Abstract
Purpose
In this study the aim was to analyze the seasonal concentration, groundwater quality, usage areas and arsenic-related health risk of major ions and heavy metals in groundwater samples collected from the Salda Lake basin.
Methods
In this study, 42 groundwater samples were collected from springs and wells in dry and wet seasons in 2015. Hydrogeochemical evaluations were made using different diagrams such as Piper and Gibbs diagrams. Groundwater quality was determined by the water quality index method (WQI) and different diagrams. Finally, health risk assessments related to arsenic were performed.
Results
The dominant water types are Mg-Ca-HCO3 and Mg-HCO3 in the wet season and Mg-HCO3 and Mg-HCO3-CO3 in the dry season. According to calculated WQI values ranged from 24.14 to 56.93 in the wet season ranged from 25.27 to 145.87 in dry season. This situation indicates that the quality of water samples is mostly good both seasons. AsT concentrations were between 2.1–6.3 μg/L in the dry season and 2.9–10.5 μg/L in the wet season. The risk of developing cancer due to arsenic exposure in healthy adults or children is very low. But arsenic has high non-carcinogenic and carcinogenic potentially harmful effect in the study area. In addition, water samples are not appropriate for use as drinking water in terms of fertilizers and trace element concentrations. Also, MH will be an important problem in waters that will be used as irrigation water. The use of some samples is not recommended as it may cause crusting on metal surfaces in industrial areas.
Conclusions
According to the results obtained, the quality of groundwater in the study area should be monitored and the usage areas should be determined accordingly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is one of the most important obstacles to the sustainable development of countries. Water is an extremely valuable, economic and strategic natural resource in our country as in the whole world [1,2,3]. In total, 95% of domestic wastewater and 70% of industrial wastewater are discharged to receiving environments without treatment in underdeveloped and developing countries. As a result, clean water sources become contaminated and access to safe drinking water becomes a major problem [4]. Groundwater is especially preferred as drinking water because it is less likely to be affected by pollutants [5,6,7,8]. Therefore, about half of the world’s drinking water is taken from groundwater aquifers [9]. However, groundwater quality and chemistry are controlled both by natural processes (weathering and soil erosion) and by anthropogenic inputs (agricultural activities, municipal and industrial wastewater discharge, solid wastes etc.) [10, 11]. The chemical composition of groundwater is determined based on the physical properties, composition and contact time of the substances it comes into contact with. The longer the contact time of the water with the substances in question, the more minerals dissolve in the water [12,13,14]. These processes also play an effective role in the formation of groundwater quality. Differences in groundwater chemistry are related to rock-water interaction during infiltration until it reaches the aquifer. In addition, water-borne pathogens and toxic and non-toxic pollutants, which are indicators of water quality, are carried by groundwater movement and cause changes in water quality [15].
The quality of groundwater reflects the combined effect of many processes along the groundwater flow path at any point underground [15,16,17]. Under normal conditions, seasonal and spatial changes in groundwater chemistry are determined by geochemical processes in the environment. It is possible to divide the factors affecting these geochemical processes into geogenic and anthropogenic factors. Geogenic factors are related to geological, hydrological and hydrogeological conditions. Anthropogenic factors that change the chemistry and quality of groundwater are the result of domestic, irrigation and industrial uses [18]. The deterioration of groundwater quality affects and limits its use in drinking, domestic, agricultural and industrial activities. Today, almost 80% of diseases worldwide and one third of deaths in developing countries are caused by contaminated or degraded drinking water [19]. Therefore, the determination of groundwater quality is important for the suitability of the water for use.
In addition, groundwater quality, especially when used as drinking water, directly affects human health. One of the important parameters affecting, groundwater quality is arsenic pollution. Groundwater arsenic (As) pollution is a problem frequently observed in many countries worldwide. Over 100 million people face the risk of arsenic poisoning due to naturally occurring groundwater arsenic pollution in many countries around the world. It has been widespread for many years especially in countries such as Bangladesh, Chile, India and Taiwan, and it is one of the important groundwater pollution problems in our country [19,20,21].
Arsenic is a toxic and carcinogenic substance that can be found in nature, with both natural and anthropogenic sources. The presence of natural As in groundwater is controlled by mineral dissolution/precipitation, adsorption/desorption, oxidation/reduction reaction mechanisms and biological transformation. The most important mechanism that restricts and increases arsenic mobility is generally adsorption and desorption. Arsenic has significant contaminant properties due to its oxidizable and reducible structure [22, 23]. The use of pesticides and mining activities are among the most important human activities that cause arsenic formation [24, 25]. The use of pesticides containing arsenic is among the non-point anthropogenic sources of arsenic. Also, copper, nickel, gold mining and ore disposal operations, fossil fuels, and landfill leachate are among the anthropogenic sources of arsenic [26]. Inorganic arsenic compounds vary depending on the redox conditions and the pH of the water and are generally seen in surface waters as arsenate (As5+) and in the groundwater as arsenite (As3+) [27]. In order to prevent drinking water pollution with arsenic in many countries around the world and prevent the health effects caused by it, the maximum pollution level accepted for arsenic in drinking water was decreased from 0.05 mg/L to 0.01 mg/L [28, 29].
As species in surface water and groundwater have become worldwide health problems due to their carcinogenic and many other toxic features. As, which is included in the Group A classification by the United States Environmental Protection Agency (USEPA), is an important carcinogen. As, which threatens human health, is increasing gradually in the water cycle today. This situation seriously increases the threats to human health. Research about the effects of As on health show that As in drinking water mainly causes skin cancer, but it also causes wide variety of other cancer types like liver, bladder, and lung cancers. Inorganic As is more toxic than organic As [30]. In our country, the limit value for arsenic was accepted as 10 ppb as of February 2008 in the Regulation on “Standards for drinking waters” [31]. Accordingly, previous studies about water resources in the west and center of Turkey showed the presence of arsenic as natural and/or anthropogenic contamination [32, 33].
Generally, drinking water containing different anions and heavy metals has significant adverse effects on human health either through deficiency or toxicity due to excessive intake. For example, like many elements, fluoride which generally occurs in nature is beneficial to human health in trace amounts, but can be toxic in excess. The links between low intakes of fluoride and dental protection are well known; however, fluoride is a powerful calcium-seeking element and can interfere with the calcified structure of bones and teeth in the human body at higher concentrations causing dental or skeletal fluorosis. Previous studies show that health problems associated with excess fluoride in drinking water are experienced in our country. Including the study area, Isparta and Burdur provinces are among the regions where this problem is most experienced [34].
In this study, the Salda Lake basin was selected as the study area. The Salda Lake basin is a region where intensive agricultural activities and animal husbandry are carried out. Groundwater is used as drinking, domestic and irrigation water in the study area especially. Also, Salda Lake is located in the basin. Salda Lake is one of Turkey’s two alkaline lakes with pH values ranging between 9 and 12. Salda Lake is located in SW Turkey and is known as Turkey’s deepest and cleanest lake. This lake is a natural analog for mineralogic carbon storage [35, 36], as meteoric waters feeding the lake dissolve adjacent ultramafic rocks and precipitate hydromagnesite in shallow littoral zones [37]. Salda Lake is extensively used for tourism and fishing. In addition, it is planned that the water of the Salda Lake will be used as drinking water in the future. Groundwater is one of the most important recharge sources for Salda Lake. Agricultural activities are the most important nonpoint pollution sources observed in the basin. The most intensive crop species are wheat, barley, corn, oats, nectarine, vegetables and fruits (especially apples), sugar beet, anise, poppy, chickpeas, beans and potatoes. During these activities, fertilizers (synthetic and natural) and pesticides are used extensively to increase product quality and quantity. Livestock in the study area is an additional source of income. Livestock wastes are another pollutant in the basin [38]. Pollutants mixed into groundwater as a result of these activities also pollute Salda Lake.
Therefore, it is important for the region to determine the chemical properties, pollution status and usage areas of groundwater and determine the effects on human health depending on the use of these waters. In relation to this, the fact that the effect of agricultural activities on the water resources (Lake and groundwater) in the region has not been studied in detail before is a major deficiency for the study area. This study aims to overcome this deficiency with current scientific techniques. As a result, the main purposes of the present study are to determine the basic hydrochemical properties of the groundwater in the basin, to evaluate the groundwater quality for drinking and irrigation by calculating the water quality index (WQI) and to evaluate the human health risk related to high arsenic concentrations in the groundwater.
Material and methods
Study area
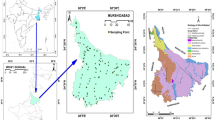
The Salda Lake basin is located between 4,259,404–444,624 N and 1,799,785–734,837 E. The study area has 207.14 km2 basin area. Generally, the Mediterranean climate is observed in the study area.
The Salda Lake is located in a depression basin with closed basin characteristics, formed by the effect of tectonism at the end of the Neogene [39]. The Quaternary alluvium in the Salda Lake basin covers up to 34.84 km2. Yeşilova is located southeast of Salda Lake and has the largest agricultural area in the basin. In addition, the wastewater from settlements in the Salda Lake basin discharges into streams or into septic tanks and the streams flow into the lake, too. The thickness of the aquifer is 5–60 m in Yeşilova and surroundings. The aquifer system is composed of sand, gravel, clay and mudstone levels (Table 1). The mean annual precipitation and real evaporation are approximately 494.10 mm/year and 345.91 mm/year in the basin, respectively [38].
Geology and hydrogeology
The interaction time of groundwater within the stratigraphic units controls the chemical compounds in the groundwater. Therefore, firstly the lithological units in the study area were investigated. The lithological formations are autochthonous and allochthonous in the study area. Allochthonous units are Marmaris peridotite and Dunite member, Kızılcadag ophiolitic mélange, İğdir metamorphites, Dutdere limestones and Orhaniye formation. The autochthonous units are alluvium and slope debris in the Çameli formation (Table 1, Fig. 1).
The lithological formations in the study area have different hydrogeological properties. Therefore, lithological units in the study area were classified as permeable-1 (granular aquifer) and permeable-2 (karstic aquifer), semipermeable (aquitard-1, 2, 3) and impermeable (aquifuge). The properties of the formations in the study area and their hydrogeological classification are given in Table 1. The Kızılcadag ophiolitic mélange and İğdir metamorphic rocks are classified as impermeable (aquifuge) units since they cannot contain water. Peridotite and dunites are generally impermeable rock types. However, fractures and cracks found in these rocks ensure that they can contain significant amounts of water depending on their spread and frequency. A large number of springs were also observed in the locations with outcrops of Marmaris peridotite and Dunite member units, which are widely distributed in the study area. Most of these springs are in the shoreline areas of Lake Salda. At the same time, these spring waters recharge Salda Lake. Therefore, the Marmaris peridotite and dunit member units in the Salda Lake basin are classified as semi-permeable units (aquitard-3) in terms of their hydrogeological properties.
The Orhaniye formation, which is observed in a small area east of Doğanbaba village to the north of the study area, is classified as semipermeable (aquitard-2). Semipermeable (aquitard-1) characteristics were identified by considering the lithology of the Çameli formation (Plç), which is observed in a very small area at the southern boundary of the study area. Limestones allow groundwater to be retained in significant amounts in cracks and melting spaces and allow transport of water. Therefore, the Dutdere limestone unit, which is observed in Kayadibi village and south of Yeşilova in the study area, represents permeable-2 (karstic aquifer) features. Quaternary alluvium and slope debris that are exposed in large areas of the basin are classified as permeable-1 (granular aquifer). The most important unit with aquifer features in the study area is alluvium. The area of the alluvium in the basin is approximately 34.84 km2. When well logs are examined, the thickness of alluvium is between 5 and 60 m in the study area.
Sampling and analysis
A total of 42 groundwater samples were analyzed in November 2015 (dry season) and June 2015 (wet season) in the study area. The water samples taken from each sampling point were collected in two plastic bottles which were pre-washed with deionized water. During sampling, two bottles were filled with water from each spot, with the first filtered and a few drops of HNO3 added for use for major cations, while the second sample was not acidified and used for major anions. All the samples were transported and kept in the dark at 4 °C before analysis. The physical parameters like hydrogen ion concentration (pH), electrical conductivity (EC), total dissolved solids (TDS), oxidation-reduction potential (Eh) and discharge temperature (°C) were determined in situ by using a YSI multi-parameter water quality probe (YSI 6050).
The major cations were analyzed at the Bureau Veritas Commodities Canada Ltd. (ACME Laboratory Vancouver, Canada, an ISO 9002 accredited company) with ICP-MS (inductively coupled plasma-mass spectrometer). Bicarbonate (HCO3), and carbonate (CO3) concentrations were determined, while chlorine (Cl), sulfate (SO4), nitrate (NO3), nitrite (NO2) and ammonium (NH4) were determined using ion chromatography in the Hacettepe University Water Chemistry Laboratory (Ankara, Turkey) with the titrimetric method (Table 2). The charge–balance error in the water samples was detected below 5%, which is within the limits of acceptability. All mathematical calculations were performed with Excel 2007 software (Microsoft Office). Statistical assessments such as correlation analysis were performed using the SPSS software version 15.
Methods
Hydrogeochemical evaluations
Hydrogeochemical evaluations were made using different diagrams. Among these diagrams, the Piper diagram especially was used to investigate and identify the hydrogeochemical facies of groundwater in the study area and the order of the main ionic abundance [40]. In addition, mechanisms controlling groundwater chemistry were determined by using Gibbs ratios [41]. The results of the analysis were evaluated by comparing with the permitted limit values for drinking water determined by [29, 31] (Table 3). These ratios were calculated using the following formulae (Eqs. 1 and 2):
-
$$ Gibbs\kern0.5em Ratio\kern0.5em I\kern0.5em \left( for\kern0.5em Anion\right)= Cl/\left( Cl+{HCO}_3\right) $$(1)
-
$$ Gibbs\kern0.5em Ratio\kern0.5em I\kern0.5em \left( for\kern0.5em Cation\right)=\left( Na+K\right)/\left( Na+K+ Ca\right) $$(2)
Water quality index (WQI)
The relative weight (Wi) is computed from the following Eq. (3).
Where Wi is the relative weight, wi is the weight of each parameter, and n is the number of parameters. In the next step, a quality rating scale (qi) for each parameter is assigned by dividing its concentration in each water sample by its respective standard according to the guidelines laid down by the World Health Organization [29] and Turkish Standards Institution [31] and the result is multiplied by 100 (Eq. 4):
-
$$ qi=\left( Ci/ Si\right)X100 $$(4)
Where qi is the quality rating, Ci is the concentration of each chemical parameter in each water sample in mg/L. Si is the drinking water standard for each chemical parameter in milligrams per liter according to the guidelines in [29, 31]. To compute the WQI, the SI is first determined for each chemical parameter, which is then used to determine the WQI as per the following (Eqs. 5 and 6)
Where SIi is the sub-index of i th parameter, qi is the rating based on concentration of i th parameter, and n is the number of parameters.
Arsenic risk assessment
Exposure assessment
Arsenic can enter the human body in many different ways such as food chain, dermal contact and inhalation. However, compared to oral intake, the effect of intake by all other means can be neglected. Therefore, exposure to AsT is measured in two main ways: oral (ingestion) and dermal (absorption) [42]. [43] recommends a lifelong average daily dose as an exposure measure to estimate a person’s daily exposure [34]. The following equation is a similar representation of daily exposure for the ingestion route modified from Eq. 7:
According to the formula, chronic daily intake (mg/kg/day) is ADD, drinking water contaminant concentration (mg/L) is C, ingestion rate per unit time (L/day) is IR, exposure duration (years) is ED, frequency of exposure (day/year) is EF, body weight (kg) is BW and average time (30/70 × 365 days) is AT. Deterministic exposure assessment is made to estimate individual exposures for each trace element using Eq. (7) [34]. As a result of this formulation, risk characterization must be done in order to make a risk assessment. Toxicity and exposure assessment results provide cancer risk and hazard indices with numerical estimates.
Human health risk assessment
In this study, both chronic and carcinogenic risk levels due to trace element concentrations in water sources were evaluated. HQ noncancer can be calculated by the following equation from [44] (Eq. 8):
-
$$ {HQ}_{noncancer}= ADD/ RfD $$(8)
AsT toxicity reference dose (RfD) used in the formula was determined as 0.0003 mg kg− 1 day−1 by [44]. Non-cancer risk, which is hazard quotient (HQ noncancer), is represented a single substance and/or exposure pathways for multiple substances. If a substance’s exposure level exceeds the specified reference dose value (RfD), that is, if the HQ noncancer value exceeds 1, it indicates that it may have potential non-carcinogenic but harmful effects. The higher this value, the higher the negative and carcinogenic effects on health [34, 44, 45]. Cancer risk (Rc) of trace elements on human health is calculated using the formula (Eq. 9) given below.
According to the formula, the carcinogenic risk is Rc, chronic daily intake (mg/kg/day) is ADD and pollutant’s slope factor (mg/kg/day) −1 is SF ([46]; Eq. 9). The slope factor (SF) of the contaminant for AsT was specified by [43] as 1.5 mg kg−1 day−1.
Statistical analysis
Basic descriptive statistical analysis and Pearson correlation analysis were done by using SPSS (Version 15.0, SPSS Inc., Chicago, IL).
Results and discussion
Hydrogeochemistry
Groundwater quality and chemical properties depend on the properties of the aquifer rocks and anthropogenic inputs. Therefore, hydrochemical properties and water quality information are needed to determine the suitability of the water for different purposes [47, 48]. In this study, the physicochemical, major ion and trace element contents of groundwater in the study area were determined and the mechanisms controlling their geochemical properties are detailed below under the following headings.
Seasonal evaluation of physico-chemical parameters
The physicochemical parameters and major ions in the groundwater samples, including temperature (°C), Eh, EC, pH and TDS, are statistically summarized and presented in Table 3. Temperatures (°C) of groundwater samples were 11.50–21.40 °C in the wet season and 8.50–16.10 °C in the dry season. Eh indicates the degree to which a substance is capable of oxidizing or reducing another substance [18]. Also, Eh, along with pH, is an important factor for determining arsenic (As) species. In the study area, Eh values in groundwater samples range from 86.40 to 255.80 (mV) in the wet season, and from 19.80 to 160.50 (mV) in the dry season. Electrical conductivity (EC) is a measure of the water’s ability to conduct electricity. The EC value increases due to increases in temperature and ion concentration of the water [33]. EC values of groundwater samples ranged from 265.70 (μS/cm) to 925.00 (μS/cm) in the wet season and 181.20 (μS/cm) to 929.00 (μS/cm) in the dry season. The high EC values in the water samples indicate enrichment of salts and the presence of anthropogenic effects resulting from domestic and agricultural activities in the study area. The pH values of the groundwater were measured as 7.38–9.74 in the wet season and 7.09–9.84 in the dry season. This shows that the waters are alkaline water. Generally, the pH values of all water samples increased in the wet season (Table 3). Also, all other samples, except water sample S16 well water, in the study area are drinkable in terms of pH values in the dry (9.84) and wet (9.74) season. Sample S16 is not suitable for use as drinking water. Since the pH values of other spring and well waters are within the limits specified in drinking water standards, they are suitable in terms of pH value as drinking water [29, 31]. In addition, TDS values of groundwater ranged from 200 to 660 (mg/L) in the wet season, and from 220 to 670 (mg/L) in the dry season. The TDS limit value stated in drinking water standards is 500 mg/L [29]. Accordingly, the water samples in the study area S7, S12, S14, S15 and S20 exceeded the limit values in November 2015 and June 2105.
All other samples in the study area are suitable for use as drinking water. Also, the TDS values of water samples are relatively high in the dry season. High TDS value is an indicator of the presence of anthropogenic inputs, especially agricultural pollutants in the study area.
Seasonal evaluation of major ions
The major cations most commonly found in groundwater are Ca, Mg, Na and K. The major anions are HCO3, Cl and SO4 (Table 3). The main source of these ions in groundwater is the decomposition of rock-forming minerals in water. Another reason for the presence and increase in these ions in groundwater is the contribution of atmospheric and anthropogenic chemical components [49].
According to analysis results, the relative abundance of cations in groundwater was in the order of Mg > Ca > Na > K and Mg > Na > Ca > K in the dry and wet season, while the anions were in the order of HCO3 > CO3 > Cl > SO4 and HCO3 > Cl > SO4 > CO3 in the dry and wet seasons, respectively (Table 4). The Ca ion concentration of the water samples in the study area ranged from 1.63 to 64.28 mg/L in the wet season and from 1.89 to 64.12 mg/L in the dry season. Mg ion concentration in the water samples ranged from 34.47 mg/L to 208.02 mg/L in the wet season and from 29.25 to 196.78 mg/L in the dry season. Na ion concentration of water samples in the study area ranged from 1.24 to 32.57 mg/L in wet season and from 1.10 to 25.09 mg/L in the dry season. K ion concentration of water samples ranged from 0.09 to 5.13 mg/L in the wet season and from 0.08 to 5.18 mg/L in the dry season.
Analysis results show that the dominant cations in the study area were determined as Mg, Ca and Na ions. In general, the reason for the presence of Ca and Mg ions in groundwater are carbonate-rich rocks such as limestone and dolomitic limestone in the environment. In the study area, high Mg ion concentrations in groundwater were associated with Marmaris peridotite and Kızılcadağ ophiolite and mélange units because ultrabasic rocks such as gabbro, peridotite and serpentine also contain abundant Mg, Fe and Ca ions. Accordingly, magnesium ion is dominant in the waters discharged from this type of rock. The source of Na and K ions in water are generally decomposition products of minerals such as albite, potash feldspar, mica and other silicate and aluminosilicate minerals. K concentrations in natural waters are generally much lower than Na ions. According to the results of the analysis, the increase in Na ion in the groundwater is remarkable, especially in the wet season. This increase is related to ion exchange due to water–rock interaction.
The bicarbonate (HCO3) ion concentration in groundwater samples ranged from 12.20 to 890.89 mg/L in the wet season and from 30.50 to 915.30 mg/L in the dry season. Carbonate (CO3) ion concentrations varied from 0.00 to 126 mg/L in the wet season and from 0.00 to 132 mg/L in the dry season (Table 3). According to the results of the analysis, HCO3 and CO3 ions increased during the dry season (Table 3). HCO3, which is the main indicator of alkalinity in water, usually occurs due to the dissolution of CO2 and carbonates, the reaction of silicates with carbonic acid and oxidation of organic matter [50]. The concentration of chloride (Cl) ranged from 1.57 to 49.84 mg/L in the wet season and from 1.76 to 56.66 mg/L in the dry season (Table 3). The main sources of chloride ions in natural waters are geogenic sources such as precipitation, water-rock interaction, and anthropogenic sources such as sewage, animal waste, synthetic fertilizers, chemicals and other industrial sources [51].
According to the results of the analysis, an increase in Cl concentrations in groundwater in the basin was observed in the dry season. The presence of Cl ions in the study area is associated with water-rock interactions and synthetic fertilizer mixing into the water. The SO4 concentration of the groundwater samples was between 0.71 and 66.93 mg/L in the wet season and from 0.13 to 73.68 mg/L in the dry season (Table 3). In natural waters, SO4 can be found by decomposition of sulfur-containing minerals and storage due to evaporation [52]. In addition, another reason for the presence of sulfate in waters is the mixing of manure and sewage waste water into the water [51]. Accordingly, when the analysis results were evaluated, it was determined that Cl and SO4 concentrations increased during the rainy season. This increase in Cl ions, especially in the wet season, may result from agricultural activities and animal wastes in the study area [38]. Chemical characteristics of water such as anions and cations are considered to be an important part in drinking water quality that can influence human health. As an adverse consequence of excessive use of fertilizer in farms, high concentration of SO4 can be observed in drinking water [45, 53]. Drinking water with high concentration of SO4 may cause health problems due to laxative properties.
Seasonal evaluation of fertilizers and trace elements
In the study, NO2, NO3, NH4 and heavy metal analysis (aluminum (Al), arsenic (As), fluoride (F), iron (Fe), manganese (Mn) and lead (Pb)) were completed to determine types and sources of pollutants (Table 3).
NO2, NO3, and NH4 are pollutants which mainly come from agricultural and industrial effluents, contain a high concentration of nitrogen and are some of the largest contributors to groundwater pollution in the world [47]. According to the results of the analysis, NO2 concentrations were determined as <0.01 mg/L in both periods. The detection limit for nitrite is ±0.001 mg/L. For this reason, the mean and standard deviation values for nitrite were not calculated (Table 3). NO3 concentrations ranged from 0.32 to 88.31 mg/L in the wet season and from 0.03 to 102.77 mg/L in the dry season. NO3 limit value stated in drinking water standards is 50 mg/L [29, 31]. Fertilizers, manures, and sewage are considered significant sources of NO3. Methemoglobinemia or blue baby disease is related to high concentrations of nitrate in drinking water. However, high concentration of nitrate (more than 10 mg/l) has no serious toxicity effects in adults [45, 54,55,56]. Accordingly, S7 well water among the water samples in the study area exceeded the limit values in November 2015 and June 2105. All other samples in the study area are suitable for use as drinking water.
In addition, NH4 concentrations were 0.06–0.10 mg/L in the wet season and 0.05–0.08 mg/L in the dry season (Table 3). From the point of view of NH4, all samples in the study area are suitable for use as drinking water [29, 31]. Accordingly, seasonal variation in NO2, NO3 and NH4 concentrations is thought to be related to infiltration of rainwater and agricultural activities.
When trace element analysis results are examined, fluoride (F) concentration of the groundwater samples was between 0.01 and 5.87 mg/L in the wet season and between 0.03 to 0.27 mg/L in the dry season (Table 3). The increase in F concentrations in the study area, especially in the wet season, is associated with water-rock interaction. The F limit value stated in drinking water standards is 1.5 mg/L [29, 31]. Accordingly, the F concentration in sample S9 spring water exceeded the limit values in June 2015. It is not appropriate to use this water as drinking water. All other samples in the study area are suitable for use as drinking water. Al concentrations were determined to be 1–369 μg/L in the wet season, and 1–517 μg/L in the dry season. The Al limit value stated in drinking water standards is 200 μg/L for [29] and 500 μg/L for [31]. Accordingly, the water samples from S2 well water in the study area exceeded the limit values in the dry and wet seasons. Therefore, it is not appropriate to use sample S2 as drinking water. In addition, AsT concentrations ranged from 2.90 to 10.50 μg/L in the wet season and from 2.10 to 6.30 μg/L in the dry season. The increase in arsenic concentrations of groundwater is associated with lithological units throughout the basin and around Salda Lake and is of geogenic origin. In addition, it is thought that the increase in arsenic concentrations in groundwater may be caused by agricultural activities. The AsT limit value stated in drinking water standards is 10 μg/L [29, 31]. Accordingly, the water samples from S14 well water and S19 well water exceed the limit values in the wet season. Due to the carcinogenic effect of arsenic, it is not appropriate to use these waters as drinking water. The FeT concentration ranged from 9.00 to 200 μg/L in the wet season and ranged from 10.00 to 437 μg/L in the dry season. FeT limit value stated in drinking water standards is 200 μg/L [31]. Accordingly, the water samples from S5 spring water exceed the limit values in wet season and from S2 well water, S5 spring water and S9 spring water exceed the limit values in dry season. Therefore, it is not appropriate to use these samples as drinking water. The Mn concentration ranged from 0.13 to 143.55 μg/L in the wet season and from 0.18 to 804.82 μg/L in the dry season. The Mn limit value stated in drinking water standards is 400 μg/L for [29] and 50 μg/L for [31]. Accordingly, the water samples from S5 spring water and S19 spring water exceed the limit values in the wet season. In addition, S5 samples exceeded the limit values in dry season. Therefore, it is not appropriate to use these samples as drinking water. Finally, the PbT concentrations were determined to be 0.10–1.00 μg/L in the wet season, and 0.10–39.50 μg/L in the dry season. The PbT limit value stated in drinking water standards is 10 μg/L [29, 31]. Accordingly, the water samples from S5 spring water and S19 spring water exceed the limit values in the wet season. Samples from S5 exceeded the limit values in the dry season. Accordingly, in terms of PbT concentrations, all water samples are drinkable during the wet season. However, in the dry season, PbT concentrations exceed the limit value in samples from S3 spring water and S10 spring water. Therefore it is not appropriate to use these samples as drinking water.
As a result, it is thought that seasonal variations in the presence and concentration of trace elements in waters may be of geogenic origin, due to the fact that the most common elements found in ultrabasic rocks (containing gabbro, peridotite, serpentine) are Mg, Fe, Mn and Ca. In addition, elements in rainwater are thought to have anthropogenic origin by infiltrating groundwater through agricultural activities.
Hydrogeochemical facies
One of the important tools used to determine the chemical history and origins of groundwater is hydrogeochemical facies. Hydrogeochemical facies determined using major ions (cations and anions) in water are classifications used to show similarities and differences between the chemical properties of water (Table 5). The hydrogeochemical facies of groundwater in the study area was evaluated with the Piper diagram [40] using major cations (Ca, Mg, Na and K) and anions (HCO3, SO4, and Cl) as meq/L unit and separately for both seasons (Fig. 2). According to the Piper diagram, dominant water types are Mg-Ca-HCO3 and Mg-HCO3 in the wet season. Also, Mg-HCO3 and Mg-HCO3-CO3 are the main water facies in the dry season (Table 5). The interaction time of groundwater with rocks plays an important role in the formation of different water types in the region. The high Mg concentrations of groundwater in the region are associated with peridotite and serpentine, which are commonly found throughout the basin. Mg ion is the main component of groundwater in terms of chemical structure.
According to the Piper diagram, water facies in dry and wet season [18]
Mechanisms controlling groundwater geochemistry
Information on the origin of dissolved ions in groundwater can be accessed with the Gibbs diagrams [41]. These diagrams are plots of (Na + K)/(Na + K + Ca) vs TDS (Gibbs Ratio I) and Cl/(Cl + HCO3) vs TDS (Gibbs Ratio II). These diagrams are mostly used to determine chemical processes such as precipitation, rock and evaporation dominance, which reveal the chemical composition of water.
In this study, Gibbs diagrams were used to determine the factors affecting the chemical structure of the waters in the region. The Gibbs diagrams show that almost all water samples in the study area fall in the rock dominance and evaporation dominance areas (Fig. 3). This means that the chemical decomposition of rock-forming minerals and evaporation are the main processes that contribute to water chemistry. Depending on the ion content of the samples plotting in the area of rock domination indicates interaction between rock chemistry and groundwater chemistry. The evaporation field is associated with high rates of dominant cations and anions, especially sodium ions, as well as increases in TDS value, in relation to precipitates caused by the combination of Ca and HCO3 [57].
Gibbs diagram [19]
Statistical analysis
Correlation matrix
The correlation analysis between the physicochemical and trace elements of the water samples can reveal the effects of the factors in the origin and composition of the water on each other [15]. In this study, the Kolmogorov–Smirnov (K-S) test was initially used to determine the compatibility of the data to normal distribution [58]. According to the K-S test, all the variables were normally distributed with 95% confidence. Therefore, Pearson correlation analysis was applied to determine the relationship between physicochemical parameters and trace elements in water samples. The correlation matrix of the parameters (Temp, Eh, EC, pH, TDS, Ca, Mg, Na, K, CO3, HCO3, Cl, SO4, NO3, NH4, F, Al, AsT, FeT, Mn and PbT.) is given in Tables 6 and 7. A high correlation coefficient (near +1 or + 1) means a good positive relationship between two variables and a value around zero means no relationship between them at a significant level of p < 0.05. More precisely, it can be said that parameters showing r > 0.7 are considered to strongly correlate whereas r between 0.5 and 0.7 shows moderate correlation. When the correlation coefficient decreases, the relationship ratio between the variables decreases [20, 59,60,61,62]. The correlation matrix of the parameters is given in Table 6 (wet season), and Table 7 (dry season). The analysis results for NO2 in the dry and wet season were 0.00 mg/l, and owing to this results were not evaluated in the correlation matrix.
According to the physicochemical Pearson correlation analysis results, pH showed negative moderate correlation with EC in the wet season and negative moderate correlation with EC and Eh in the dry season. TDS showed positive strong correlation with EC and negative moderate correlation with pH in wet season. In addition, TDS showed positive strong correlation with EC in dry season. This indicates that the total dissolved solid matter in the water samples and the electrical conductivity values increase in direct proportion with each other. This increase may be related to anthropogenic inputs as well as rock water interaction.
According to the Pearson correlation analysis results for the major ions, Ca ion showed negative strong correlation with pH in the wet season. In addition, Ca ion showed positive moderate correlation with Eh and negative strong correlation with pH. Mg ion showed positive strong correlation with EC and TDS in the wet and the dry seasons. This shows that Ca and Mg salt in water samples increase the TDS and EC values. This increase is particularly related to rock-water interaction due to limestones and dolomitic limestones in the study area. Na ion showed positive moderate correlation with Ca in the wet and dry seasons. This indicates that ion exchange processes occur between Ca and Na ions, and there may also be anthropogenic input into the water. The CO3 ion showed positive strong correlation with pH and negative moderate correlation with Ca in the wet season. It also showed a negative moderate correlation with Eh in the dry season, in addition to the correlations during the wet season. The HCO3 ion showed positive strong correlation with EC, TDS, and Mg, showed a negative moderate correlation with pH and CO3 in the dry and wet seasons. This indicates that the CO3 and HCO3 ions in the water samples and the Ca ion, Mg ion, electrical conductivity and TDS values increase in direct proportion with each other due to water-rock interaction. The Cl ion showed positive strong correlation with Na in the wet and dry seasons and positive moderate correlation with Ca in the wet season. SO4 ion showed positive strong correlation with Ca, Na and Cl ions in wet season. In addition, SO4 ion showed positive strong correlation with Cl and positive moderate correlation with Ca and Na ions. The SO4 and Cl ion concentration may also be related to rock-water interaction in the study area and particularly inputs related to agricultural activities.
According to the Pearson correlation analysis results of the trace elements and pollution parameters, NO3 ion showed positive strong correlation with Ca, Na, Cl and SO4 ions in wet season. In addition, NO3 ion showed positive strong correlation with Cl, SO4 ions and positive moderate correlation with Ca and Na in the dry season. This shows that there is a strong and directly proportional relationship between the increase in NO3 ion concentration in water samples and especially Na and Cl concentrations. The presence and increase of the NO3 ion concentration are also associated with inputs related to agricultural activities in the study area. NH4 ion showed positive strong correlation with K ion only in the dry season. The direct proportional increase relationship between ammonium salts with K ion also shows that there are inputs related to agricultural activities in water. F ion showed positive strong correlation with Ca ion positive moderate correlation with Na and negative moderate correlation with EC in the dry season. The F ion did not show a significant correlation with any parameters in the wet season. The direct proportional relationship between the amount of fluoride (F) in water samples with Ca and Na concentrations shows us that rock-water interaction is high in the study area. Al ion showed positive moderate correlation with Ca ion only in the wet season. AsT showed a negative moderate correlation with Al only in the wet season. The AsT did not show a significant correlation with any parameters in the dry season. FeT showed positive moderate correlation with Al ion in dry and wet season. Mn ion showed negative strong correlation with Eh showed positive moderate correlation with NH4 and FeT in the wet season. In addition, the Mn ion showed positive moderate correlation with F and FeT in the dry season. PbT ion showed negative moderate correlation with temperature in the dry season. PbT ion did not show a significant correlation with any parameters in the wet season. Among the trace elements, especially the positive relationship of FeT and Mn ion may be due to rock water interaction or pesticides in water. As a result statistical analysis results show that rock-water interaction, climate conditions, ion exchange processes and excessive application of fertilizer and agricultural chemicals in the study area affect water resources.
Evaluation of Water Quality
In order to determine the suitability of the water samples in the study area for drinking, irrigation and industrial purposes, detailed evaluations are made in this section.
Evaluation of water quality for drinking water
Water quality index (WQI) evaluations
WQI is a kind of grading technique prepared to show the total effect of each water quality parameter on the overall quality of the water to be used for human consumption [63]. The standards for drinking purposes as recommended by [64] were considered for the calculation of WQI. In this study, WQI was computed in three steps. The analysis results were evaluated and compared with [29, 31] (Table 3). Each of the 16 parameters (pH, TDS, Ca, Mg, Na, K, HCO3, Cl, SO4, NO3, F, Al, FeT, PbT, AsT, Mn) were assigned a weight (wi) according to its relative importance for drinking water quality (Table 8). Much weight is assigned to parameters which have critical health effects and whose presence above certain critical concentration limits could limit the usability of the resource for domestic purposes. [65]. For this reason, parameters such as TDS, Cl, SO4, NO3, PbT and AsT are given the maximum weight value of 5 due to their deterioration of water quality and effect on consumption [66]. Since it has the least effect on water quality, HCO3 ion is given a value of 1 with a minimum weight value. Other parameters are evaluated among themselves and weight values between 1 and 5 are given to these parameters according to their importance.
Finally, the WQI values obtained from the calculations were evaluated together with the water quality types in Table 9. In addition, the WQI values and water types for each sample are shown separately in Table 10 for the dry and wet season. According to Table 10, the calculated WQI values ranged from 24.14 to 56.93 in the wet season. In addition, 71.42% of groundwater samples represent “excellent water” and 28.57% of groundwater samples represent “good water” in the wet season. In the dry season, WQI values ranged from 25.27 to 145.87 in the study area. In this season, 80.95% of groundwater samples represent “excellent water”, 14.28% of groundwater samples represent “good water” and 4.76% of groundwater samples represent “poor water”. This situation indicates that, the quality of water samples is mostly good in both seasons. However, when the quality of groundwater in the dry season and the groundwater quality in the wet season are compared, the water quality in the dry season is relatively deteriorated. The increase of ion concentrations in the dry season is associated with the infiltration of anthropogenic pollutants into groundwater in agricultural areas.
In addition, fertilizers and trace element concentrations in water samples were compared and assessed with the limit values determined by the World Health Organization [29] for the usability of drinking water. Accordingly, it is not appropriate to use as drinking water in terms of fertilizers and trace element analysis results (Table 3).
Evaluation of arsenic pollution in groundwater
Arsenic is the 20th most abundant element found in the earth’s crust, and its presence in the continental crust is 1–2 mg/kg [67]. Arsenic is released organically by natural processes such as decomposition and volcanic eruptions (geogenic) and from anthropogenic (human activities) sources and can be transported over long distances as water- or air-suspended particles and aerosols. However, inorganic arsenic, which constitutes the human health risk, is primarily present in the form of arsenate (As5+) or arsenite (As3+). In general, arsenate is dominant in oxidizing conditions, whereas arsenite is dominant in reducing conditions. AsT concentrations were between 2.1–6.3 μg/L in the dry season and 2.9–10.5 μg/L in the wet season. AsT limit value stated in drinking water standards is 10 μg/L [29 and 31]. Accordingly, the water samples in the study area from S14 well water and S19 well water exceed the limit values in the wet season.
Therefore, the reason for the increase in arsenic concentration in groundwater in the study area was investigated. There are four main geochemical processes that cause arsenic formation in water. These reactions are enrichment by dissolution, desorption and evaporation through oxidation and reduction. Accordingly, the high AsT concentration in groundwater in the study area may be caused by an oxidative and reductive dissolution reaction of Fe and Mn hydroxides in the Kızılcadağ ophiolitic mélange and Marmaris peridotite [20]. The Kızılcadağ ophiolite and mélange and Marmaris peridotite cover wide areas in the Salda Lake basin and groundwater wells are located within the alluvium formed by materials detached from the Kızılcadağ ophiolite and mélange and Marmaris peridotite. (Fig. 1). This unit consists of extremely serpentinized ophiolitic material, and can be considered as a geogenic source of AsT in groundwater [68]. In addition to all this, groundwater in the study area may be polluted by anthropogenic pollution as well as geogenic pollution because agricultural activities are carried out intensively in the study area, especially in the alluvial environment around Salda Lake.
Accordingly, the arsenic species found in groundwater in the study area were determined using the pH-Eh diagram (Fig. 4). According to this diagram, arsenate (H2AsO4−2) was identified as the dominant arsenic type in groundwater in the study area. Increasing the pH of the water also affects the solubility of arsenic in the water. Studies show that arsenic can be adsorbed to colloidal iron oxides when it enters an oxidizing environment with a pH higher than 9.4 [69,70,71,72]. Accordingly, arsenic can be a contaminant in water sources in relation to oxidation under alkaline conditions. The pH values of the water samples in the study area ranged from 7.38 to 9.74 in the wet season and 7.09 to 9.84 in the dry season and all of the waters were alkaline in terms of pH. This situation creates a potential environment for arsenic contamination in the study area. According to this, the reason for arsenic pollution in the region is accepted as geogenic due to the Kızılcadağ ophiolite and mélange and Marmaris peridotite which are widely distributed in the study area and is accepted as anthropogenic due to the chemicals used in agricultural activities.
Arsenic health risk assessment
The consumption of drinking water contaminated with arsenic plays an important role in increasing diseases related to arsenic exposure. Risk assessment is defined in two groups as carcinogenic risk and non-carcinogenic risk. Geographical differences, arsenic (AsT) concentrations within drinking water, differences in water consumption and anthropometric features such as height and body weight are indicative of the variability of arsenic exposure. Therefore, exposure and carcinogenic effect are determined by taking into account the daily intake, the amount of concentration, water consumption rate, body weight and all variations in sex [73].
In the study area, groundwater is used as drinking, irrigation and domestic water by local people from wells. Therefore, in this study, a health risk assessment (chronic and carcinogenic effects such as average daily dose (ADD), HQnoncancer and carcinogenic risk (Rc)) was made for arsenic exposure in groundwater through drinking water intake in Tables 11 and 12. In addition, health risk values were calculated separately for adults and children and are given in Table 12 because children are more vulnerable to the harmful effects of arsenic compared to adults.
According to Table 12, the values of ADD for AsT were between 0.0000–0.0001 (mg/kg) for an adult and between 0.0002–0.0007 (mg/kg) for a child in the wet season. In addition, the values of ADD for AsT were between 0.0000–0.0001 (mg/kg) for an adult and between 0.0001 and 0.0004 (mg/kg) for a child in the dry season. The values of HQnoncancer were between 0.1324 and 0.4795 for an adult and between 0.6180 and 2.2374 for a child in the wet season. In addition, the values of HQnoncancer were between 0.0959–0.2877 for an adult and between 0.4475–1.3425 for a child in the dry season. Also, values of Rc, were between 0.0001–0.0002 for an adult and between 0.0002–0.0007 for a child in the wet season. In addition, values of Rc, were between 0.0000–0.0001 for an adult and between 0.0002–0.0006 for a child in the dry season.
The carcinogenic risk is the possibility of developing cancer in the body if a person is exposed to a substance that has a toxic effect throughout their life. Tolerable risk values for toxic substances were determined by experts and legal regulators. Accordingly, the tolerable risk values determined for arsenic are between 10−6 and 10−4 [18, 19, 74, 75].
According to Table 12, the risk of developing cancer due to arsenic exposure in healthy adults or children is very low. But arsenic has high non-carcinogenic and carcinogenic potentially harmful effects. According to drinking water standards, concentrations of AsT exceeded the limit values in only S14 and S19. This also supports the health risk assessment results.
Evaluation of water quality for irrigation use
Values of parameters such as sodium adsorption rate (SAR), permeability index (PI), sodium percentage (% Na), magnesium hazard (MH) and residual sodium carbonate (RSC) were calculated to determine the suitability of the waters in the study area for use as irrigation water in agricultural activities. The results obtained are evaluated in Table 13.
Sodium adsorption ratio
SAR values, which are one of the most important parameters in the use of groundwater in the study area for irrigation water in agricultural areas, were between 0.03 and 0.71 in the wet season and between 0.03 and 0.77 in the dry season. Accordingly, groundwaters are in the “no problem” class in terms of SAR value in both periods. In short, the waters are suitable for irrigation in terms of SAR value (Table 13). In addition, the United States Salinity Laboratory (USSL 1954) diagram was prepared according to the relationship between the EC and SAR values of water. According to the diagram, 85.71% of the groundwater in the study area was in the C2-S1 water class and 14.25% was in the C3-S1 water quality class in the wet season. This shows that most of the groundwater in the wet season has medium salinity and low sodium hazard and is suitable for use as irrigation water. In this period, only samples from S7 well water, S12 spring water and S15 spring water had high salinity and low sodium hazard. Also, 76.19% of the groundwater was classified as C2-S1, 19.04% C1-S1 and 4.76% C3-S1 water quality in the dry season (Fig. 5). This shows that most of the groundwater in the dry season has low-medium salinity and low sodium hazard and is suitable for use as irrigation water. In this period, only sample S12 had high salinity and low sodium hazard. When evaluated in general, samples S7, S12 and S15 are limited for use as irrigation water.
Permeability index
In the study, permeability index values were calculated and [76] classification was prepared according to these values. PI values of groundwater in the study area vary between 22.24 and 56.32 in the rainy season and between 23.66 and 46.51 in the dry season. According to these results, all water samples are in the category of “suitable” waters and are suitable for use as irrigation water (Table 13).
Sodium percentage
In all natural waters, the percentage of sodium in water is a parameter commonly used to evaluate the suitability of water for agricultural purposes [77]. Sodium combined with carbonate ion causes the formation of alkaline soils. In addition, sodium combined with chloride ion forms soils with high salt content. Both of these soils prevent plant growth and reduce crop yield [78]. Therefore, in order to determine the suitability of groundwater for use as irrigation water in the study area, sodium percentage values of individual samples were calculated for dry and wet seasons (Table 13). The sodium percentage of these samples was plotted against EC in a Wilcox diagram (Fig. 6) and are given in Table 13. According to the Wilcox diagram, 95.23% of all samples were in the “excellent to good” irrigation water class and 4.76% (S16) were in the “good to permissible” irrigation water class in the dry season. In addition, all samples were in the “excellent to good” irrigation water class in the wet season.
According to the [55] diagram, irrigational suitability of groundwater in the dry and wet season
Magnesium hazard (MH)
Another method developed to evaluate the suitability of irrigation water quality is the “magnesium hazard” ratio (MH) developed by [79]. Ca and Mg ions are generally in equilibrium in water and tend to maintain this equilibrium state. If the Na ions or other salts in the water are high, the magnesium concentration in the water increases and this damages the soil structure. This situation affects crop yields [15]. Accordingly, MH values for groundwater in the study area were calculated. MH values varied between 49.64 and 98.78 in the wet season and between 49.41 and 98.91 in the dry season. When the water samples in the study area are evaluated with the classification according to MH given in Table 13, all water samples are not suitable for use as irrigation water. It was determined that if all groundwater throughout the basin is used as irrigation water, it will have negative effects on agricultural land and crop productivity.
Residual sodium carbonate (RSC)
RSC is another important parameter used in evaluating the use of water as irrigation water [15, 80]. If magnesium and calcium ions are high, these ions tend to precipitate as carbonate. While carbonate precipitation takes place, the sodium concentration increases and the sodium in the water is fixed to the soil. Thus, soil permeability is reduced; [18] classified irrigation water in three main groups according to RSC (Table 13). According to the RSC values, 95.23% of all samples were in the “suitable” irrigation water class and 4.76% (S20 spring water) were in the “marginal” irrigation water class in the dry season. In addition, all samples were in the “suitable” irrigation water class in the wet season (Table 13).
Evaluation of water quality for industry
Classifications for the quality of water to be used in industrial areas are quite diverse and have their own standards for each industrial unit. The most common problems due to low water quality and related chemical reactions in industrial areas are crusting and corrosion. Crusting is a condition that occurs on metal surfaces in industrial materials and vehicles due to excessive CaCO3. Corrosion, on the other hand, is the wear of metal surfaces due to the chemical properties of water as a result of contact of water with metals in the industrial environment. There is no known large-scale industrial activity in the study area. However, in this study, an attempt was made to determine whether groundwater is suitable for use in small-scale industrial activities and industries planned for the future according to the following water quality criteria [18];
-
(a)
If the water contains more than 400 mg/L of HCO3 or more than 100 mg/L of SO4 ions, a crusting problem may occur;
-
(b)
If the water has pH <7, TDS more than 1000 mg/L or Cl more than 500 mg/L, this water may cause corrosion in its environment.
When the groundwater in the study area was evaluated according to these criteria, + approximately 42.85% of HCO3 concentrations of the samples (S2, S5, S7, S8, S12, S13, S14, S15 and S20) exceeded 400 mg/L in the wet season. Also 38.09% of HCO3 concentrations of the samples (S2, S5, S12, S13, S14, S15, S17 and S20) exceeded 400 mg/L in the dry season. In addition, SO4 concentrations in none of the water samples exceeded 100 mg/L in dry and wet seasons. The results show that the use of the mentioned samples is not recommended as it may cause crusting on metal surfaces in industrial areas. In the evaluation made according to other criteria for industrial use, pH > 7, TDS values were lower than 1000 mg/L and Cl concentrations were lower than 500 mg/L in both dry and rainy seasons in the study area. This shows that the groundwater of the region has no corrosion effect.
Conclusions
In the present study, hydrochemical properties, water quality and its suitability for drinking and irrigation were assessed using water quality index methods in the Salda Lake basin, and arsenic pollution of the groundwater and effects on human health were examined. The groundwaters are used especially as drinking, domestic and irrigation water in the study area. At the same time, these groundwaters are one of the most important recharge sources for Salda Lake located in the basin. It is also known that Salda Lake basin is a region where intensive agricultural activities and animal husbandry are carried out.
In this study, according to the analysis results, the physical properties and major ions in groundwater show that seasonal changes in the physicochemical properties of groundwater occur due to rock-water interaction. According to the Piper diagram, the dominant water types are Mg-Ca-HCO3 and Mg-HCO3 in the rainy season. In addition, Mg-HCO3 and Mg-HCO3-CO3 are dominant water types in the dry season. In the results obtained from the Gibbs diagrams, the chemical decomposition and evaporation of rock-forming minerals are the main processes that contribute to water chemistry. Pearson correlation analysis results also showed that dissolution/precipitation reactions, concentration effects and anthropogenic inputs are mainly effective in the formation of physicochemical properties of groundwater, in relation to simultaneous increase/decrease in cations.
In this study, WQI was applied to determine the drinking water quality of groundwater samples in the study area. According to the calculated WQI values, 71.42% of groundwater samples represent ‘excellent water’ and 28.57% of groundwater samples represent ‘good water’ in the wet season and 80.95% of groundwater samples represent ‘excellent water’, 14.28% of groundwater samples represent ‘good water’ and 4.76% of groundwater samples represent ‘poor water’ in the dry season. This situation indicates that the quality of water samples is mostly good in both seasons. However, fertilizers and trace element concentrations in water samples were compared and assessed with the limit values determined by the World Health Organization (2011) for the usability of drinking water. Accordingly, it is not appropriate to use the groundwater as drinking water in terms of fertilizers and trace element analysis results. Especially, AsT pollution was detected in samples S14 and S19 from the groundwater of Salda Lake basin during the wet season. According to the Eh-pH graphs, the dominant type is arsenate. According to this, the reason for arsenic pollution in the region is accepted as geogenic due to the Kızılcadağ ophiolite and mélange and Marmaris peridotite which are widely distributed in the study area and as anthropogenic due to the chemicals used in agricultural activities. Therefore, health risk assessment related to arsenic was performed for adults and children separately. According to this, the risk of developing cancer due to arsenic exposure in healthy adults or children is very low. But arsenic has high non-carcinogenic and carcinogenic potentially harmful effects.
In addition, the sodium adsorption ratio (SAR), permeability index (PI), sodium percentage (Na %), magnesium hazard (MH), and residual sodium carbonate (RSC) indices were used to determine the suitability of groundwater for agricultural irrigation. As a result, none of the water samples in the study area are suitable for use as irrigation water in terms of MH values. Use of this water will cause a decrease in soil quality and crop yield in the region. In addition, almost all of these waters are suitable for use as irrigation water in terms of the other parameters. Also, groundwater quality was also evaluated in terms of suitability for industrial use. The results show that the use of some samples (S2, S5, S7, S8, S12, S13, S14, S15 and S20) is not recommended as it may cause crusting on metal surfaces in industrial areas. In addition, the groundwater of the region has no corrosion effect.
Besides all these, the water quality in Salda Lake, which is recharged from groundwater, will gradually deteriorate due to the pollution of groundwater. This situation will negatively affect the ecological balance of Salda Lake and its surroundings together with human health in the Salda Lake basin. Therefore, the results obtained from this study show that for the sustainability of water resources in Salda Lake and its basin, groundwater quality should be monitored, usage areas should be determined accordingly and necessary measures should be taken.
Ultimately, it is suggested that the knowledge of people and farmers in the study area be improved about using organic and inorganic materials as fertilizer for protecting groundwater sources with emphasis on groundwater contamination and human health risks. Furthermore, strict governmental management and administration of effective water treatment, as well as monitoring of selected water supply sources far from contaminated sources, should be considered.
References
Gümrükçüoğlu M, Baştürk O. A study on river pollution in sustainable water management, TMMOB 2. Water Policy Congress. 2008;1:52–529.
Abbasnia A, Radfard M, Mahvi AH, Nabizadeh R, Yousefi M, Soleimani H, Alimohammadi M. Groundwater quality assessment for irrigation purposes based on irrigation water quality index and its zoning with GIS in the villages of Chabahar, Sistan and Baluchistan, Iran. Data in Brief. 2018a;19:623–31.
Yousefi M, Ghoochani M, Mahvi AH. Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol Environ Saf. 2018a;148:426–30.
Boateng TK, Opoku F, Acquaah SO, Akoto O. Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben municipality. Ghana Environ Earth Sci. 2016;75(6):489.
Babiker SI, Mohamed AA, Mohamed TH. Assessing groundwater quality using GIS. Water Resour Manag. 2007;21:699–715.
Arya S, Kumar V, Sharma S. Analysis of water quality parameters of groundwater in and around diamond cement industry, Jhansi. Central India Int J Curr Res. 2012;4(3):75–7.
Dahiya S, Kaur A. Physico chemical characteristics of underground water in rural areas of Tosham subdivision, Bhiwani district. Haryana J Environ Poll. 1999;6(4):281.
Şener Ş, Şener E, Davraz A, Varol S. Hydrogeological and hydrochemical investigation in the Burdur saline Lake Basin, Southwest Turkey. Geochemistry. 2020;80:125592.
Li F, Zhu J, Deng X, Zhao Y, Li S. Assessment and uncertainty analysis of groundwater risk. Environ Res. 2018;160:140–51.
Varol S, Davraz A. Assessment of geochemistry and hydrogeochemical processes in groundwater of the Tefenni plain (Burdur/Turkey). Environ Earth Sci. 2014;71(11):4657–73.
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ. Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf. 2009;72:301–9.
Sargın AH. Groundwaters. Ankara, Turkey: General Directorate of State Hydraulic Works (SHW) Geotechnical Services and Groundwater Department; 2010;200p.
Yetiş AD. Determination of Ceylanpınar plain groundwater quality and pollution potential. Adana, Turkey: Çukurova University, Institute of Science, Department of Environmental Sciences PhD thesis; 2013. (Ç. U. Supported by Research Projects Unit. Project No: MMF2012D5)
Nadiri AA, Moghaddam AA, Tsai FT, Fijani E. Hydrogeochemical analysis for Tasuj plain aquifer. Iran J Earth Syst Sci. 2013;122(4):1091–105.
Varol S, Davraz A. Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci. 2015;73(4):1725–44.
Abbasnia A, Alimohammadi M, Mahvi AH, Nabizadeh R, Yousefi M, Mohammadi AA, Pasalari H, Mirzabeigi M. Assessment of groundwater quality and evaluation of scaling and corrosiveness potential of drinking water samples in villages of Chabahr city, Sistan and Baluchistan province in Iran. Data in Brief. 2018b;16:182–92.
Yousefi M, Yaseri M, Nabizadeh R, Hooshmand E, Jalilzadeh M, Mahvi AH, Mohammadi AA. Association of hypertension, body mass index, and waist circumference with fluoride intake; water drinking in residents of fluoride endemic areas, Iran. Biol Trace Elem Res. 2018b;185(2):282–8.
Varol S, Şekerci M. Hydrogeochemistry, water quality and health risk assessment of water resources contaminated by agricultural activities in Korkuteli (Antalya, Turkey) district center. J Water Health. 2018;16(4):574–99.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, & International Agency for Research on Cancer. Some drinking-water disinfectants and contaminants, including arsenic, 2004; Vol. 84.
Varol S, Köse İ. Effect on human health of the arsenic pollution and hydrogeochemistry of the Yazır Lake wetland (Çavdır-Burdur/Turkey). Environ Sci Pollut Res. 2018;25(16):16217–35.
Lone SA, Jeelani G, Mukherjee A, Coomar P. Geogenic groundwater arsenic in high altitude bedrock aquifers of upper Indus river basin (UIRB), Ladakh. Appl Geochem. 2020;113:104497.
Claesson M, Fagerberg J. Arsenic in groundwater of Santiago Del Estero, Argentina: a minor field study. Royal Ins. of Tech. Inter. Office, TRITA-LWR-EX-03-5, 2003;59.
Yazıcı M, Değirmenci M, Sözüdoğru O, Ekmekçi M, Atmaca E, Tezcan L, et al. Groundwater of Kayseri evaluation in term of arsenic contamination. Karaelmas Sci Eng J. 2015;5(1):16–25.
Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58(1):201–35.
Moore K. Treatment of arsenic contaminated groundwater using oxidation and membrane filtration. Master of Applied Science in Civil Engineering, University of Waterloo, 2005.
Başkan MB, Pala A. Arsenic pollution in drinking water: an evaluation from our country’s perspective. Pamukkale Univ J Eng Sci. 2009;15(1):69–79.
Alpaslan MN, Dölgen D, Boycaoğlu H, Sarptaş H. Arsenic removal from drinking water by chemical methods. ITU, Water Poll Count J. 2010;1:15–25.
Lee Y, Um I, Yoon J. Arsenic(III) oxidation by iron(VI) (ferrate) and subsequent removal of arsenic(V) by iron(III) coagulation. Env Sci Tech. 2003;37:5750–6.
WHO (World Health Organization). Guidelines for drinking-water quality. 4th edn. Geneva: 2011-07-04, 2011.
Nguyen CM, Bang S, Cho J, Kim KW. Performance and mechanism of arsenic removal from water by a nanofiltration membrane. Desalination. 2009;245(1–3):82–94.
TSI -266 Standards for drinking waters. Turkish Standards Institution, Ankara, Turkey, 2005.
Baba A, Sözbilir H. Source of arsenic based on geological and hydrogeochemical properties of geothermal systems in Western Turkey. Chem Geol. 2012;334:364–77.
Şener Ş, Karakuş M. Investigating water quality and arsenic contamination in drinking water resources in the Tavşanlı District (Kütahya, Western Turkey). Environ Earth Sci. 2017;76(21):750.
Varol S, Davraz A. Evaluation of potential human health risk and investigation of drinking water quality in Isparta city center (Turkey). J Water Health. 2016;14(3):471–88.
Oelkers EH, Schott J. Geochemical aspects of CO2 sequestration. Chem Geol. 2005;217:183–6.
Oelkers EH, Gislason SR, Matter J. Mineral carbonation of CO2. Elements. 2008;4:333–8.
Shirokova LS, Mavromatis V, Bundeleva IA, Pokrovsky OS, Bénézeth P, Gérard E, Pearce CR, Oelkers EH. Using mg isotopes to trace cyanobacterially mediated magnesium carbonate precipitation in alkaline lakes. Aquat Geochem. 2013;19:1–24.
Varol S, Davraz A, Şener Ş, Şener E, Aksever F, Kırkan B, et al.. Determination of pollution and monitoring of the hydrogeochemical properties, hydrogeology of Salda Lake Wetland, TÜBİTAK ÇAYDAG project report, project no: 114Y084, 2017 (in Turkish).
Lise Y, Gülle İ, Kesici E, Dişli E, Akarsu F, Küçükala A, et al. Salda Lake Wetland Basin biodiversity survey. Ministry of Forestry and Water Management, General Directorate of Nature Conservation and National Parks, 2013 (in Turkish).
Piper AM. A graphic procedure in the chemical interpretation of water analysis. Am Geophys Union Trans. 1944;25:914–23.
Gibbs RJ. Mechanisms controlling worlds water chemistry. Science. 1970;170:1088–90.
Ehsan N, Shan A, Riaz S, uz Zaman Q, Javied S, Jabeen M. Health risk assessment due to exposure of arsenic contamination in drinking water of district Shiekhupura, Punjab, Pakistan. Hum Ecol Risk Assess: An Int J. 2020;26(1):162–76.
USEPA. Guidelines for Carcinogen Risk Assessment. EPA/ 630/P-03/001F. US Environmental Protection Agency, Risk Assessment Forum, 2005. Washington, DC.
USEPA. Risk Assessment Guidance for Superfund, Vol. I. Human Health Evaluation Manual, EPA/540/1-89/002. 1989. Washington, DC.
Radfard M, Yunesian M, Nabizadeh R, Biglari H, Nazmara S, Hadi M, Yousefi N, Yousefi M, Abbasnia A, Mahvi AH. Drinking water quality and arsenic health risk assessment in Sistan and Baluchestan, Southeastern Province, Iran. Hum Ecol Risk Assess: An Int J. 2019;25(4):949–65.
Kavcar P, Sofuoglu A, Sofuoglu S. A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health. 2009;212:216–27.
Li P, Tian R, Xue C, Wu J. Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res. 2017;24(15):13224–34. https://doi.org/10.1007/s11356-017-8753-7.
Adimalla N. Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health. 2019;11(2):109–23.
Zhang Q, Kang S, Wang F, Li C, Xu Y. Major ion geochemistry of Nam Co Lake and its sources, Tibetan Plateau. Aquat Geochem. 2008;14(4):321–36.
Ranjan RK, Ramanathan AL, Parthasarthy P, Kumar A. Hydrochemical characteristics of groundwater in the plains of Phalgu river in Gaya, Bihar. India Arab J Geosci. 2013;6:3257–67.
Khatri N, Tyagi S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci. 2015;8(1):23–39.
Mallick J. Hydrogeochemical characteristics and assessment of water quality in the Al-Saad Lake. Abha Saudi Arabia Appl. Water Sci. 2017;7:2869–82.
Mora A, Mac-Quhae C, Calzadilla M, Sanchez L. Survey of trace metals in drinking water supplied to rural populations in the eastern Llanos of Venezuela. J Environ Manag. 2009;90:752–9. https://doi.org/10.1016/j.jenvman.2008.01.005.
WHO (World Health Organization). Guidelines for drinking-water quality: recommendations. Geneva, Switzerland: World Health Organization; 2004.
Chowdary V, Rao N, Sarma P. Decision support framework for assessment of non-pointsource pollution of groundwater in large irrigation projects. Agric Water Manag. 2005;75:194–225. https://doi.org/10.1016/j.agwat.2004.12.013.
Mohammadi AA, Yaghmaeian K, Faraji H, Nabizadeh R, Dehghani MH, Khaili JK, et al. Temporal and spatial variation of chemical parameter concentration in drinking water resources of Bandar-e Gaz City using geographic information system. Desalin Water Treat. 2017;68:170–6. https://doi.org/10.5004/dwt.2017.20341.
Laxmankumar D, Satyanarayana E, Dhakate R, Saxena PR. Hydrogeochemical characteristics with respect to fluoride contamination in groundwater of Maheshwarm Mandal, RR district, Telangana state, India. Groundw Sustain Dev. 2019;8:474–83.
Varol M, Şen B. Assessment of surface water quality using multivariate statistical techniques: a case study of Behrimaz stream. Turk Environ Monit Assess. 2009;159:543–53.
Manish K, Ramanathan A, Rao MS, Kumar B. Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J Environ Geol. 2006;50:1025–39.
Kibena J, Nhapi I, Gumindoga W. Assessing the relationship between water quality parameters and changes in land use patterns in the Upper Manyame River. Zimbabwe Phys Chem Earth. 2014;67–69:153–63.
Bu H, Song X, Zhang Y. Using multivariate statistical analyses to identify and evaluate the main sources of contamination in a polluted river near to the Liaodong Bay in Northeast China. Environ Pollut. 2019;245:1058–70.
Varol M. Spatio-temporal changes in surface water quality and sediment phosphorus content of a large reservoir in Turkey. Environ Pollut. 2020;259:113860.
Reza R, Singh G. Assessment of ground water quality status by using water quality index method in Orissa. India World Appl Sci J. 2010;9(12):1392–7.
WHO (World Health Organization). Guidelines for drinking water quality, vol. 1. 2nd ed. Geneva: Switzerland: Recommendations; 1993. p. 188.
Yidana SM, Banoeng-Yakubo B, Akabzaa TM. Analysis of groundwater quality using multivariate and spatial analyses in the Keta basin Ghana. J Afr Earth Sci. 2010;58(2):220–34.
Srinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavihar M, Peter J, Anandhan P. Identification of major sources controlling groundwater chemistry from a hard rock terrain—a case study from Mettur taluk, Salem district, Tamil Nadu. India J Earth Syst Sci. 2008;117(1):49–58.
Taylor SR, McLennan SM. The continental crust: its composition and evolution. London: Blackwell Scientific; 1985.
Teker Y. The Manganese occurences of Isparta and Burdur compared with different Manganese origin types of Turkey by geological, mineralogıcal and geochemical properties. Isparta, Turkey (Turkish): Suleyman Demirel University Graduate School of Applied and Natural Sciences Department of Geological Engineering, 293 page; 2010.
Hem J. Study and interpretation of the chemical characteristics of natural water, U.S. Geological Survey Water-supply Paper 2254. Reston: USGS; 1985. p. 264.
Anh NV, Bang S, Viet PH, Kim KW. Contamination of groundwater and risk assessment for arsenic exposure in Ha Nam province. Vietnam Environ Int. 2016;35(3):466–72.
McArthur JM, Ravenscroft P, Safiulla S, Thirlwall MF. Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour Res. 2001;37(1):109–17.
Gasemi A, Razmara M, Nazari E, Nematollahi MJ, Borabadi R. Hydrological and geochemical processes controlling the origin and transport of arsenic in the Pangi area (NW Torbat Hydarieh, Iran). J Middle East Appl Sci Technol (JMEAST). 2014;15(2):29–34.
Liang CP, Wang SW, Kao YH, Chen JS. Health risk assessment of groundwater arsenic pollution in southern Taiwan. Environ Geochem Health. 2016;38(6):1271–81.
USEPA Water Quality Standards. Establishment of numeric criteria for priority toxic pollutants for the state of California. Washington, DC: US Environmental Protection Agency; 2000.
Muhammad S, Shah MT, Khan S. Arsenic health risk assessment in drinking water and source apportionment using multivariate statistical techniques in Kohistan region, northern Pakistan. Food Chem Toxicol. 2010;48:2855–64.
Doneen LD. Notes on water quality in agriculture. Published as a Water Science and Engineering Paper 4001. Department of Water Science and Engineering, University of California, 1964.
Wilcox LV. Classification and use of irrigation waters, USDA Circular No. 969. Washington, DC: USDA; 1955. p. 19.
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S. Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur City. Tamil Nadu, India, Environ Monit Assess. 2010;171:289–308.
Paliwal KV. Irrigation with saline water. In: Monogram No. 2 (New Series). IARI, Water Technology Centre, New Delhi, pp. 198, 1972.
Siddiqui A, Naseem S, Jalil T. Groundwater quality assessment and around Kalu Khuhar, super highway. Sindh Pakistan J Appl Sci. 2005;5:1260–5.
Acknowledgments
This study has been supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) with Project No: 114Y084. The authors would like to thank TÜBİTAK (Project No: 114Y084) for contributing to the financial portion of the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varol, S., Davraz, A., Şener, Ş. et al. Assessment of groundwater quality and usability of Salda Lake Basin (Burdur/Turkey) and health risk related to arsenic pollution. J Environ Health Sci Engineer 19, 681–706 (2021). https://doi.org/10.1007/s40201-021-00638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-021-00638-5