Abstract
The aim of this review article is to summarize the current knowledge about mechanisms that connect blood pressure regulation and hypercholesterolemia, the mutual interaction between hypertension and hypercholesterolemia, and their influence on atherosclerosis development. Our research shows that at least one-third of the population of Western Europe has hypertension and hypercholesterolemia. Several biohumoral mechanisms could explain the relationship between hypertension and hypercholesterolemia and the association between these risk factors and accelerated atherosclerosis. The most investigated mechanisms are the renin-angiotensin-aldosterone system, oxidative stress, endothelial dysfunction, and increased production of endothelin-1. Arterial hypertension is frequently observed in combination with hypercholesterolemia, and this is related to accelerated atherosclerosis. Understanding the mechanisms behind this relationship could help explain the benefits of therapy that simultaneously reduce blood pressure and cholesterol levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hypercholesterolemia and hypertension have synergistic pro-oxidant, pro-inflammatory, and pro-thrombotic effects. |
Renin-angiotensin system inhibitors are highly effective in the reduction of hypercholesterolemia-induced atherosclerosis. |
Lipid-lowering therapy with statins significantly decreases blood pressure via a pleiotropic effect. |
Combined blood pressure-lowering and lipid-lowering therapy has an important synergistic effect on the regulation of blood pressure and cholesterol levels. |
1 Introduction
The term ‘risk factor’ was first introduced into medical literature during the 1960s [1]. This was followed by the results of the Framingham study, published in 1961, which emphasized the relationship between hypertension/hypercholesterolemia and left ventricular hypertrophy and coronary artery disease [2]. ‘Risk factor’ was defined as a cause of pathophysiological events that affect prognosis, the modification of which significantly reduces the risk of major cardiovascular and cerebrovascular events. Many major investigations have been conducted to illuminate all aspects of the adverse effects of risk factors and to find the best solution to improve them. One particular problem is that a risk factor is rarely seen in isolation but is usually one of a cluster of factors that significantly increase global cardiovascular risk.
According to the MONICA (Monitoring of Trends and Determinants in Cardiovascular Disease) study, 35 % of the population of Western Europe has at least two risk factors [3]. Results of the Framingham study showed that 78 % of men and 82 % of women with hypertension incurred at least one further risk factor [4]. Egan et al. [5] analyzed three reports (1988–1994, 1999–2004, and 2005–2010) from the National Health and Nutrition Examination Surveys (NHANES) and showed that 60.7–64.3 % of hypertensive patients also had hypercholesterolemia [5]. Williams et al. [6] reported a somewhat lower prevalence of concomitant hypertension and hypercholesterolemia (36 %) [6]. NHANES 1988–2010 showed that the prevalence of concomitant hypertension and hypercholesterolemia consistently increased: hypertension and increased low-density lipoprotein (LDL) from 5.0 to 30.7 %, and the frequency of combined hypertension, LDL, and non–high-density lipoprotein (HDL) cholesterol increased from 1.8 to 26.9 % [5]. One of the possible reasons for this relationship is that hypercholesterolemia, in subjects who are predisposed for the development of arterial hypertension, increases sensitivity to some of the mechanisms involved in blood pressure elevation.
The aim of this review article is to summarize the current knowledge about mechanisms that connect blood pressure regulation and hypercholesterolemia, the mutual interaction between hypertension and hypercholesterolemia, and their influence on atherosclerosis development.
2 Mechanisms of the Causal Relation Between Hypercholesterolemia and Hypertension
The effect of hypercholesterolemia on blood pressure elevation is based on several mechanisms [7]. One is the effect of hypercholesterolemia on the reduction of nitric oxide (NO) bioavailability. Hypercholesterolemic conditions increase LDL oxidation and the formation of free radicals, which decreases the transcription of endothelial NO synthase (eNOS). The increase in free radical production is mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, cyclooxygenase, and mitochondrial electron transport, inactivation of the antioxidant system, and uncoupling of endothelial NO synthase. Plasma cholesterol level significantly correlates with the concentration of dimethylarginine, which is a natural analog of l-arginine, which competitively inhibits the production of NO [8].
NO plays an important role in the regulation of vascular tone, and normal production of NO has an important role in the homeostasis of the cardiovascular system [9]. Physiologically, NO preserves normal function of the vascular endothelium by inhibiting leukocyte–endothelial cell adhesion, platelet aggregation, and vascular smooth muscle cell proliferation and migration. A decrease in NO reduces vasodilatory capacity and induces hypertension. NO plays an important role in antagonizing the effects of angiotensin (Ang)-II, endothelin, and reactive oxygen species. The balance between NO and Ang II appears to have an important role in the regulation of the sympathetic tone.
The hypercholesterolemia is also associated with the increased secretion of vasoconstrictor molecules [7]. Hypercholesterolemia stimulates plasma and tissue activity of the renin-angiotensin-aldosterone system, and the synthesis of Ang II. In addition, hypercholesterolemia increases the activity of Ang I. The consequences of hypercholesterolemia also include increased endothelin-1 level and amplified activity of endothelin-1 receptors [7]. On the other hand, Kurtel et al. [8] found that hypercholesterolemia and hypertension does not show an additive effect of vasomotor dysfunction compared with either risk factor alone. These apparently contrasting findings may result from differences in animal species, vessels, and/or models of hypertension studied. The other reason is that hypercholesterolemia and hypertension exert their actions on endothelial cells via common signaling pathways. However, the authors revealed that H2O2 and cell-associated Ang II type 1 receptors (AT1) were related with endothelium-dependent dilation in the combination of these risk factors [8].
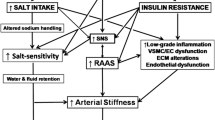
Decreased NO bioavailability and elevated levels of vasoactive substance induce endothelial dysfunction, but also increase salt sensitivity [7]. Furthermore, endothelial dysfunction is one of the most important mechanisms for the induction of salt sensitivity. In hypercholesterolemia, the transfer between lipoprotein and cell membrane affects the fluidity of the membrane and its transport activity through ion channels. Thus, cholesterol affects the renal cell membrane by reducing sodium efflux from the nephron, with a consequent reduction of the sodium clearance.
An additional mechanism that could contribute to elevated blood pressure in hypercholesterolemia is microvascular constriction, which represents the consequence of the increased calcium influx into smooth myofibrils. The increased activity of the L-type calcium channels in the smooth myofibril membrane, ‘enhanced’ with cholesterol, is the essential modification that could contribute to hypertension in individuals with hypercholesterolemia [7].
Kalayci et al. [10] investigated the impact of hypercholesterolemia and/or hypertension on endothelial cells of brain microvessels. They suggested that hypercholesterolemia may affect blood–brain barrier integrity by increasing the expression of tight junction proteins, leading to the production of vascular endothelial growth factor via, at least partly, increased NO, tumor necrosis factor (TNF)-α, and catalase in hypertensive conditions. These findings potentially could explain the greater brain damage in subjects with both risk factors.
The causal relationship between hypercholesterolemia and hypertension is less important than their combined effect on atherosclerosis development. These two risk factors have their own well-known mechanisms in the development of atherosclerosis. However, synergism in pro-oxidant, pro-inflammatory, and pro-thrombotic effects increases the risk of major cardiovascular and cerebrovascular events.
3 The Mechanisms Responsible for Atherosclerosis Development in Arterial Hypertension and Hypercholesterolemia
Numerous studies conducted on animal and human models and published 2 decades ago investigated the mechanisms of these two risk factors in the development of endothelial dysfunction, oxidative stress, and vascular inflammation [11]. The mechanisms by which hypercholesterolemia induces endothelial dysfunction partly overlap with the mechanisms that lead to elevated blood pressure [7]. The possible mechanisms of interaction between hypertension and hypercholesterolemia are provided in Table 1.
Recently, Alexandru et al. [12] reported that endothelial progenitor cells could reduce platelet activation and modify their pro-inflammatory and anti-thrombogenic properties in hypertension associated with hypercholesterolemia, which could potentially explain the mechanism of atherosclerosis in patients with these two conditions. Rodrigues et al. [13] suggested that AT1 and reactive oxygen species contribute to the vascular responses induced by hypercholesterolemia and hypertension. The authors indicated that the combination of these risk factors results in a severe but reversible inflammatory and thrombogenic phenotype in the cerebral microvasculature [13].
Reduction in the bioavailability of NO has a key role in the development of endothelial dysfunction, which is why hypercholesterolemia has an important role [14]. The oxidized LDL particles reduce the expression of eNOS, whereas the increased level of asymmetrical dimethylarginine inhibits eNOS [14–16]. On the other hand, increased Ang II activity, which correlates closely with hypertension, is responsible for reducing the NO production by reducing the expression of eNOS responsible for the degradation of NO [17, 18].
Oxidized LDL particles contribute to oxidative stress by increasing the activity of endothelial oxidase and levels of superoxide. NO reacts with superoxide and creates peroxynitrite, which has pro-oxidant toxic effects on proteins. Ang II is also a powerful oxidant that participates in the synthesis of superoxide via NADPH oxidase. In addition, Ang II contributes to the formation of free radicals via the activation of various intracellular signaling cascades, including the mitogen-activating protein kinase and the transcription factor nuclear factor (NF)-κB. These compounds have a cytotoxic effect on endothelial cells by inducing a reduction of NO production, which could be the consequence of direct cell damage with consequent reduced synthesis or increased degradation of NO [14, 19]. Inactivation of NO due to increased superoxide anion is considered to be the main mechanism in the impairment of endothelial function in arterial hypertension and hypercholesterolemia.
Furthermore, Ang II enhanced secretion of monocyte chemoattractant protein (MCP)-1, which has been demonstrated to have a role in the development of experimental atherosclerosis [20]. Borghi et al. [21] even found that the presence of hypercholesterolemia can promote the development of stable hypertension through its interaction with the circulating renin–angiotensin system in patients with high–normal blood pressure [21]. High plasma renin activity increased the overall rate of hypertension in subjects with normal and increased cholesterol levels. The interaction between cholesterol and plasma renin activity was more evident in patients with borderline high cholesterol levels, with the adjusted relative risk (RR) of new onset of hypertension 2.17 (95 % confidence interval [CI] 1.2–3.74; p < 0.05) in high plasma renin activity subjects and 1.17 (95 % CI 0.67–2.23; p = 0.87) in subjects with normal plasma renin activity [21].
Under conditions of endothelial dysfunction, vascular permeability is increased for LDL particles, which are oxidized in the arterial wall where they are taken up by macrophages, which are transformed into the foam cells. In this process, the renin–angiotensin system is involved by increasing the activity of Ang-1 receptor, which contributes to the increase of LDL oxidation [22]. In addition, atherosclerotic plaque is rich in angiotensin-converting enzyme [23].
Inflammatory cells in the vascular wall play an important part in the development of atherothrombosis. This process involves adhesion and chemotactic molecule activity, and an important role in this process is assigned to the transcription factor NF-κB that is activated by free radicals and oxidized LDL, while HDL has an inhibitory effect [24]. Experiments have indicated that Ang II also activates NF-κB in the culture of monocytes and smooth muscle cells on one side and induces formation of free radicals on the other side [25]. Furthermore, Ang II via the Ang-1 receptor induces the expression of monocyte chemotactic factor, interleukin-6, and interleukin-8 [18, 20, 25].
Thrombosis is a key component in the development of major cardiac and cerebral events. Activated tissue factor, which is the first step in the coagulation cascade, is one of the components of the lipid core of the atherosclerotic plaque. Ang II is involved in the induction of tissue factor expression through protein kinase C. Endogenous fibrinolytic system activity is also affected by Ang II, which reduces plasminogen activator inhibitor (PAI)-1 [16, 18, 20, 26].
Further studies have confirmed that hypertension and hypercholesterolemia induce structural and functional changes of various segments of the vascular bed by different mechanisms.
3.1 The Effect of Arterial Hypertension and Hypercholesterolemia on the Development of Atherosclerosis in Various Segments of the Vascular Bed
Research conducted more than 2 decades ago investigated the influence of age, hypertension, hypercholesterolemia, and concomitant hypertension and hypercholesterolemia on structural changes of the aorta and carotid and brachial arteries [27]. Different ultrasonographic methods revealed that hypertension was associated with increases in arterial stiffness, whereas investigators found a higher prevalence and severity of carotid atherosclerosis in patients with hypercholesterolemia additional to arterial stiffness change. Recently, Dobsak et al. [28] reported that the cardio-ankle vascular index, a sensitive non-invasive marker of arterial stiffness and atherosclerosis, was significantly increased in normotensive patients with hyperlipidemia (primary hypercholesterolemia, combined hyperlipidemia). Furthermore, Kanaki et al. [29] showed that low-dose atorvastatin treatment improved arterial stiffness and exerted a reduction on central aortic pressures in patients with mild hypertension and hypercholesterolemia [29]. On the other hand, Saba et al. [30] found that carotid intimal-medial thickness and stiffness were not affected by hypercholesterolemia in subjects with uncomplicated hypertension. However, these findings could be explained by the results of a more recently published study that showed that pulse wave velocity, but not intima-media thickness, was an early indicator of vascular damage in hypercholesterolemic subjects [31].
Investigations have also discovered a relationship between hypertension and increased arterial elasticity compliance due to an increase of smooth muscle cells in the vascular wall and reduced collagen [32]. Studies have shown arterial elasticity to be significantly impacted by hypertension, dyslipidemia, obesity, and diabetes [33]. Independent research has also shown that vascular compliance in hypercholesterolemia is reduced due to an increase in collagen and calcium in lipid deposits of the intima and a reduction of the secretion of endothelial factors that induce relaxation under the influence of elevated cholesterol [34].
Based on these findings it would be interesting to research the impact of the mutual influence of hypertension and hypercholesterolemia on the structure and function of distributing arteries. It was found that concomitant influence of these two risk factors was associated with a reduction in isobaric compliance and distensibility of the radial artery due to the influence of hypercholesterolemia over hypertension [35]. Additionally, Sharman et al. [36] found that hypercholesterolemia significantly decreased pulse pressure amplification during exercise.
Versari et al. [37] in experimental research in an animal model confirmed a different influence of hypercholesterolemia and hypertension on the structural and functional changes of the carotid arteries. They found that both risk factors induced an increase in systemic and vascular oxidative stress, with a consequent reduction in vasorelaxation. They also found that hypertension was related to a reduced response on endothelial-independent stimuli, increased vascular fibrosis, and thickening of the media. On the other hand, hypercholesterolemia was associated with vasa vasorum neovascularization, which is considered to be important in the progression of atherosclerotic disease. In another study in an animal model of atherosclerosis, Georgescu et al. [38] showed that hypertension associated with hypercholesterolemia induced considerable changes consisting of lipid, collagen, and macrophage accumulation, activation of endothelial and smooth muscle cells, and synthesis of hyperplasic-multilayered basal lamina in the thoracic aortic arch and arteriole regions. Moreover, a considerably altered reactivity of the arterial wall was observed to be closely correlated with microparticles and endothelial progenitor cells adherence.
Toikka et al. [39] examined the impact of borderline hypertension in men on oxidation of LDL, development of asymptomatic atherosclerosis, and cardiac remodeling. They found a relationship between prehypertension, increased LDL oxidation, and asymptomatic atherosclerosis. An experimental study explained these findings, demonstrating that mechanical stretching of the culture of smooth muscular cells, equivalent to arterial hypertension, induced significant absorption of oxidized LDL particles in the arterial wall and increased the oxidation of LDL particles and increased superoxide production [40]. Based on the abovementioned studies, it could be hypothesized that hypertension increases atherogenicity of LDL particles.
Rodriguez-Porcel et al. [41] investigated the effects of arterial hypertension and/or hypercholesterolemia on coronary artery endothelial function in an animal model. They found decreased NO bioavailability in the presence of isolated hypertension or hypercholesterolemia. However, the authors also found increased ‘sensitivity’ to NO in animals with isolated hypercholesterolemia. This was explained by the increased cyclic guanosine monophosphate (cGMP) level, which represents the main second messenger of NO. Poor response in cGMP production in arterial hypertension was explained by a direct unfavorable effect of Ang II on smooth muscle cells. Furthermore, the authors showed that a combination of hypertension and hypercholesterolemia impaired endothelial function more than each of these factors individually. The investigators demonstrated the vascular damage by studying the endothelial response to the receptor- and non-receptor-mediated vasodilatation. The synergism between different biohumoral factors was responsible for the increased incidence of coronary events in patients with both risk factors. The same authors investigated whether arterial hypertension led to exacerbation of myocardial perfusion defects in hypercholesterolemia [42]. They found that the combined effect of hypercholesterolemia and hypertension led to a reduced response to dobutamine as well as altered in vivo microvascular permeability. These functional changes were explained by the synergistic unfavorable effect of these two factors on systemic and myocardial oxidative stress.
In addition to endothelial dysfunction, these two risk factors are responsible for the remodeling of coronary vessels in different ways. Herrmann et al. [43] showed that adventitia remodeling occurs in the early stages of hypercholesterolemia and hypertension, before intima and media remodeling. The investigators showed that these two factors affect the arterial wall in significantly different manners. They have shown that hypercholesterolemia increases the adventitial vasa vasorum density, while hypertension increases collagen III. The investigation additionally revealed that hypercholesterolemia is associated with increased necrotic core volume in coronary artery plaque [44], which suggests an important role in the development of complex atherosclerotic plaque.
It is evident that these two risk factors, using different mechanisms, stimulate multiple changes in the vascular bed as the result of synergistic or cumulative effects. Some differences in the aforementioned studies might be the result of different methodologies and durations of hypercholesterolemia and hypertension.
4 Implications for Drug Treatment
Atherosclerosis is the most important underlying cause of ischemic cardiac disease. Epidemiological, genetic, and clinical studies have shown that hypertension and hypercholesterolemia are consistently related to the development of atherosclerotic diseases. A large number of clinical trials have demonstrated that treatment of either hypercholesterolemia or hypertension decreased the incidence of cardiovascular and cerebrovascular events. Given that the prevalence of these two risk factors is constantly increasing, it is of a great clinical importance to achieve the target values of blood pressure and cholesterol in primary and secondary cardiovascular prevention.
4.1 Benefits of Lowering Blood Pressure and Low-Density Lipoprotein (LDL)-Cholesterol
Investigators agree on the beneficial effect of blood pressure lowering on cardiovascular morbidity and mortality. A recently published meta-analysis that included 153,825 individuals demonstrated that all outcomes were significantly reduced by blood pressure lowering: stroke (−36 %), heart failure (−43 %), coronary artery disease (−16 %), cardiovascular mortality (−18 %), and all-cause mortality (−11 %) [45]. Absolute risk reductions were 17 strokes, 28 cardiovascular events, and eight deaths prevented in every 1000 patients treated for 5 years [45]. Interestingly, the risks for stroke and composite cardiovascular events were related to systolic, diastolic and pulse pressure reductions. An additional meta-analysis of the same data demonstrated that blood pressure-lowering treatment has more favorable effects in patients with a higher cardiovascular risk [46]. However, a higher risk level was also associated with higher absolute residual risk [46]. The systematic review that compared renin–angiotensin inhibitors as first-line treatment with other first-line antihypertensive drugs revealed that first-line thiazides caused less heart failure and stroke than first-line renin–angiotensin inhibitors [47]. The authors also found a somewhat lower risk of total cardiovascular events and stroke seen with renin–angiotensin inhibitors compared with beta-blockers [47]. They demonstrated that renin–angiotensin inhibitors had reduced the risk of heart failure but increased the risk of stroke compared with first-line calcium channel blockers. The degree of reduction in heart failure exceeded the increase in stroke. The minor differences in blood pressure reduction effect achieved by different drug classes did not correlate with the primary outcomes [47].
Similarly, investigators agree about the positive effect of lipid lowering, especially LDL cholesterol lowering, for prevention of vascular events. A recently published meta-analysis that involved 174,000 subjects demonstrated that the proportional reductions per 1.0 mmol/l reduction in LDL cholesterol in major vascular events were similar overall for women (RR 0.84, 99 % CI 0.78–0.91) and men (RR 0.78, 99 % CI 0.75–0.81), and also for those women and men at <10 % predicted 5-year absolute cardiovascular risk [48]. The proportional reductions in major coronary events, coronary revascularization, and stroke did not differ significantly by sex. All-cause mortality was also reduced with statin therapy for both women (RR 0.91, 99 % CI 0.84–0.99) and men (RR 0.90, 99 % CI 0.86–0.95) [48].
Several trials suggested that combined statin plus renin–angiotensin inhibitor reduces cardiovascular events more than does a statin alone and considerably more than renin–angiotensin inhibition alone [49–52]. This benefit seems to be related not only to the reduction of blood pressure and LDL cholesterol, but also to the effects on endothelial function, vascular inflammation, and the initiation, progression, and rupture of atheromatous plaques.
4.2 Pleiotropic Effects Not Explained by Simple Reduction of Blood Pressure and LDL Cholesterol
The renin–angiotensin–aldosterone system blockers—angiotensin-converting enzyme inhibitors and AT1 receptor blockers—irrespective of blood pressure reduction, significantly decrease oxidative stress by inhibiting NADPH oxidase activity [53]. It has been shown that long-term usage of the renin–angiotensin–aldosterone system blockers, in addition to the reduction of blood pressure, also stops or significantly slows vascular remodeling by repairing the vasodilatory response to acetylcholine [54, 55]. AT1 receptor blockers could improve endothelial function in hypercholesterolemia by reducing oxidative stress [55, 56]. Thus, losartan has been shown to normalize the production of superoxide, whereas candesartan significantly improved flow-mediated vasodilation and reduced plasma levels of oxidant stress and markers of inflammation and hemostasis independent of blood pressure reduction [56, 57]. Furthermore, renin–angiotensin inhibitors improve insulin sensitivity in hypertensive patients and could also potentially reduce cholesterol level [58, 59].
Statins are known to have pleiotropic effects that go beyond lowering the cholesterol level [60–63]. They improve endothelial-dependent vasodilation, increase the bioavailability of NO, decrease levels of oxidized LDL cholesterol, reduce levels of endothelin-1, affect the immunological system, stabilize atherosclerotic plaque, decrease synthesis of aldosterone, inhibit the proliferation of vascular smooth muscle cells and platelet aggregation, and reduce vascular inflammation. Furthermore, statins reverse the elevated blood pressure response to Ang II infusion, accompanied by decreased AT1 receptor density [64, 65].
Investigations have shown that some calcium channel blockers also lead to the restoration of endothelial dysfunction. Amlodipine has a very high affinity for the lipid membrane and induces electronegativity of the membrane (protons are given and the peroxidation process is blocked) that reduces the accumulation of LDL cholesterol and formation of the foam cells [66]. By inhibiting protein kinase C, amlodipine reduces the adhesion of monocytes to endothelial cells. Amlodipine causes a dose-dependent increase of NO. However, the combination of renin–angiotensin blockers and/or calcium channel blockers with statins is far more efficient for the prevention of cardiovascular events than the use of any of these drugs alone [49–52, 59, 67, 68].
Among the beta-blockers, only nebivolol, a third-generation highly selective beta-1 receptor blocker, also causes vasodilatation and endothelial protection via interaction with the endothelial l-arginine-NO pathway [69] and potentially has a synergistic effect with statins in the prevention of atherosclerosis. However, to our knowledge, no study has investigated the combined effect of nebivolol and statins. Benefits and risks of different drug classes in the treatment of concomitant hypertension and hypercholesterolemia are presented in Table 2.
4.3 Future Directions
The great prevalence of coexisting hypertension and hypercholesterolemia raises the inevitable question about the use of statins in hypertensive patients in the absence of hypercholesterolemia and the use of antihypertensives (primarily renin–angiotensin inhibitors) in normotensive subjects with hypercholesterolemia. Recently published guidelines provide the answer to this question [70]. Primary prevention in non-diabetic patients with hypertension and normal levels of LDL cholesterol is recommended when 10-year ASCVD risk (risk of coronary heart disease, stroke and peripheral arterial disease) is >7.5 % (moderate- or high-intensity statin therapy) or 5–7.5 % (moderate-intensity therapy) [70]. When 10-year ASCVD risk is <5 %, statins should be used only in selected individuals (family history of premature ASCVD, high lifetime ASCVD risk, abnormal coronary artery calcium score or ankle-brachial index, or high-sensitivity C-reactive protein [hs-CRP] ≥2 mg/l). Any ASCVD risk <5 % should be re-evaluated after 5–6 years [70]. Given that 10-year and lifetime ASCVD risks include sex, age, race, smoking status, diabetes, hypertension treatment (yes/no), and systolic blood pressure level, in addition to HDL and total cholesterol, it is understandable that a hypertensive patient could be a candidate for statin therapy even with a normal total cholesterol level. It is much easier to make a decision in secondary prevention where moderate- or high-intensity statin therapy is recommended in almost all patients. Furthermore, patients who have already experienced a cardiovascular event or have cardiovascular disease are also candidates for renin–angiotensin inhibitors due to their favorable effect on cardiovascular remodeling. Thus, in secondary prevention, the concomitant usage of statins and renin–angiotensin inhibitors is more the rule than the exception. However, the adverse effects of the drugs should be monitored.
Koh et al. [71] recently showed that the combination of a statin and an AT1 blocker (pravastatin and valsartan) in normotensive patients with hypercholesterolemia increased plasma adiponectin, lowered fasting insulin levels, and improved insulin sensitivity in an additive manner compared with monotherapy. These findings imply the benefit of concomitant usage of statin and renin–angiotensin inhibitor in hyperlipidemic normotensive subjects. However, the official guidelines do not stress this topic [70], and further large studies are needed to support this hypothesis.
Future studies should investigate the positive effects of concomitant usage of statins and highly selective beta-blockers such as nebivolol, or combinations of other lipid-lowering drugs (other than statins) and renin–angiotensin inhibitors, calcium channel blockers, or beta-blockers.
5 The Target Value of Blood Pressure and Cholesterol in Clinical Practice
The crucial clinical question is ‘how low should we go with blood pressure and lipid lowering?’ Randomized trials have shown that intensive blood pressure reduction does not have advantages over conventional treatment, even in patients with diabetes, coronary artery disease, stroke, or renal disease without proteinuria. In the latest European guidelines and the Eighth Joint National Committee (JNC 8) guidelines, the target blood pressure value for all these groups is 140/90 mmHg and is even higher in the elderly [72, 73]. The main problem with intensive blood pressure reduction is the existence of a J-shape and U-shape relationship between achieved blood pressure level and cardiovascular mortality risk [74, 75].
Blood pressure regulation in the elderly presents a particular problem. That is, the isolated systolic hypertension is dominant form of hypertension in this population and insisting on systolic blood pressure lowering could significantly reduce diastolic blood pressure and induce a U-shape phenomenon. This is why the latest European guidelines recommend the maintenance of systolic blood pressure in the range of 140–150 mmHg in patients aged >80 years [72]. The same is valid for the JNC 8 in patients aged ≥60 years [73].
The ‘lower is better’ rule is more convenient for LDL cholesterol level, especially in the secondary cardiovascular prevention. A meta-analysis of data from 170,000 participants in 26 randomized trials showed that each 1.0 mmol/l reduction in LDL cholesterol reduces the annual rate of heart attack, revascularization, and ischemic stroke for >20 % [76]. The authors claimed that reduction of LDL cholesterol by 2–3 mmol/l would reduce the risk of these major vascular events by 40–50 %. The same study group recently re-analyzed the results of this meta-analysis and showed that there is no difference in effect of LDL cholesterol lowering between sexes [77]. The authors also showed that LDL cholesterol reduction significantly decreased the risk of these major vascular events even in normotensive patients (<140/80 mmHg) [76, 77]. In a meta-analysis Messerli et al. [78] confirmed that lipid-lowering therapy effectively decreases cardiovascular morbidity and mortality to the same extent in both hypertensive and non-hypertensive patients.
Clinical trials and meta-analyses have shown the reduction of cardiovascular risk with blood pressure lowering [79–81] or cholesterol lowering [82]. However, the ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm) trial demonstrated that lipid lowering together with blood pressure lowering decreases the incidence of major cardiovascular events more effectively than regulation of hypertension or hypercholesterolemia separately [83].
6 The Role of the Polypill in Blood Pressure and Cholesterol Lowering
The concept of the polypill—a fixed combination of aspirin, statin, and blood pressure-lowering drug with/without folic acid—was introduced more than a decade ago, preferably for use in cardiovascular prevention [84]. The authors calculated the theoretical risk reductions of ischemic heart disease and stroke using the polypill [84]. The main advantages of the polypill are increased effectiveness, enhanced adherence, and lower cost [67]. However, the use of the polypill in primary prevention is controversial. TIPS (The Indian Polycap Study) showed that the polypill is non-inferior in comparison with its individual components in blood pressure lowering [85]. On the other hand, the polypill did not reduce LDL levels as much as a statin given alone. Malekzadeh [86] demonstrated modest reductions in LDL cholesterol and blood pressure, which reduced the RR, but still less than predicted [86]. Unexpectedly, the authors found a relatively high non-adherence rate of 30–35 %. Nevertheless, the benefit of polypill usage in primary prevention is still in the theoretical domain, and additional follow-up analysis with a large number of participants is needed to be able to reach a final conclusion.
The usage of the polypill in secondary cardiovascular prevention is supported by more evidence. Lafeber et al. [87] showed that the polypill is associated with a lower risk of vascular events and total mortality in patients with coronary artery disease. The recently published UMPIRE (Use of a Multidrug Pill in Reducing Cardiovascular Events) trial [88] showed that adherence to medication in the polypill group was 85 % compared with 60 % in the standard-care group. Other authors obtained similar results [89, 90]. Furthermore, blood pressure and LDL cholesterol levels were reduced with the polypill strategy to a greater extent than with standard care, but the differences were modest [88].
7 Conclusion
The benefits of treatment with statins and renin–angiotensin inhibitors is not only based on hemodynamic improvement and reduction in cholesterol level but also on their pleotropic action and possible mutual interaction. Statins may contribute to the significant reduction of blood pressure, whereas antihypertensives reduce the atherogenicity of hypercholesterolemia. The mechanisms of these effects are still unknown, with no evidence that cholesterol has a direct influence on any of the proteins in the angiotensin peptide biosynthesis pathway. Further investigation is necessary to evaluate the possible favorable effects of concomitant usage of statins and highly selective beta-blockers, or other lipid-lowering drugs and antihypertensive drugs.
References
Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nat Health. 1957;47:4–24.
Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham Study. Ann Int Med. 1961;55:33–50.
Tunstall-Pedoe H, Chen R, Kramarz P. Prevalence of individuals with both raised blood pressure and raised cholesterol in WHO MONICA Project population surveys 1989–97. Pharmacoepidemiol Drug Saf. 2004;13(Suppl 1):S307–8.
Kannel WB. Fifty years of Framingham Study contributions to understanding hypertension. J Hum Hypertens. 2000;14:83–90.
Egan BM, Li J, Qanungo S, Wolfman TE. Blood pressure and cholesterol control in hypertensive hypercholesterolemic patients: national health and nutrition examination surveys 1988–2010. Circulation. 2013;128(1):29–41.
Williams B, Wilson K, Lacey L, et al. The prevalence and management of patients with co-existing hypertension and hypercholesterolaemia in the UK. Eur Heart J. 2004;25:528–9.
Spasito AC. Emerging insights into hypertension and dyslipidaemia synergies. Eur Heart J Suppl. 2004;6 (Supplement G):G8–12.
Kurtel H, Rodrigues SF, Yilmaz CE, Yildirim A, Granger DN. Impaired vasomotor function induced by the combination of hypertension and hypercholesterolemia. J Am Soc Hypertens. 2013;7(1):14–23.
González J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: new insights. World J Cardiol. 2014;6(6):353–66.
Kalayci R, Kaya M, Uzun H, Bilgic B, Ahishali B, Arican N, Elmas I, Küçük M. Influence of hypercholesterolemia and hypertension on the integrity of the blood-brain barrier in rats. Int J Neurosci. 2009;119(10):1881–904.
John S, Schmieder RE. Potential mechanisms of impaired endothelial function in arterial hypertension and hypercholesterolemia. Current Hypertens Rep. 2003;5:199–207.
Alexandru N, Popov D, Dragan E, Andrei E, Georgescu A. Circulating endothelial progenitor cell and platelet microparticle impact on platelet activation in hypertension associated with hypercholesterolemia. PLoS One. 2013;8(1):e52058.
Rodrigues SF, Almeida-Paula LD, Granger DN. Synergistic effects of high blood cholesterol and hypertension on leukocyte and platelet recruitment in the cerebral microcirculation. Hypertension. 2014;63(4):747–52.
Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10(1):4–18.
Böger RH, Bode-Böger SM, Szuba A, et al. Asymetric Dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction—its role in hypercholesterolemia. Circulation. 1998;98:1842–7.
Päivä H, Laakso J, Laine H, et al. Plasma asymmetric dimethylarginine and hyperemic myocardial blood flow in young subjects with borderline hypertension or familial hypercholesterolemia. J Am Coll Cardiol. 2002;40:1241–7.
Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured smooth muscle cells. Circ Res. 1994;74:1141–8.
Drapala A, Sikora M, Ufnal M. Statins, the renin-angiotensin-aldosterone system and hypertension - a tale of another beneficial effect of statins. J Renin Angiotensin Aldosterone Syst. 2014;15(3):250–8.
Griendling KK, Alexander RW. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–5.
Daugherty A, Lu H, Rateri DL, Cassis LA. Augmentation of the renin-angiotensin system by hypercholesterolemia promotes vascular diseases. Future Lipidol. 2008;3(6):625–36.
Borghi C, Veronesi M, Cosentino E, Cicero AF, Kuria F, Dormi A, Ambrosioni E. Interaction between serum cholesterol levels and the renin-angiotensin system on the new onset of arterial hypertension in subjects with high-normal blood pressure. J Hypertens. 2007;25(10):2051–7.
Warnholtz A, Nickenig G, Schulz E, et al. Increased NADHoxidase-mediated superoxide production in the early stages of atherosclerosis:evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–33.
Diet F, Pratt RE, Berry GJ, et al. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation. 1996;94:2756–67.
Matsunaga T, Hokari S, Koyama I, Harada T, Komoda T. NF-kappa B activation in endothelial cells treated with oxidized high-density lipoprotein. Biochem Biophys Res Commun. 2003;303(1):313–9.
Hernández-Presa M, Bustos C, Ortego M, et al. Angiotensin converting enzyme inhibition prevents arterial NF-kB activation, MCP-1 expression and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–41.
Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–62.
Alva F, Samanego V, Gonzalez V, Mogler R, Aney E. Structural and dynamic changes in the elastic arteries due to arterial hypertension and hypercholesterolemia. Clin Cardiol. 1993;16:614–8.
Dobsak P, Soska V, Sochor O, Jarkovsky J, Novakova M, Homolka M, Soucek M, Palanova P, Lopez-Jimenez F, Shirai K. Increased cardio-ankle vascular index in hyperlipidemic patients without diabetes or hypertension. J Atheroscler Thromb. 2015;22(3):272–83.
Kanaki AI, Sarafidis PA, Georgianos PI, Kanavos K, Tziolas IM, Zebekakis PE, Lasaridis AN. Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. Am J Hypertens. 2013;26(5):608–16.
Saba PS, Roman MJ, Longhini C, Scorzoni D, Pini R, Devereux RB, Ganau A. Carotid intimal-medial thickness and stiffness are not affected by hypercholesterolemia in uncomplicated essential hypertension. Arterioscler Thromb Vasc Biol. 1999;19(11):2788–94.
Riggio S, Mandraffino G, Sardo MA, Iudicello R, Camarda N, Imbalzano E, Alibrandi A, Saitta C, Carerj S, Arrigo T, Saitta A. Pulse wave velocity and augmentation index, but not intima-media thickness, are early indicators of vascular damage in hypercholesterolemic children. Eur J Clin Invest. 2010;40(3):250–7.
Laurent S, Hayoz D, Trazzi S, Boutouyrie P, Waeber B, Omboni S, Brunner HR, Mancia G, Safar M. Isobaric compliance of the radial artery is increased in patients with essential hypertension. J Hypertens. 1993;11:89–98.
Cernes R, Zimlichman R, Shargorodsky M. Arterial elasticity in cardiovascular disease: focus on hypertension, metabolic syndrome and diabetes. Adv Cardiol. 2008;45:65–81.
Hayoz D, Weber R, Rutschmann B, Darioli R, Burnier M, Waeber B, Brunner HR. Post-ischemic blood flow response in hypercholesterolemic patients. Hypertension. 1995;26:497–502.
Giannattasio C, Mangoni A, Failla M, Stella M, Carugo S, Bombelli M, Sega R, Mancia G. Combined effects of hypertension and hypercholesterolemia on radial artery function. Hypertension. 1997;29:583–6.
Sharman JE, McEniery CM, Dhakam ZR, Coombes JS, Wilkinson IB, Cockcroft JR. Pulse pressure amplification during exercise is significantly reduced with age and hypercholesterolemia. J Hypertens. 2007;25(6):1249–54.
Versari D, Gossl M, Mannheim D, Daghini E, Galili O, Napoli C, Lerman L, Lerman A. Hypertension and hypercholesterolemia differentially affect the function and structure of pig carotid artery. Hypertension. 2007;50:1063–8.
Georgescu A, Alexandru N, Andrei E, Titorencu I, Dragan E, Tarziu C, Ghiorghe S, Badila E, Bartos D, Popov D. Circulating microparticles and endothelial progenitor cells in atherosclerosis; pharmacological effects of irbesartan. J Thromb Haemost. 2012;10:680–91.
Toikka JO, Laine H, Ahotupa M, Haapanen A, Viikari JSA, Hartiala JJ, Raitakari OT. Increased arterial intima-media thickness and in vivo LDL oxidation in young men with borderline hypertension. Hypertension. 2000;36:929–36.
Klima Ł, Kawecka-Jaszcz K, Stolarz-Skrzypek K, Menne J, Fijorek K, Olszanecka A, Wojciechowska W, Bilo G, Czarnecka D. Structure and function of large arteries in hypertension in relation to oxidative stress markers. Kardiol Pol. 2013;71(9):917–23.
Rodriguez-Porcel M, Lerman LO, Herrmann J, Sawamura T, Napoli C, Lerman A. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003;23(5):885–91.
Rodriguez-Porcel M, Lerman A, Herrmann J, Schwartz RS, Sawamura T, Condorelli M, Napoli C, Lerman LO. Hypertension exacerbates the effect of hypercholesterolemia on the myocardial microvasculature. Cardiovasc Res. 2003;58(1):213–21.
Herrmann J, Samee S, Chade A, Porcel MR, Lerman LO, Lerman A. Differential effect of experimental hypertension and hypercholesterolemia on adventitial remodeling. Arterioscler Thromb Vasc Biol. 2005;25:447–53.
Seo YH, Lee CS, Yuk HB, Yang DJ, Park HW, Kim KH, Kim WH, Kwon TG, Bae JH. Hypercholesterolemia and in-vivo coronary plaque composition in patients with coronary artery disease: a virtual histology - intravascular ultrasound study. Korean Circ J. 2013;43(1):23–8.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32(12):2285–95.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk–overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2305–14.
Xue H, Lu Z, Tang WL, Pang LW, Wang GM, Wong GW, Wright JM. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev. 2015;1:CD008170.
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174000 participants in 27 randomised trials. Lancet. 2015 Jan 8. pii: S0140-6736(14)61368-4.
Athyros VG, Mikhailidis DP, Papageorgiou AA, Bouloukos VI, Pehlivanidis AN, Symeonidis AN, Elisaf M, GREACE Study Collaborative Group. Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease. J Hum Hypertens. 2004;18(11):781–8.
Kohro T, Hayashi D, Okada Y, Yamazaki T, Nagai R, JCAD Investigators. Effects of medication on cardiovascular events in the Japanese coronary artery disease (JCAD) study. Circ J. 2007;71(12):1835–40.
Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58.
Athyros VG, Ganotakis E, Kolovou GD, Nicolaou V, Achimastos A, Bilianou E, Alexandrides T, Karagiannis A, Paletas K, Liberopoulos EN, Tziomalos K, Petridis D, Kakafika A, Elisaf MS. Mikhailidis DP; Assessing The Treatment Effect in Metabolic Syndrome Without Perceptible Diabetes (ATTEMPT) Collaborative. Assessing the treatment effect in metabolic syndrome without perceptible diabetes (ATTEMPT): a prospective-randomized study in middle aged men and women. Curr Vasc Pharmacol. 2011;9(6):647–57.
Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16.
Schiffrin EL, Deng LY. Comparison of effects of angiotensin I converting enzyme inhibition and ß-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995;25:699–703.
Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–9.
Wassmann S, Hilgers S, Laufs U, et al. Angiotensin II type 1 receptor antagonism improves hypercholesterolemia-associated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22:1208–12.
Koh KK, Ahn JY, Han SH, Kim DS, Jin DK, Kim HS, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–10.
Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Koh Y, et al. Distinct vascular and metabolic effects of different classes of antihypertensive drugs. Int J Cardiol. 2010;140:73–81.
Lee HY, Sakuma I, Ihm SH, Goh CW, Koh KK. Statins and renin-angiotensin system inhibitor combination treatment to prevent cardiovascular disease. Circ J. 2014;78(2):281–7.
Joo SJ. Anti-inflammatory effects of statins beyond cholesterol low- ering. Korean Circ J. 2012;42:592–4.
Profumo E, Buttari B, Saso L, Rigano R. Pleiotropic effects of statins in atherosclerotic disease: focus on the antioxidant activity of atorvastatin. Curr Top Med Chem. 2014;14(22):2542–51.
Moon GJ, Kim SJ, Cho YH, Ryoo S, Bang OY. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J Clin Neurol. 2014;10(2):140–7.
Nickenig G, Bäumer AT, Temur Y, Kebben D, Jockenhövel F, Böhm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100(21):2131–4.
Correale M, Abruzzese S, Greco CA, Concilio M, Biase MD, Brunetti ND. Pleiotropic effects of statin in therapy in heart failure: a review. Curr Vasc Pharmacol. 2014;12(6):873–84.
Tuñón J, Martín-Ventura JL, Blanco-Colio LM, Tarín N, Egido J. Common pathways of hypercholesterolemia and hypertension leading to atherothrombosis: the need for a global approach in the management of cardiovascular risk factors. Vasc Health Risk Manag. 2007;3:521–6.
Mason RP, Marche P, Hintze TH. Novel vascular biology of third-generation l-type calcium channel antagonists ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol. 2003;23:2155–67.
Ivanovic B, Tadic M. Fixed combination of amlodipine/atorvastatin: from mechanisms to trials. J Cardiovasc Pharmacol Ther. 2013;18(6):544–9.
Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Combination of statin plus renin angiotensin system inhibition for the prevention or the treatment of atherosclerotic cardiovascular disease. Curr Pharm Des. 2014;20(40):6299–305.
Toblli JE, DiGennaro F, Giani JF, Dominici FP. Nebivolol: impact on cardiac and endothelial function and clinical utility. Vasc Health Risk Manag. 2012;8:151–60.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45.
Koh KK, Lim S, Choi H, Lee Y, Han SH, Lee K, Oh PC, Sakuma I, Shin EK, Quon MJ. Combination pravastatin and valsartan treatment has additive beneficial effects to simultaneously improve both metabolic and cardiovascular phenotypes beyond that of monotherapy with either drug in patients with primary hypercholesterolemia. Diabetes. 2013;62(10):3547–52.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23(1):3–16.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Ivanovic B, Tadic M. When does low normal blood pressure become too low? The J-curve phenomenon. Acta Cardiol. 2014;69(2):121–9.
Rutter MK, Nesto RW. Blood pressure, lipids and glucose in type 2 diabetes: how low should we go? Re-discovering personalized care. Eur Heart J. 2011;32(18):2247–55.
Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Cholesterol Treatment Trialists’ (CTT) Collaboration, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–405.
Messerli FH, Pinto L, Tang SS, Thakker KM, Cappelleri JC, Sichrovsky T, Dubois RW. Impact of systemic hypertension on the cardiovascular benefits of statin therapy: a meta-analysis. Am J Cardiol. 2008;101(3):319–25.
Poulter NR, Wedel H, Dahlöf B, Sever PS, Beevers DG, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, Pocock S, ASCOT Investigators. Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA). Lancet. 2005;366(9489):907–13.
Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62.
Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M, Neal B, Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med. 2015;162(3):184–91.
Blood Pressure Lowering Treatment Trialists’ Collaboration1, Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, Bulpitt C, Chalmers J, Fagard R, Gleason A, Heritier S, Li N, Perkovic V, Woodward M, MacMahon S. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336(7653):1121–3.
Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16.
Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80 %. BMJ. 2003;326:1419e24.
Indian Polycap Study (TIPS), Yusuf S, Pais P, Afzal R, Xavier D, Teo K, Eikelboom J, Sigamani A, Mohan V, Gupta R, Thomas N. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373(9672):1341–51.
Malekzadeh A. pilot double-blind randomized placebo-controlled trial of the effects of fixed-dose combination therapy (‘polypill’) on cardiovascular risk factors. Int J Clin Pract. 2010;64:1220e7.
Lafeber M, Spiering W, van der Graaf Y, Nathoe H, Bots ML, Grobbee DE, Visseren FL. The combined use of aspirin, a statin, and blood pressure-lowering agents (polypill components) and the risk of vascular morbidity and mortality in patients with coronary artery disease. Am Heart J. 2013;166(2):282–9.
Thom S, Poulter N, Field J, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD:the UMPIRE randomized clinical trial. JAMA. 2013;310:918–29.
Patel A, Cass A, Peiris D, Usherwood T, Brown A, Jan S, Neal B, Hillis GS, Rafter N, Tonkin A, Webster R, Billot L, Bompoint S, Burch C, Burke H, Hayman N, Molanus B, Reid CM, Shiel L, Togni S, Rodgers A; for the Kanyini Guidelines Adherence with the Polypill (Kanyini GAP) Collaboration. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. 2015;22(7):920–30. doi:10.1177/2047487314530382.
Selak V, Elley CR, Bullen C, Crengle S, Wadham A, Rafter N, Parag V, Harwood M, Doughty RN, Arroll B, Milne RJ, Bramley D, Bryant L, Jackson R, Rodgers A. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ. 2014;348:g3318.
Acknowledgments
No external funding was received to prepare this manuscript. BI has given lectures and attended conferences sponsored by various pharmaceutical companies. Neither BI nor MT have any potential conflicts of interest that might be relevant to the contents of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanovic, B., Tadic, M. Hypercholesterolemia and Hypertension: Two Sides of the Same Coin. Am J Cardiovasc Drugs 15, 403–414 (2015). https://doi.org/10.1007/s40256-015-0128-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-015-0128-1