Abstract
A dielectric barrier discharge system with a discharging zone where degradation processes happen is designed to remove 4-chlorophenol from water. The removal of 4-chlorophenol was influenced by the processing parameters such as gas flow rate, flow ratio of oxygen and argon, applied voltage and total applied power. Increasing the power or gas flow rates within a certain range enhanced the removal efficiency. 99% of 4-chlorophenol was removed in 6.5 min at reactor’s efficient point which is set by adjusting the flow ratio of introduced gases and voltage. The removal percent was about 95% at 5 min of non-thermal plasma treatment with peak voltage of 10 kV and oxygen and argon flow rate of 20 SCCM and 200 SCCM respectively. Then by adjusting the flow ratios in order to find the optimum point. At this point the efficiency reached its peak due to excessive introduction oxygen gas which results in production of more oxidative agents. HPLC and GC-MS analysis have been carried out in order to investigate the by-products of degradation process. After 6.5 min of treatment at efficient point of degradation reactor, a 64% decrease in COD index has been indicated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental pollution by emerging pollutants has been considered as the most important concerns in the globe and therefore, the legislation requirements for discharging of industrial wastewater have becoFfigme stricter [1,2,3]. Constituting an important category of persistent organic pollutants and some of them being identified as carcinogens and environmental endocrine disruptors, Chlorophenols (CPs) are repeatedly detected in industrial wastewater generated by manufacturing of plastics, resins, textile, steel and paper [4]. Chlorophenols are pernicious to organisms even at ppb levels and listed as priority pollutants. Although the advanced oxidation processes have been successfully used to remediate different organic pollutants or disinfection purposes, they are commonly require the addition of chemical agents [5,6,7,8,9]. and the full scale application of other methods such as adsorption processes require high contact time and limited by the slow rate of process [10,11,12]. Meanwhile, the advanced oxidation processes based on plasma discharges are novel and promising alternatives. Alongside with newly established efficient wastewater treatment techniques such as ozonation, carbon adsorption and photocatalytic generation of oxidants; discharge technique has been of great interest, demonstrating that it could induce an oxidation process culminating in the effective degradation of persistent organic contaminants that could not be eliminated efficiently in order that their toxicity and bioaccumulation can cease to exist and be mineralized [13, 14].
Duo to CPs resistance to biological and classical physicochemical processes, conventional water treatment techniques are proved to be ineffective in degradation and hence mineralization of these persistent pollutants. Therefore, Advanced Oxidation Processes (AOPs) have been proposed as a promising method for CPs eradication since these processes can generate highly reactive species (e.g. hydroxyl radical) leading to mineralization of persistent organic pollutants to carbon dioxide and water [15]. AOPs frequently are conventional techniques that are developed and optimized either by modification of their treatment process (e.g. increasing surface contact) or combining two or more oxidation process in order to employ their synergistic effects. For the conventional ozonation treatment, ozone is the oxidative species introduced into the degradation reactor in which degradation of persistent organic pollutants can be carried out solely by direct ozonation. Moreover, in order to escalate degradation efficiency, conventional ozonation has been combined with UV, H2O2, Fenton and TiO2 photocatalytic oxidation [16,17,18]. Plasma also can be a source of UV emission which can be utilized to enhance degradability of photocatalysts while employing synergistic effects of them [19, 20]. In addition, in order to prevent main drawbacks of photocatalysts such as recombination of e- and h+, which hampers photocatalytic reaction process, noble metal Pt-doped TiO2 has been proposed and investigated to increase catalytic reactions to enhance degradation of persistent contaminants [21].
Along with multitudinous of AOPs proposed to escalate degradation, electrical discharge technologies such as glow discharge and dielectric barrier discharge have been regarded as promising processes. Applications of Dielectric barrier discharge plasma (DBD) vary within a broad range from ozone generation and surface treatment to wastewater treatment and fuel reforming [22, 23]. The two electrodes comprising a DBD system are separated by a dielectric layer that limits charge particles movement, hence, micro discharges known as plasma streams can be distributed uniformly between the two electrodes. Reactive oxidant species are mainly O3 and hydroxyl radical which are generated by plasma discharges, leading to effective elimination of pollutants. In a Non-Thermal Plasma (NTP) DBD system, Perchloroethylene (PCE) degradation investigation has been carried out in within two stainless steel electrodes with a quartz dielectric barrier between them. Optical emission spectroscopy illustrated that O•, H• and OH are responsible for oxidation processes leading to degradation of PCE [24]. The elimination of PCE was about 80% at optimized reaction time after 3 min. In a dual discharge zone reactor which is comprised of two coaxial quartz tubes, two stainless steel disk electrodes and one stainless steel mesh electrode with water flowing as thin film to be treated by NTP, an elimination of 94% Methyl Orange in 80 mg/L concentration of 4 L solution was observed in 80 min [25]. Veterinary antibiotics (e.g. lincomycin, enrofloxacin) with initial concentration of 5 mg/L were degraded by a DBD system using gas air and O2 as working gas diffused into solution [26]. Energy requirement for degradation of 60% of 5 mg/L antibiotic solution was between the range of 0.26–1.49 kJ/mg while for 90% the energy requirement was 0.36 to 2.06 kJ/mg.
Wang et al. developed a spraying DBD system to decolorize indigo carmine aqueous solution which was used as a grounded spraying water electrode [27]. When the air gaps were 30 mm and the voltage was 30 kV, the decoloration percent was about 95% at 18 min. Methyl violet 5BN (MV-5BN) was effectively decolorized by a DBD plasma plume array [28].
In this paper, the degradation of 4-chlorophenol (4CP) was investigated in a DBD system that utilizes stainless steel electrodes and quartz glass as dielectric barrier. The process of 4CP removal and degradation is undergone in discharge zone. When voltage is introduced into reactor, NTP is generated between coaxial cylindrical electrodes where degrading zone is located. Treated by NTP, 4CP is effectively degraded and wastewater was half mineralized when processed at optimum set of parameters. The effects of multiple treatment variables including applied voltage and power, gas flow rates and ratio of flows and duration of treatment were studied. Quantitative and qualitative assessments of reactive oxidant species responsible for degradation process were also carried out. By-products generated as result of 4CP degradation process were also identified by HPLC and GC-MS techniques, which were illustrated as in-toxic and not persistent. Furthermore, quantitative comparison of two Advanced Oxidation Processes (AOP), ozonation and NTP, was drawn to illustrate designed reactor’s effectiveness and potential. This contribution distinctly revolves around the effect of fed gases in efficiency of degradation process. Various ratios of argon and oxygen flow rates have been fed through the discharge zone in order to establish the optimum point at which generation rate of active species is maximum, leading to maximum degradation efficiency. Besides, active species are detected qualitatively in the discharge zone during the treatment and the role hydroxyl radicals in degradation process is specifically corroborated.
Materials and methods
Materials and solution
The synthetic effluent was prepared with distilled water and 4-chlorophenol. 4-chlorophenol was purchased from Merck® with concentration of 99% suitable for analysis. Stock solution of 100 mg/L concentration was prepared by adding liquid 4-chlorophenol in 40 C to distilled water and solving in water in a heat bath set at 40 C using a magnetic stirrer. Oxygen and argon gases were fed from two steel cylinders (both 99.999% concentration suitable for analysis) to the discharging zone and gas flow meters were used to control the flow rate.
Experimental setup
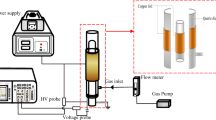
The schematic diagram of the DBD system is depicted in Fig.1. a. It consists of a homemade high voltage power supply and a coaxial cylindrical reactor with a discharging zone in which degradation is undergone. Before any treatment and introduction of gases, 50 mL of 4-chlorophenol solution was poured into the reactor between the two coaxial electrodes where discharges were to be produced. Afterward, the gases were introduced to the discharge zone and bubbles of the fed gases were observed in the discharge zone and the high voltage power supply was set to a particular voltage value. The peak voltage was set in a corresponding frequency, however, before any discharge took place the power supply was tried to maximize the voltage until first discharges happened. After first discharge and generation of streamers, the peak voltage was set and controlled in order to investigate its role on degradation efficiency. Trying to find the most efficient point on which the reactor performs effectively with least power consumption, the degradation of 4-chlorophenol was found to be most efficacious at 10 KV peak voltage, then the peak voltage was fixed for evaluating other parameters and their roles in the efficiency of the reactor. Furthermore, frequency of the high voltage power supply was constant in 50 kHz during all the study. After wastewater treatment by reactor, samples were taken out the reactor for material analysis. The applied voltage, current and its other characteristics were recorded by a digital oscilloscope (Tektronix, DPO3012) with a high voltage probe (Tektronix, P6015) and a current probe (Tektronix, TCP202). Figure 2 shows the typical waveforms of the applied voltage and current.
The structure of the reactor is also shown in Fig.1. b. The ground electrode is a stainless-steel mesh enclosed on outer surface of the quartz discharge barrier. The high voltage electrode is a hollow stainless-steel rode through which fed gases introduced to reactor from the top of the reactor after their flows were fixed and their ratio were set. The high voltage electrode was connected to a high voltage power supply and the ground electrode was connected to ground wire of laboratory. The length and gap of the discharging zone was 300 mm and 4 mm respectively. The bubbling fed gases were introduced to the discharging zone from the bottom of the high voltage electrode where they appeared as bubbles due to the several small holes carved in the bottom which serve as gas diffuser.
Material analysis and plasma characterization methods
The initial concentration of 4-chlorophenol was adjusted and its concentration after each experiment was all identified by a Brucker 450 GC equipped by FID Varian capillary CP-Sil 5CB (30 m× 0.25 mm ×1 μm) column. The by-products and the effect of plasma treatment on the solution were identified by a Knauer HPLC equipped with a 2800 Smartline photo-diode array UV-Vis detector and a Waters Spherisorb 5 μm ODS2 C18 column (5 μm× 4.6 mm× 250 mm). The injection volume of the sample was 10 μL and the flow rate of eluent was 1.0 mL/min. The compounds were eluted with a binary system of acetonitrile–water. The mobile phase gradient was initially 30% acetonitrile, increasing linearly to 80% during 20 min. The detector wavelength was set at 280 nm. To identify compounds the retention time was compared with standard (tR= 11.12 min). The error for HPLC analyses was not more than 1%.
In order to investigate and identify by-products of treated wastewater, a ThermoQuest-Finnigan Trace MS Quadrupole GC-MS with 70 eV ionization energy equipped with Agilent J&W DB-5 ms capillary column was used. In addition, to measure the effectiveness of the designed reactor in mineralization of wastewater, COD analysis using Aqualytic AL 125 thermoreactor equipped with AL 250 photometer has been performed.
In addition, Optical Emission Spectroscopy (OES) was used to examine the excited high energy species in the plasma matrix. In order to identify excited species during NTP treatment, the optical spectra probe, located in front of NTP reactor, detected plasma emission’s wavelengths ranging from 200 to 1100 nm by an optical spectrometer (Avantes Ava Spec-3648).
Results and discussion
Degradation kinetics of 4-chlorophenol in different peak voltage
In the DBD system, peak voltage is one of the key parameters affecting mostly the input power. Figure 3 shows the 4-chlorophenol degradation at various peak voltages. Meanwhile, the oxygen and argon flow rates were fixed at 20 SCCM and 200 SCCM respectively. The degradation rate increases quickly by increasing peak voltage from 8 kV to 14 kV, which intuitively is deducted. Only 71% of 4-chlorophenol was removed in 5 min with peak voltage 8 kV while 97% was removed in same duration with 14 kV peak voltage. In general, increasing peak voltage results in an increase in the input energy which generates more and stronger streamers. Peak voltages of 8 kV, 10 kV, 12 kV and 14 kV correspond with input energy of 400 W, 500 W, 600 W, 700 W respectively due to constant 50 mA current. Weak corona spots are observed at 8 kV voltage with few streamers, while illuminating corona accompanied by multitudinous streamers having more diameters were observed. Stronger corona means more formation of active species [29]. Hence, the contaminant degradation rate could be accelerated by increasing peak voltage in certain range. In the present setup, it was difficult to heighten the peak voltage above 14 kV. However, increasing applied voltage leads overheating of aqueous solution and reactor parts, which results in instability of reactor. Aside from energy consumption, overheating plays a limiting role in exerting voltages above 10 kV to reactor. Hence, the optimum voltage indeed is determined to be 10 kV from both energy consumption and stability perspective.
The k1 (s−1), defined as degradation kinetic rate constant in first-order kinetic expression in Eq. (2) which ln (C/Co) versus time (s) is plotted to calculate degradation rate of 4-chlorophenol:
The degradation action was demonstrated using a first-order expression. The actual degradation kinetic rate was estimated from the slope of ln (C/Co) versus time (s) plot. Least-squares regression values (r2) of the degradation rate constant data for 4-chlorophenol removal (r2 > 0.9867) shows a proper fit between ln (Ce/Co) and time. Based on Table (1) Kinetic of 4-chlorophenol degradation in peak voltage of (14 kV) was the highest and equal to 0.013 s−1.
peak voltage (kv) | first-order degradation kinetic (s−1) | R2 |
|---|---|---|
8 | 0.0047 | 0.9627 |
10 | 0.0065 | 0.9842 |
12 | 0.0081 | 0.9743 |
14 | 0.0135 | 0.9867 |
Optimization of oxygen and argon flow rate
Figure 4 shows the removal of 4-chlorophenol at two different flow rate ratios of oxygen and argon while applied peak voltage is fixed at 10 kV. It was observed that the removal rate was improved by increasing oxygen gas flow rate from 20 SCCM to 50 SCCM while decreasing argon gas flow rate from 200 SCCM to 170 SCCM. During 3 min of treatment, the removal was 58% and 78% at 20 SCCM oxygen flow rate and 50 SCCM argon flow rate, respectively. There were negligible variations on the removal rate by further increasing the oxygen flow rate ratio; however, different gas flow rate means a different amount of gas molecules are passing through the discharge zone at the same time. As the oxygen gas flow rate is increased in a certain range and ratio, more oxygen gas molecules will pass through the DBD discharging reactor, culminating in more dissociation of gas molecules at the same interval. As a result, more radicals are formed (either atomic oxygen or its products such as hydroxyl radical), which results in more oxidation and decontamination of 4-chlorophenol either by breaking the aromatic ring and mineralization or replacing Cl atom which renders chlorophenols toxic [30]. Meanwhile, it was observed that degradation of 4-chlorophenols was accelerated during the first minutes of degradation and the overall degradation was not so. This could be attributed to saturation of both energy and active species. Active species formed by streamers cause more damage to the persistent organic molecules when concentrations are high due to more collision between radicals and organic molecules. Collisions plummeted when a majority of molecules were degraded; besides, direct collision of streamers and corona with contaminants was decreased. What is more, increasing the oxygen flow rate more than 50 SCCM was shown to be ineffective to degradation rate, which can be attributed to either the limit of applied energy in the discharge field or saturation of radicals. Considering all the aforementioned factors, the oxygen and argon gas flow rates of 50 SCCM and 170 SCCM corresponding to flow ratio of 5:17 respectively were suggested in the following experiments. Fig. 4 demonstrates the effect of oxygen and argon flow rate on the removal rate against time. In other studies, using a NTP reactor and employing synergistic effects of Fe+2 and H2O2 catalysts, over 95% of 4CP was removed using H2O2 catalyst applying 150 W at 17 kV into DBD system [31]. In these studies, catalysts and chemical compound (e.g. iron sulfate) must be added to solution before any treatment, which imposes limitation when scaling up for industrial purposes is planned. Besides, after plasma treatment solution must be left for hours in order that catalysts’ maximum efficiency can be reached.
Comparison between ozonation and NTP reactor
Demonstrating proposed NTP DBD reactor’s effectiveness and potential, a comparison between this advanced oxidation process of contaminant removal and a conventional facility was drawn. Concentration of 4-chlorophenol was set 100 mg/L for both ozonation and DBD samples. The processed samples both had 50 mL volume. Ozone was bubbled into 4CP solution using ozone generator (ANSEROS, COM-AD-01-OEM) generating 5 g/h ozone. DBD reactor was set at its optimum removal point with 10 KV applied voltage and 50 SCCM and 170 SCCM of oxygen and argon fed gases respectively. Fig. 5 shows the removal of 4CP against time. As it is shown, while the whole 4CP was removed from sample after 6 min when DBD reactor was exploited, the complete removal 4CP using ozonation technique was not achieved during 20 min of treatment and 47% removal was recorded. This exemplarily shows the effectiveness of NTP reactor in removal of persistent organic pollutant 4CP. In a catalytic degradation system exploiting ozone and ultra-small β-FeOOH nano-rods catalysts removal rate of 99% and 67% was achieved after 40 min and 2 l min in the presence of combined ozone and catalyst and ozone only, respectively. [32]
The role of active species
The plasma discharge was characterized using optical emission spectroscopy (OES). During water treatment time, the optical spectra emitted from plasma were recorded in wavelength range between 180 to 500 nm simultaneously using optical spectrometer. Each observed peak individually has been corresponded to a reference peak in order to detect each active species dwelling in discharge zone during the treatment. From Fig. 6, it can be seen that small peaks of ∙OH were recorded at 295–320 nm and corresponded with previous observations [33]. Emissions from N2 and excited species of N2 exhibited distinct peaks in the UV region and were the highest peaks recorded [34]. As expected, the atmospheric discharge is an effective source of reactive nitrogen oxygen species (RNOS). NO and oxygen based oxidative species were observed due to discharge in atmospheric pressure and oxygen fed gas. Argon was also detected in spectrum since it was fed through discharge zone. Radical species such as superoxides and hydroxyl radicals are short-lived whereas hydrogen peroxide is relatively more stable even up to 24 h. Such active species play a key role in the degradation of 4CP.
HPLC analysis
Using HPLC equipped with UV-Vis spectrophotometer, not only were the evidences for removal of 4CP in optimum point of reactor given, but it was also confirmed that 4CP was degraded due to the change in UV adsorption of molecule. Figure 7 shows the HPLC chromatogram for 4CP solution before and after the NTP treatment. The red and black trajectories indicate the solution chromatogram before and after the treatment, respectively, at optimum point. The chromatogram illustrates two peaks for pre-treatment solution, the first relates to moving phase used for indicating the chemicals while the second peak relates to 4CP. The post-treatment chromatographic trajectory shows 3 peaks, among which the peak corresponding to tR = 8.04 min is related to by-product resulted from NTP treatment of the solution. Evaluating the under peak areas of tR = 10.89 min peak, which was related to 4CP in solution, the results of GC analysis for concentration of 4CP were precisely confirmed. Furthermore, UV-Vis detector of HPLC column illustrated the degradation of 4CP by confirming opening of its benzene ring.
Photocatalytic disassociation of the C-Cl bond in 4CP has been reported using graphite and graphene oxide activated with the UV lamp [35]. The reactions initiated by OH radical are the main stage in the C-Cl bond cleavage of 4CP, with subsequent decomposition of the intermediate ion or radical. It is important to consider the bond at 280 nm, which defines the generation of intermediate compounds, in the progression of 4-chlorophenol degradation. In Fig.8 the UV-Vis spectrum corresponding to tR = 8.04 min is shown. Comparing two 280 nm absorption peaks of pre and post-treatment solution, it was apparent that this peak is intact, indicating no generation of C-Cl containing by-products. Moreover, the 225 nm peak was diminished considerably after NTP treatment, suggesting that 4CP’s benzene ring was opened. What is more, formation of another peak at 202 nm was attributed to generation of maleic acid as the final by-product of 4CP degradation [36].
GC-MS analysis
In order to investigate degradation pathways of 4CP during NTP treatment, Gas chromatography equipped with mass spectrometer analysis has been carried out. After treatment of 4CP there were produced several kinds of intermediates a majority of which were transient (i.e. were degraded after short period of time). [37] However, although the main purpose of the reactor was to mineralize persistent organic pollutant 4CP, persistent by-products remained in the treated solution. Distinguishing between transient and persistent by-products, the analyzed sample was taken from optimally treated solution of 4CP after 3 min of treatment. As it is shown in Fig. 9, two major peaks were observed in the chromatogram. The second peak (tR = 39.21 min) was attributed to 4CP remnant in the solution while the first peak (tR = 39.12 min) was related to another persistent organic pollutant detected as 2,3-dihydrofuran. The second peak was observed in the initial sample of 4CP. Furthermore, Fig. 9 shows the first peak’s (tR = 39.12 min) mass spectra and closest analogue of this mass spectra pattern which was interpreted as 2,3-dihydrofuran by the library. Although the detected by-product found to be a persistent organic pollutant, it was not reported as an endocrine disruptor since it does not contain chlorine atoms attached to benzene ring [38]; thus, it is concluded that endocrine disruption characteristic of treated solution ceased to exist. In an assessment of by-products resulted from 4-chlorophenol, several kinds of opened benzene ring and organic molecules were reported, all of which did not possess chlorine atom attached to benzene ring. [39]
Intermediate by-products were assessed at the optimum conditions of this study. At this study up to 96% 4-CP degradation was achieved. GC-MS examination was used to identify the 4-CP degradation by-products in DBD set up. The main compounds during 4-CP degradation were aromatic structure such as Hydroquinone, 1, 4-Benzoquinone, 4-chlorocatechol, and 2,3-dihydrofuran, along with aliphatic acids such as, 2, 4-Hexadiene-1-OL, 1-Hexanone, oxalic acid, maleic acid, acetic acid and formic acid. The most important reason for the decomposition of organic pollutants in DBD set up is formation of OH· and O· free radicals in the plasma environment. Figure 10 shows the anticipated pathway of 4-CP degradation. As can be seen, first, free radicals (superoxide and hydroxyl) attack to 4-CP and remove chlorine (Cl) atom from the 4-CP molecule and produce hydroquinone. Then hydroquinone dehydrogenation led to the 1, 4-Benzoquinone generation. Regarding that extra amounts of free radicals existed in the DBD reactor, 1, 4-Benzoquinone ring decomposed and converted to the aliphatic acids such as oxalic, maleic, acetic and formic acid. Finally, if enough contact time is provided for this setup, complete mineralization possibly will happen and aliphatic acids excess degradation will lead to H2O and CO2 molecules production in DBD reactor.
Mineralization of 4-chlorophenol evaluated by COD
The COD analysis was carried out in order to assess the total quantity of oxygen-consuming substances during the complete chemical breakdown of organic substances in water. Table. 1 illustrates mineralization of 4CP achieved by NTP treatment after 6.5 min under optimal conditions mentioned above corresponding to 99% removal of 4CP. It can be observed that NTP degradation process eliminates 64% of organic matter. In addition, it is worthy of indicate that organic elimination achieved by other degradation research was 25% after 15 min treatment [40]. In another reactor designed to eliminate pharmaceutical compound an elimination of 56% was achieved after 120 min of treatment [25].
Conclusions
Degradation of 4-chlorophenol in solution of 100 mg/L is achieved employing non-thermal plasma generated by a DBD system in 3 min at peak voltage of 10 kV at optimum condition of 50 SCCM and 170 SCCM of feeding oxygen and argon gases respectively. 95% of 4-chlorophenol was removed after 5 min of treatment in DBD reactor at optimum point. It was shown that degradation of 4CP depends on applied voltage and flow rate of fed gases, which determines the optimum point at which degradation is most efficient and effective. A comparison was drawn between proposed AOP reactor and ozonation concluding excellence and effectiveness of proposed process. Carrying out OES, the role of different active species such as hydroxyl radical generated by NTP in degradation of 4CP was investigated.
Furthermore, performing HPLC and GC-MS analysis to detect by-products resulted from degradation process in reactor, it was illustrated that detected by-products do not exhibit endocrine disruption characteristics. Finally, it was illustrated that 4CP solution is mineralized due to 64% reduction in COD index of solution after NTP treatment in 6.5 min at optimum conditions.
Based on presented results and discussions, it can be corroborated that the proposed reactor poses as potential water purifier specifically designed for persistent organic pollutants decontamination in aqueous media. The reactor is highly efficacious at removing 4CP, albeit being complicated in terms of characterization and optimization of the discharge process.
References
Ahmadi E, Yousefzadeh S, Ansari M, Ghaffari HR, Azari A, Miri M, et al. Performance, kinetic, and biodegradation pathway evaluation of anaerobic fixed film fixed bed reactor in removing phthalic acid esters from wastewater. Sci Rep. 2017;7:41020.
Yousefzadeh S, Ahmadi E, Gholami M, Ghaffari HR, Azari A, Ansari M, et al. A comparative study of anaerobic fixed film baffled reactor and up-flow anaerobic fixed film fixed bed reactor for biological removal of diethyl phthalate from wastewater: a performance, kinetic, biogas, and metabolic pathway study. Biotechnol Biofuels. 2017;10:139.
Esrafili A, Rezaei Kalantary R, Azari A, Ahmadi E, Gholami M. Removal of diethyl phthalate from aqueous solution using persulfate-based (UV/Na2S2O8/Fe2+) advanced oxidation process. J Mazandaran Univ Med Sci. 2016;25:122–35.
Czaplicka M. Sources and transformations of chlorophenols in the natural environment. Sci Total Environ. 2004;322(1–3):21–39.
Yousefzadeh S, Nabizadeh R, Mesdaghinia A, Nasseri S, Hezarkhani P, Beikzadeh M, et al. Evaluation of disinfection efficacy of performic acid (PFA) catalyzed by sulfuric and ascorbic acids tested on Escherichia coli (ATCC, 8739). Desalin Water Treat. 2014;52:3280–9.
Sabeti Z, Alimohammadi M, Yousefzadeh S, Aslani H, Ghani M, Nabizadeh R. Application of response surface methodology for modeling and optimization of Bacillus subtilis spores inactivation by the UV/persulfate process. Water Sci Technol Water Supply. 2016;17:342–51.
Yousefzadeh S, Matin AR, Ahmadi E, Sabeti Z, Alimohammadi M, Aslani H, Nabizadeh R. Response surface methodology as a tool for modeling and optimization of bacillus subtilis spores inactivation by UV/ nano-Fe0 process for safe water production, Food Chem Toxicol, 2018.
Matin AR, Yousefzadeh S, Ahmadi E, Mahvi A, Alimohammadi M, Aslani H, et al. A comparative study of the disinfection efficacy of H2O2/ferrate and UV/H2O2/ferrate processes on inactivation of Bacillus subtilis spores by response surface methodology for modeling and optimization. Food Chem Toxicol. 2018;116:129–37.
Ahmadi M, Rahmani K, Rahmani A, Rahmani H. Removal of benzotriazole by photo-Fenton like process using nano zero-valent iron: response surface methodology with a box-Behnken design. Polish J Chem Technol. 2017;19(1):104–12.
Gholami M, Rahmani K, Rahmani A, Rahmani H, Esrafili A. Oxidative degradation of clindamycin in aqueous solution using nanoscale zero-valent iron/H2O2/US. Desalin Water Treat. 2016;57(30):13878–86.
Azari A, Esrafili A, Ahmadi E, Gholami M. Performance evaluation of magnetized multiwall carbon nanotubes by iron oxide nanoparticles in removing fluoride from aqueous solution. J Mazandaran Univ Med Sci. 2015;25:128–42.
Ahmadi E, Kakavandi B, Azari A, Izanloo H, Gharibi H, Mahvi AH, et al. The performance of mesoporous magnetite zeolite nanocomposite in removing dimethyl phthalate from aquatic environments. Desalin Water Treat. 2016;57:27768–82.
Magureanu M, Piroi D, Mandache NB, David V, Medvedovici A, Parvulescu VI. Degradation of pharmaceutical compound pentoxifylline in water by non-thermal plasma treatment. Water Res. 2010;44(11):3445–53.
Tang S, et al. Removal of bisphenol a in water using an integrated granular activated carbon preconcentration and dielectric barrier discharge degradation treatment. Thin Solid Films. 2012;521:257–60.
Zhang YZ, Xiong XY, Han Y, Yuan H, Deng SH, Xiao H, et al. Application of titanium dioxide-loaded activated carbon fiber in a pulsed discharge reactor for degradation of methyl orange. Chem Eng J. 2010;162:1045–9.
Bailey PS. Ozone in water and wastewater treatment. Michigan: Ann Arbor Science Publishers; 1972.
Prousek J. Advanced oxidation processes for water treatment, chemical processes. Chem List. 1996;90:229–37.
Manojlovic D, Ostojic DR, Obradovic BM, Kuraica MM. Removal of phenol and chlorophenols from water by new ozone generator. Desalination. 2007;213:116–22.
Kuo WS. Synergistic effects of combination of photolysis and ozonation on destruction of chlorophenols in water. Chemosphere. 1999;39:1853–60.
Friedemann AER, et al. Photocatalytic activity of TiO2 layers produced with plasma electrolytic oxidation. Surf Coat Technol. 2018;344:710–21.
Chen Y, et al. Synergistic degradation performance and mechanism of 17β-estradiol by dielectric barrier discharge non-thermal plasma combined with Pt–TiO2. Sep Purif Technol. 2015;152:46–54.
Wang TC, Lu N, Li J, Wu Y. Degradation of pentachlorophenol in soil by pulsed corona discharge plasma. J Hazard Mater. 2010;180:436–41.
Khani MR, et al. Investigation of cracking by cylindrical dielectric barrier discharge reactor on the n-hexadecane as a model compound. IEEE Trans Plasma Sci. 2011;39(9):1807–13.
Karimaei M, et al. Dielectric barrier discharge plasma as excellent method for Perchloroethylene removal from aqueous environments: degradation kinetics and parameters modeling. J Mol Liq. 2017;248:177–83.
Tao X, et al. A novel two-level dielectric barrier discharge reactor for methyl orange degradation. J Environ Manag. 2016;184:480–6.
Dojčinović BP, Roglić GM, Obradović BM, Kuraica MM, Kostić MM, Nešić J, et al. Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J Hazard Mater. 2011;192(2):763–71.
Wang ZH, Xu DX, Chen Y, Hao CX, Zhang XY. Plasma decoloration of dye using dielectric barrier discharges with earthed spraying water electrodes. J.Electrostat. 2008;66:476–81.
Chen GL, Zhou MY, Chen SH, Chen WX. The different effects of oxygen and air DBD plasma byproducts on the degradation of methyl violet 5BN. J Hazard Mater. 2009;172:786–91.
Zhang R, Cheng X, Wang L, Wu Y, Guan Z. Kinetics of dechlorination of azo dye by bipolar pulsed barrier discharge in a three-phase discharge plasma reactor. J Hazard Mater. 2007;142:105–10.
Dorathi PJ, Kandasamy P. Dechlorination of chlorophenols by zero-valent iron impregnated silica. J Environ Sci. 2012;24(4):765–73.
Marković MD, et al. Degradation and detoxification of the 4-chlorophenol by non-thermal plasma-influence of homogeneous catalysts. Sep Purif Technol. 2015;154:246–54.
Oputu O, et al. Catalytic activities of ultra-small β-FeOOH nanorods in ozonation of 4-chlorophenol. J Environ Sci. 2015;35:83–90.
Bingyan C et al. Yield of hydrogen peroxide, ozone and nitrite nitrogen with DBD arrays in water mist spray. 2015 IEEE International Conference on Plasma Sciences (ICOPS). IEEE, 2015.
Heise M, Lierfeld T, Franken O, Neff W. Single filament charge transfer and UV-emission properties of a cascaded dielectric barrier discharge (CDBD) setup. Plasma Sources Sci Technol. 2004;13:351.
Bustos-Ramírez K. Et al. "4-chlorophenol removal from water using graphite and graphene oxides as photocatalysts." journal of environmental health science and. Engineering. 2015;13(1):33.
Vargas R, Borrás C, Mostany J, Scharifker BR. Measurement of phenols dearomatization via electrolysis: the UV-Vis solid phase extraction method. Water Res. 2010;44(3):911–7.
Bian W, Song X, Liu D, Zhang J, Chen X. The intermediate products in the degradation of 4-chlorophenol by pulsed high voltage discharge in water. J Hazard Mater. 2011;192.3:1330–9.
Rathna R, Varjani S, Nakkeeran E. Recent developments and prospects of dioxins and furans remediation. J Environ Manag. 2018;223:797–806.
Chen X, et al. Degradation of 4-chlorophenol in a dielectric barrier discharge system. Sep Purif Technol. 2013;120:102–9.
Magureanu M, Piroi D, Mandache NB, David V, Medvedovici A, Bradu C, et al. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011;45.11:3407–16.
Acknowledgements
The authors gratefully acknowledge Department of Biology, Medicinal Plants and Drugs Research Institute for their contribution to material analyses performed in this research and Dr. Alireza Ghassempour.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasani, M., Khani, MR., Karimaei, M. et al. Degradation of 4-chlorophenol in aqueous solution by dielectric barrier discharge system: effect of fed gases. J Environ Health Sci Engineer 17, 1185–1194 (2019). https://doi.org/10.1007/s40201-019-00433-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-019-00433-3