) of 5 evaluable infants in LAL-CL08 [7] and 5 (14%) of 35 evaluable children and adults during the double-blind period of ARISE [22]. Three infants (two in VITAL and one in LAL-CL08) developed ADAs that inhibited both in vitro enzyme activity and cellular uptake of the enzyme [7]. In comparison, one patient developed ADAs during the double-blind period of ARISE that inhibited in vitro cellular uptake of the enzyme [7]; two additional patients developed neutralizing antibodies during the open-label period of this trial (after 20 and 52 weeks’ follow-up) [8, 25]. However, the presence of neutralizing ADAs has not impacted on the safety profile of sebelipase alfa [10].
No safety signals emerged, based on extensive evaluation of clinical laboratory data [10]. The transient and reversible hyperlipidaemia (likely due to lysosomal lipid mobilization) that occurs following initiation of treatment with sebelipase alfa (Sect. 2) is not associated with any clinical sequelae [10, 22].
6 Dosage and Administration of Sebelipase Alfa
Intravenous sebelipase alfa is indicated in various countries worldwide, including those of the EU [7], the USA [8] and Japan [9], for long-term ERT in patients of all ages with a diagnosis of LAL-D (Sect. 1). The recommended starting dosage of sebelipase alfa in infants with rapidly progressive disease presenting within the first 6 months of life is 1 mg/kg once weekly; dose escalation to 3 mg/kg should be considered, based on clinical response. The recommended starting dosage in children and adults who do not present with rapidly progressive LAL-D prior to 6 months of age is 1 mg/kg once every other week. No dose adjustment is recommended in patients with renal or hepatic impairment; no alternative dose regimens can be recommended in patients who are overweight (due to limited data) or older than 65 years (due to a lack of data) [7].
Local prescribing information should be consulted for detailed information, including instructions for administration, contraindications, special warnings and precautions and use in special patient populations.
7 Current Status of Sebelipase Alfa in the Management of LAL-D
Sebelipase alfa, the first treatment to specifically target the underlying cause of LAL-D, has been approved in the EU [7] and USA [8] for use in patients of all ages affected with this condition, based on results from two ongoing pivotal trials. In the pivotal VITAL study in infants with early-onset LAL-D, open-label treatment with sebelipase alfa significantly improved survival at 12 months of age compared with historical controls (Sect. 4.1). A substantial mortality benefit was maintained at 2 years of age, as was a reduction in disease-related activity (Sect. 4.1). In the pivotal ARISE study in children and adults with late-onset LAL-D, 20 weeks’ double-blind treatment with sebelipase alfa reduced multiple disease-related hepatic and lipid abnormalities compared with placebo (Sect. 4.2). Sustained improvements in markers of liver damage and dyslipidaemia were seen after 76 weeks’ open-label treatment in an extension of this trial and, similarly, after 2 years’ open-label treatment in an extension of another study in adults (LAL-CL04) (Sect. 4.2). Data pertaining to hard clinical outcomes (i.e. long-term progression of liver disease, cardiovascular events and mortality) in the patient population from the ARISE extension (Sect. 4.2) and other ongoing, open-label, long-term studies (see discussion below) are awaited with considerable interest [32].
To date, sebelipase alfa has been generally well tolerated, with signs and symptoms consistent with anaphylaxis being the most serious adverse reactions experienced by patients receiving the drug in ongoing clinical trials (who were not routinely premedicated) (Sect. 5). Due to the potential for anaphylaxis, appropriate medical support must be readily available when the drug is administered [7, 8]. Local prescribing information should be consulted for advice on managing suspected hypersensitivity reactions, including anaphylaxis. ADAs developed in a few patients receiving sebelipase alfa, albeit without affecting the apparent safety (and efficacy) of the drug (Sect. 5).
The long-term efficacy and safety of sebelipase alfa in the treatment of LAL-D are being investigated in a number of ongoing, open-label studies or open-label extension studies, including VITAL in patients who were infants at the time of first dosing (up to 208 weeks’ follow-up) (Sect. 4.1), ARISE extension in children and adults (up to 130 weeks’ follow-up) (Sect. 4.2) and LAL-CL04 in adults (up to 156 weeks’ follow-up) (Sect. 4.2). Two further open-label, single-arm, multinational, phase 2 studies are underway: LAL-CL08 in infants aged <8 months at the time of first dosing (up to 156 weeks’ follow-up; planned enrolment of 10 patients) [33]; and LAL-CL06 in infants aged >8 months at the time of first dosing, children and adults (up to 96 weeks’ follow-up; planned enrolment 30 patients) [34]. Of note, the objective of LAL-CL06 is to assess sebelipase alfa in a more broad population of patients with LAL-D than previously studied. Such patients may have been excluded from enrollment in other studies because of, among other reasons, age, disease progression or previous treatment by haematopoietic stem cell or liver transplantation [34].
Given that drugs developed for ultra-rare diseases are among the most expensive medicines on a cost-per patient basis [35], pharmacoeconomic analyses examining the cost-effectiveness of sebelipase alfa would be of interest.
In conclusion, available data from a limited number of patients (a reflection of the rarity of the disease) indicate that sebelipase alfa is an effective disease-specific therapy for individuals with LAL-D who have historically been managed using supportive therapies.
Data selection sources:
Relevant medical literature (including published and unpublished data) on sebelipase alfa was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 13 October 2016], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Sebelipase, Kanuma, SBC-102, LAL-CL01, LAL-CL02, LAL-CL03, LAL-CL04, ARISE trial, NCT01307098, NCT01757184, NCT01371825, NCT01488097, lysosomal, lipase, dyslipidaemia, LAL, LALD, Wolman*, cholesterol esters or cholesteryl esters.
References
Bernstein DL, Hülkova H, Bialer MG, et al. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J Hepatol. 2013;58(6):1230–43.
Reiner Z, Guardamagna O, Nair D, et al. Lysosomal acid lipase deficiency—an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235(1):21–30.
Porto AF. Lysosomal acid lipase deficiency: diagnosis and treatment of wolman and cholesteryl ester storage diseases. Pediatr Endocrinol Rev. 2014;12(Suppl 1):125–32.
Sjouke B, van der Stappen JWJ, Groener JEM, et al. Hypercholesterolaemia and hepatosplenomegaly: two manifestations of cholesteryl ester storage disease. Neth J Med. 2015;73(3):129–32.
Shirley M. Sebelipase alfa: first global approval. Drugs. 2015;75(16):1935–40.
Balwani M, Breen C, Enns GM, et al. Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease. Hepatology. 2013;58(3):950–7.
European Medicines Agency. Kanuma (sebelipase alfa): summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 18 July 2015.
Alexion Pharmaceuticals Inc. Kanuma (sebelipase alfa) injection, for intravenous use: US prescribing information. 2015. http://www.accessdata.fda.gov. Accessed 18 July 2016.
Alexion Pharmaceuticals, Inc. Kanuma® (sebelipase alfa) receives marketing approval in Japan for treatment of patients with lysosomal acid lipase deficiency (LAL-D) [media release]. http://news.alexionpharma.com. 28 Mar 2016.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report. Kanuma. International non-proprietary name: sebelipase alfa. Procedure No. EMEA/H/C/004004/0000. 2015. http://www.ema.europa.eu Accessed 18 July 2016.
Quinn AG, Harvey A, Chen M, et al. SBC-102, a recombinant enzyme replacement therapy, corrects key abnormalities due to lysosomal acid lipase deficiency [abstract]. In: American Society of Human Genetics Annual Meeting, 2010.
Leavitt M, Burt AD, Hu W, et al. Recombinant lysosomal acid lipase normalizes liver weight, transaminases and histopathological abnormalities in an in vivo model of cholesteryl ester storage disease [abstract no. 900]. J Hepatol. 2011;54(Suppl 1):S358.
Leavitt M, Hu W, Canty D, et al. Efficacy of SBC-102, a recombinant enzyme replacement therapy, across a broad range of doses in an in vivo model of lysosomal acid lipase deficiency [abstract no. PA-H-0036]. J Pediatr Gastroenterol Nutr. 2011;52(Suppl 1):E20.
Thelwall PE, Smith FE, Leavitt MC, et al. Hepatic cholesteryl ester accumulation in lysosomal acid lipase deficiency: non-invasive identification and treatment monitoring by magnetic resonance. J Hepatol. 2013;59(3):543–9.
Rutkowski J, Burt AD, Leavitt M, et al. Recombinant human lysosomal acid lipase decreases hepatic macrophage aggregates and colocalized fibrosis in a rat model of lysosomal acid lipase deficiency [abstract no. 1351]. Hepatology. 2012;56(S1):830A.
Rutkowski JV, Burt AD, Leavitt MC, et al. Co-localization of macrophage aggregation and fibrosis in a rat model of lysosomal acid lipase (LAL) deficiency and the effects of enzyme replacement with SBC-102 [abstract]. Mol Genet Metab. 2013;108(2):S80–1.
US National Institutes of Health. ClinicalTrials.gov identifier NCT01371825. 2016. https://clinicaltrials.gov. Accessed 23 June 2016.
Jones SA, Plantaz D, Vara R, et al. Effect of sebelipase alfa on survival and liver function in infants with rapidly progressive lysosomal acid lipase deficiency [abstract no. 120]. Mol Genet Metab. 2015;114(2):S59.
Jones S, Plantaz D, Vara R, et al. Impact of sebelipase alfa on survival and liver function in infants with rapidly progressive lysosomal acid lipase deficiency [abstract no. P1212]. J Hepatol. 2015;62(Suppl 2):S811.
Jones SA, Brassier A, Hughes J, et al. Effect of sebelipase alfa on survival and liver function in infants with rapidly progressive lysosomal acid lipase deficiency: 2-year follow-up data [abstract no. 150]. Mol Genet Metab. 2016;117(2):S63.
Jones S, Valayannopoulos V, Schneider E, et al. Rapid progression and mortality of lysosomal acid lipase deficiency presenting in infants. 2016;18(5):452–8.
Burton BK, Balwani M, Feillet F, et al. A phase 3 trial of sebelipase alfa in lysosomal acid lipase deficiency. N Engl J Med. 2015;373(11):1010–20.
Wilson DP, Marulkar S, Tripuraneni R, et al. Sebelipase alfa improves atherogenic biomarkers in adults and children with lysosomal acid lipase deficiency [abstract no. 133]. J Clin Lipidol. 2016;10(3):678–9.
Furuya KN, Marulkar S, Friedman M, et al. Long-term benefit of sebelipase alfa over 52 weeks in children and adults with lysosomal acid lipase deficiency (ARISE trial) [abstract no. 164]. J Pediatr Gastroenterol Nutr. 2016;63:164(Suppl 2):s50.
Furuya KN, Marulkar S, Friedman M, et al. Long-term benefit of sebelipase alfa over 76 weeks in children and adults with lysosomal acid lipase deficiency (ARISE trial) [abstract no. 564]. Hepatology. 2016;64(Suppl 1):281A.
Goodman ZD, Burton B, Alaparthi L, et al. Change in liver fibrosis in children and adults with lysosomal acid lipase deficiency after 52 weeks of sebelipase alfa (ARISE trial) [abstract no. 561]. Hepatology. 2016;64(Suppl 1):279–80A. 2016.
Valayannopoulos V, Malinova V, Honzík T, et al. Sebelipase alfa over 52 weeks reduces serum transaminases, liver volume and improves serum lipids in patients with lysosomal acid lipase deficiency. J Hepatol. 2014;61(5):1135–42.
Whitley CB, Valayannopoulos V, Malinova V, et al. Long-term clinical effect and safety of sebelipase alfa in adults with lysosomal acid lipase deficiency [abstract]. Mol Genet Metab. 2014;111(2):S113–4.
Valayannopoulos V, Malinova VERA, Sharma R, et al. Effect of sebelipase alfa after 90 weeks in adults with lysosomal acid lipase deficiency [abstract]. In: 82nd European Atherosclerosis Society Congress, 2014.
Abel F, Whitley CB, Valayannopoulos V, et al. Effect of sebelipase alfa after 2 years in adults with lysosomal acid lipase deficiency [abstract no. P-445]. J Inherit Metab Dis. 2014;37(Suppl 1):S160.
Friedman M, Valayannopoulos V, Grande CC, et al. Safety findings from 3 trials of treatment with sebelipase alfa in children and adults with lysosomal acid lipase deficiency [abstract no. 102]. Mol Genet Metab. 2016;117(2):S47.
Paton DM. Sebelipase alfa: enzymatic replacement treatment for lysosomal acid lipase deficiency. Drugs Today. 2016;52(5):287–93.
US National Institutes of Health. ClinicalTrials.gov identifier NCT02193867. 2016. https://clinicaltrials.gov. Accessed 15 July 2016.
US National Institutes of Health. ClinicalTrials.gov identifier NCT02112994. 2016. https://clinicaltrials.gov. Accessed 15 July 2016.
Schuller Y, Hollak CEM, Biegstraaten M. The quality of economic evaluations of ultra-orphan drugs in Europe—a systematic review. Orphanet J Rare Dis. 2015;10:92.
Acknowledgements
During the peer review process, the manufacturer of sebelipase alfa was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
James Frampton is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: S. Calandra, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy; O. Guardamagna, Department of Public and Pediatric Sciences, Medical School, University of Turin, Turin, Italy; S. Jones, Willink Biochemical Genetics Unit, Manchester Centre for Genomic Medicine, St. Mary’s Hospital, Manchester, UK; Ž. Reiner, Department of Internal Medicine, School of Medicine University of Zagreb, University Hospital Centre Zagreb, Zagreb, Croatia; E. Ros, Lipid Clinic, Endocrinology and Nutrition Service, Institut d’Investigations Biomèdiques August Pi Sunyer, Hospital Clinic, Barcelona, Spain.
Rights and permissions
About this article
Cite this article
Frampton, J.E. Sebelipase Alfa: A Review in Lysosomal Acid Lipase Deficiency. Am J Cardiovasc Drugs 16, 461–468 (2016). https://doi.org/10.1007/s40256-016-0203-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-016-0203-2