Abstract
Introduction
Circulating levels of C1q/TNF-α‐related protein 6 (CTRP6) is an adipokine that is involved in regulation of glucose and lipid metabolism, inflammation, and insulin sensitivity. However, the exact role of CTRP6 in metabolic processes remains unclear due to conflicting findings. To address current gap, we aimed to investigate the serum levels of CTRP6 in patients with coronary artery disease (CAD) and its association with inflammatory cytokines.
Method
In this case-control study, the serum levels of CTRP6, interlukin-6 (IL-6), tumor necrosis factor- α (TNF-α), adiponectin, and fasting insulin were measured using enzyme-linked immunosorbent assay (ELISA) kits in a total of 176 participants, consisting of 88 CAD patients and 88 control subjects. Additionally, various anthropometric and biochemical measurements were measured and compared between cases and controls.
Results
The present study found that serum levels of CTRP6 were significantly higher in the CAD group (561.3 ± 15.14) compared to the control group (429.3 ± 12.85, p < 0.001). After adjusting for age, sex, and body mass index (BMI), CTRP6 levels were found to be positively associated with the risk of CAD (p < 0.001). Correlation analysis in CAD subjects revealed a positive correlation between CTRP6 levels and BMI, systolic blood pressure (SBP), malondialdehyde (MDA), TNF-α, and IL-6, as well as a negative correlation with creatinine and total anti-oxidant capacity.
Conclusion
The findings of this study provide novel evidence that elevated serum levels of CTRP6 are significantly associated with an increased risk of developing CAD. Moreover, our results indicate a correlation between CTRP6 and various risk factors for atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is a prevalent form of cardiovascular disease (CVD) and remains a leading cause of death globally [1]. This disease is characterized by the formation of atherosclerotic plaques caused by the accumulation of cholesterol and lipids in the walls of the coronary arteries [2]. There is compelling evidence to suggest that inflammation and obesity have a significant and independent impact on the prognosis of atherosclerosis [1, 3]. In obesity, there is a persistent inflammatory state characterized by increased infiltration of macrophages in adipose tissue. These macrophages can alter the secretory profile of adipocytes, resulting in various metabolic and inflammatory processes that can affect the body [4]. In addition, being overweight or obese is associated with not only heightened systemic inflammation but also increased oxidative stress. Excessive caloric intake leads to an increase in substrate-induced tricarboxylic acid cycle activity, resulting in the overproduction of mitochondrial NADH and reactive oxygen species (ROS). Oxidative stress is recognized as a shared underlying factor in insulin resistance, type 2 diabetes, and cardiovascular disease, linking inflammation with these conditions [5, 6].
Adipose tissue releases secretory cytokines known as adipokines, which have a significant impact on whole-body metabolism, endocrine functions, and inflammation [1]. Research has demonstrated that adipokines, such as resistin, leptin, chemerin, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and adiponectin, play significant roles in both the development and progression of atherosclerosis. These adipokines can also impact diabetes, insulin resistance, coagulation, fibrinolysis, and lipid levels [1, 7]. Adiponectin has anti-inflammatory, anti-diabetic, and anti-atherogenic properties. Low circulating levels of adiponectin are associated with various cardiovascular diseases, including CAD [8, 9]. C1q/TNF-related proteins (CTRP) are a family of adipokines that have been identified as adiponectin paralogs with a significant impact on several metabolic pathways [10]. The CTRP family consists of 15 members, ranging from CTRP1 to CTRP15, which act as metabolic regulators [11], The CTRP family of adipokines regulates several metabolic pathways, including lipid and glucose metabolism and inflammation. They also play a significant role in the association between obesity and inflammation, as well as the modulation of inflammation in adipose tissue [12].. CTRP6 is a member of the CTRP family, primarily expressed in adipose tissue, brain, heart, and placenta. Reports have suggested that CTRP6 has a distinct function in comparison to other well-known CTRPs [13, 14].. A growing body of evidence has shown that CTRP6 can regulate the differentiation of adipocytes and myofibroblasts, as well as angiotensin II-induced hypertension, cardiac fibrosis, fibrogenesis, and the function of endothelial cells [13]. Furthermore, CTRP6 is associated with the regulation of glucose and lipid metabolism, inflammation, and insulin sensitivity [12, 13]. In conditions of obesity and diabetes, the expression of CTRP6 adipokine is significantly increased in adipose tissue, with stromal vascular cells and macrophages being the primary source of CTRP6 [12, 13]. In addition, an increase in CTRP6 levels has been reported in patients with rheumatoid arthritis [15]. Furthermore, CTRP6 has been shown to increase the expression of interleukin-10 in macrophages, indicating its anti-inflammatory effect [16]. Lei et al. [17]. and Wei et al. [18] have shown CTRP6 cardioprotective effects. They demonstrated that elevated levels of CTRP6 may mitigate postinfarct cardiac fibrosis and lower the incidence of acute coronary syndrome (ACS). Nevertheless, the existing literature presents contrasting evidence regarding the function of CTRP6 in metabolic processes, and no definitive findings have emerged regarding the connection between CTRP6 and risk factors for coronary artery disease (CAD). Against this backdrop, the present study was undertaken to assess the serum concentrations of CTRP6 in patients with CAD and to investigate its association with anthropometric and biochemical parameters, as well as inflammatory cytokines.
Materials and methods
Study population
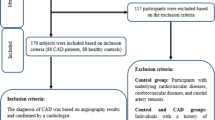
A total of 176 participants were recruited, including 88 CAD patients (55 males and 33 females) and 88 controls (46 males and 42 females) aged between 45 and 75 years. All participants underwent angiography in Shariati Hospital (Tehran, Iran) to determine the extent of coronary artery stenosis. CAD was identified by a cardiologist, and patients with more than 50% stenosis in at least one coronary artery were classified as CAD patients. The control group consisted of subjects whose coronary vessel stenosis was less than 30%. Subjects with underlying diseases, such as unstable angina, carotid artery stenosis, cerebrovascular disease, peripheral and coronary vascular disease, myocardial infarction, diabetes, and those being treated with thiazolidinedione drugs, were excluded from the control category. Additionally, none of the participants had used alcohol or cigarettes in the past three months. The study adhered to the Helsinki Declaration and was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SHARIATI.REC.1401.017). All study participants provided written consent.
Anthropometric and laboratory assessment
The body mass index (BMI) was estimated using the standard formula of weight (in kilograms) divided by height (in square meters). Systolic and diastolic blood pressures were measured in the seated position following 15 min of rest, utilizing a standard sphygmomanometer. After overnight fasting, venous blood samples were collected, and laboratory parameters such as triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), creatinine (Cr), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured via commercially available kits (Pars Azmoon; Iran) with an auto analyzer. Additionally, malondialdehyde (MDA) and total antioxidant capacity (TAC) were measured following the provided instructions of a commercially available kit (Kia zist; Iran). Fasting insulin levels were measured using an Enzyme-linked immunosorbent assay (ELISA) kit (Monobind; U.S.A.). The homeostasis model of insulin resistance (HOMA-IR) was calculated using the standard formula of [fasting blood glucose (mg/dL)] multiplied by [fasting blood insulin (µU)] divided by 405.
Cytokine and adipokine measurement
To evaluate serum levels of TNF-α (Cat# DTA00C) and IL-6 (Cat# HS600B), ELISA kits (R & D Systems; USA) were employed. The least detectable range for IL-6 was 0.11 pg/mL and for TNF-α was 0.5 pg/mL. Intra-assay and inter-assay coefficients of variation (CV) for IL-6 and TNF-α were 6.9 and 9.6, and 5.2 and 7.4, respectively. Adiponectin serum levels were measured with an ELISA kit (Adipogen; South Korea; Cat# AG-45 A-0001YEKKI01), and the least detectable range was 0.1 pg/mL, with intra- and inter-assay CV of 3.4% and 4.3%, respectively. CTRP6 levels were measured using an ELISA kit (Aviscera Bioscience; USA; Cat# SK00392-06) with an intra-assay CV of 7%, an inter-assay CV of 6%, and a detection limit of 10 pg/mL.
Statistical analysis
The categorical data were analyzed using the chi-square test and presented as frequency and percentage. The normality of variables was assessed using the Kolmogorov–Smirnov test. Variables with normal distribution were expressed as mean ± standard Error of mean (SEM), while those with non-normal distribution were presented as median ± interquartile range (IQR). Student t-test and Mann-Whitney U test were employed for data analysis with normal and non-normal distribution, respectively. The analysis of covariance (ANCOVA) test was utilized to eliminate any possible influence of covariates on CTRP6 levels. Pearson correlation analysis was used to assess the correlation of CTRP6 with biochemical and anthropometric variables. Binary logistic regression was performed to predict CAD risk based on the circulating level of CTRP6. Logarithmic transformation was performed for non-normally distributed data before correlation and regression analysis. The CTRP6 cut-off value was determined based on the receiver operating characteristic (ROC) curve to differentiate between the CAD and control groups. The statistical analysis was performed using SPSS21 (SPSS, Chicago, IL, USA), and a p-value < 0.05 was considered statistically significant.
Results
Anthropometric and biochemical measurements
Table 1 presents the descriptive statistics of study variables for the CAD and control groups. The mean age for the CAD and control groups were 57.92 and 57.5 years, respectively, and did not differ significantly (P = 0.720). The mean BMI also did not differ significantly between the CAD and control groups (P = 0.686). The CAD patients had higher levels of TG, TC, and LDL-C, and lower levels of HDL-C, compared to the control group, with significant differences observed for TG, TC, HDL-C, and LDL-C (P < 0.001, P < 0.001, P = 0.002, and P = 0.001, respectively).
MDA levels were significantly higher in CAD patients [9.27 (8.23, 11.45)] compared to controls [6.69 (5.32, 9.1), (p < 0.001)], while TAC levels were markedly lower in CAD group [93.77 ± 1.80 vs. 80.27 ± 1.37, (P < 0.001)]. No significant differences were observed for ALT (P = 0.131), FBG (P = 0.966), and creatinine (P = 0.068), while significant differences were observed for AST (P = 0.047), insulin (P < 0.001) (Fig. 1a), HOMA-IR (P < 0.001), (Fig. 1b), DBP (P = 0.005), and SBP (P = 0.009) between the groups.
Cytokine and adipokine serum levels
The study found that CAD patients had higher serum levels of IL-6 [7.9 (5.4, 12.35) pg/mL] and TNF-α [26.65 (23.4, 33.35) pg/mL] than controls [6.2 (4.3, 7.8) pg/mL and 24.8 (16.95, 28.35) pg/mL, respectively] (Table 1). In contrast, Adiponectin serum levels were significantly lower in the CAD group than in the control group [8.54 ± 0.30 µg/mL vs. 10.36 ± 0.40 µg/mL, (P < 0.001)] (Table 1). Moreover, the study observed a higher level of CTRP6 in CAD patients compared with the controls (P < 0.001, Fig. 2a).
Additionally, CTRP6 serum levels were higher in the 3-vessel disease group compared to the 1-vessel disease group, and in the 2-vessel disease group compared to the 1-vessel disease group (P < 0.001). However, no significant difference was observed between the 3-vessel disease and 2-vessel disease groups (P > 0.05), Fig. 2b).
Furthermore, we analyzed CTRP6 levels according to gender and BMI in each group. In the control group, females had higher significant levels of CTRP6 compared to males (P < 0.001), while no significant difference was observed between males and females in the CAD group (P > 0.05, Fig. 2c). We have further compared male and female CAD patients to their control counterparts. There was a significant higher CTRP6 levels in female CAD patients than controls females (P < 0.01). Similar result was found for male participants; CTRP6 levels was higher in male CAD patients compared to control males (P < 0.001, Fig. 2c).
Moreover, in both CAD and control subjects, serum levels of CTRP6 differed significantly between BMI < 25 and BMI > 25 groups (P < 0.001 and P < 0.01, respectively), with higher levels in those with BMI > 25 (Fig. 2d).
Serum levels of CTRP6 in study population. (a) Serum levels of CTRP6 were found to be higher in CAD patients compared to controls (p < 0.001). (b) CTRP6 serum levels demonstrated higher level in 3-VD and 2-VD groups than 1-VD group (p < 0.001). (C) Serum levels of CTRP6 were higher in females compared with males in control group. (d) CTRP6 serum levels were significantly higher in groups with BMI > 25 (p < 0.001)
The association of CTRP6 levels with biochemical and anthropometric parameters
The results of the correlation analysis between CTRP6 levels and other parameters in both the control and CAD groups are presented in Table 2. In the control group, serum CTRP6 levels were positively correlated with BMI, SBP, insulin, HOMA-IR, ALT, and MDA, while showing a negative correlation with HDL-C and TAC. Similarly, in the CAD group, CTRP6 levels were positively correlated with BMI, SBP, MDA, IL-6, and TNF-α, and negatively correlated with creatinine and TAC (Table 2).
The association of CTRP6 with CAD
A logistic regression analysis was conducted to explore the potential relationship between serum CTRP6 levels and the likelihood of CAD. The results of this analysis are summarized in Table 3. Specifically, the odds ratio (OR) of CAD status per 10-unit increase in CTRP6 was examined. In the unadjusted model, higher serum levels of CTRP6 were significantly associated with an increased risk of CAD (OR [95% confidence interval (CI)] = 1.078 [1. 049–1.108]; p < 0.0001). Furthermore, after controlling for the effects of BMI, sex, and age, CTRP6 remained significantly correlated with the risk of CAD (OR [95% CI] = 1.123 [1.079–1.169]; p < 0.0001).
Receiver operating characteristic (ROC) curve analysis was employed to evaluate the diagnostic ability of CTRP6 to discriminate between the CAD and control groups (area under the curve (AUC): 0.745 [95% CI: 0.674–0.816] and p < 0.0001), indicating moderate accuracy of CTRP6 in distinguishing between CAD and control subjects (Fig. 3).
Discussion
The current study represents a novel investigation into the association between CTRP6 and the risk factors of CAD. While prior research has explored the expression levels of various members of the CTRP family in different metabolic disorders such as metabolic syndrome, non-alcoholic fatty liver disease, and diabetes [19,20,21,22] this study provides unique insights into the specific relationship between CTRP6 and CAD risk factors. Among them, CTRP13, CTRP9, CTRP12, CTRP3 and CTRP1 have been stated to be linked with CAD [10, 20, 23, 24]. Previous research has suggested a correlation between CTRP6 and inflammatory markers, lipid oxidation, and glucose metabolism, among other metabolic disorders [12, 13, 25,26,27]. While there is evidence for relation of CTRP6 with risk factors of atherosclerosis in cardiometabolic diseases, there is limited data on the relationship between CTRP6 and the CAD. Given the similar structure and biological activities of the CTRP family, along with CTRP6’s role as a metabolic and immune regulator, we hypothesized that CTRP6 may play a significant role in the development of CAD.
Based on our findings, we observed that the levels of CTRP6 were higher in CAD patients and exhibited a positive correlation with the severity of the disease. Contrary to the results of the present study, in 2022, Wei et al. [18]. investigated the association of serum CTRP6 with the development and exacerbation of ACS. They indicated that the levels of CTRP6 were markedly lower in the ACS group than the control group, and the risk of developing ACS reduced with high levels of CTRP6. The reasons behind the differences between the present study and their reports may be related to the study population. In this context, cases of unstable angina were excluded in our study, while not in Wei et al’s study. Also, patient with less than 50% stenosis in coronary artery were included in the control group, but in our study, people with less than 30% stenosis in coronary artery were classified in control group. The racial differences of the participants in both studies can also have an effect on the dissimilarity in the consequences.
Several studies have reported comparable results to our findings in the context of cardiometabolic diseases, including type 2 diabetes, metabolic syndrome, and non-alcoholic fatty liver disease. Sadeghi and colleagues demonstrated a statistically significant elevation in the concentration of CTRP6, among patients diagnosed with polycystic ovary syndrome (PCOS). On the other hand, an independent study has reported similar findings, documenting a higher concentration of CTRP6 in a cohort of obese individuals [28, 29]. In addition, in 2017, Lei et al. [12]. conducted a study in mouse and human models with diabetes and obesity, demonstrating a significant up-regulation of CTRP6 expression in adipose tissue. In line with these finding, there was a positive association between circulating CTRP6 with BMI in the recent study. A number of studies have shown the regulatory mechanism of CTRP6 in lipid metabolism. Knockout of CTRP6 resulted in inhibition of adipogenesis in adipocytes by suppression of lipogenic and adipogenic markers like CCAAT/enhancer binding proteins (C/EBPs), extracellular signal-regulated kinase 1/2 (Erk1/2) signaling pathway and peroxisome proliferator-activated receptor gamma (PPARγ) [30] as well as prevented inappropriate lipogenesis in myoblasts by reducing the AdipoR1/Erk/PPARγ signaling pathway [31]. This mechanism might be a causative explanation for the relation of CTRP6 with obesity and adipose tissue.
Furthermore, the overexpression of CTRP6 has been shown to increase the levels of circulating inflammatory cytokines and pro-inflammatory macrophages in adipose tissue. Conversely, the loss of CTRP6 has been found to decrease the serum levels of TNF-α and MCP1 in mice under a high-fat diet [12]. Consistent with these findings, our study has shown a positive correlation between CTRP6 and IL-6 and TNF-α in patients with CAD. Additionally, our study found a positive correlation between CTRP6 and MDA, a marker of lipid peroxidation, and a negative correlation with total antioxidant capacity. The impact of CTRP6 on oxidative stress is inconsistent; while there is evidence for a favorable impact of CTRP6 on MDA levels and ROS production [32], there are also studies that show CTRP6 leads to an increase in oxidative stress, and loss of CTRP6 is protective against oxidative stress [33]. These studies utilized various cell types, implying that the impact of CTRP6 on oxidative stress may be specific to certain tissues. Our findings have demonstrated a correlation between CTRP6 and oxidative stress in patients with CAD, but not in healthy controls. These results collectively suggest a complex relationship between CTRP6 and oxidative stress, indicating the need for further studies in this regard.
While relation of CTRP6 and oxidative stress was limited to CAD patients, the relation of CTRP6 and insulin levels and insulin resistance was limited to controls. In 2018, Wang et al. [13]. investigated the serum levels of CTRP6 in patients with type 2 diabetes for the first time. Their results showed that CTRP6 level was higher in diabetic group than healthy group, and its serum levels had a positive correlation with BMI, fat%, WHR, fasting insulin, FBG, HOMA-IR as well as TNF-α and a negative association with adiponectin [34, 35].
Chi et al. [36]. in 2017 showed that CTRP6 serum levels were notably decreased in spontaneously hypertensive rats (SHR), and conversely overexpression of CTRP6 significantly reduced angiotensinII-mediated hypertension and inflammation of vascular endothelial. CTRP6 can reduce hypertension caused by angiotensin II and dysfunction of vascular endothelial in SHR by activating the Erk1/2 signaling pathway and PPARγ expression, which negatively regulates angiotensin II [36]. These findings are contrary to our results that show a positive correlation of CTRP6 with SBP in CAD patients. The reason for these discrepancies can be attributed to the different CTRP6 mechanism of action in humans and mice. On the other hand, it can be assumed that the body increases circulating CTPP6 levels as a compensatory response to hypertension.
Conclusion
In conclusion, the study provides unique insights into the specific relationship between CTRP6 and CAD risk factors. The findings indicate that the levels of CTRP6 were higher in CAD patients and exhibited a positive correlation with the severity of the disease. Our study has also shown a positive correlation between CTRP6 and circulating inflammatory cytokines, oxidative stress, and insulin resistance. However, the impact of CTRP6 on oxidative stress may be specific to certain tissues, indicating the need for further studies. The regulatory mechanism of CTRP6 in lipid metabolism may be a causative explanation for the relationship of CTRP6 with obesity and adipose tissue.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Liu L, Shi Z, Ji X, Zhang W, Luan J, Zahr T, et al. Adipokines, adiposity, and atherosclerosis. Cell Mol Life Sci. 2022;79(5):272. https://doi.org/10.1007/s00018-022-04286-2.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66. https://doi.org/10.1161/circresaha.114.302721.
Montarello NJ, Nguyen MT, Wong DTL, Nicholls SJ, Psaltis PJ. Inflammation in coronary atherosclerosis and its therapeutic implications. Cardiovasc Drugs Ther. 2022;36(2):347–62. https://doi.org/10.1007/s10557-020-07106-6.
Bai Y, Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev. 2015;16(2):127–36. https://doi.org/10.1111/obr.12242.
Maddux BA, See W, Lawrence JC Jr., Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50(2):404–10. https://doi.org/10.2337/diabetes.50.2.404.
Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–23. https://doi.org/10.1161/01.Atv.0000122852.22604.78.
Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, et al. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2. https://doi.org/10.1186/1475-2840-10-2.
Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr. 2009;4(1):44–9. https://doi.org/10.1111/j.1559-4572.2008.00030.x.
Zaidi H, Byrkjeland R, Njerve IU, Åkra S, Solheim S, Arnesen H, et al. Adiponectin in relation to exercise and physical performance in patients with type 2 diabetes and coronary artery disease. Adipocyte. 2021;10(1):612–20. https://doi.org/10.1080/21623945.2021.1996699.
Fadaei R, Moradi N, Kazemi T, Chamani E, Azdaki N, Moezibady SA, et al. Decreased serum levels of CTRP12/adipolin in patients with coronary artery disease in relation to inflammatory cytokines and insulin resistance. Cytokine. 2019;113:326–31. https://doi.org/10.1016/j.cyto.2018.09.019.
Schäffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab. 2012;23(4):194–204. https://doi.org/10.1016/j.tem.2011.12.003.
Lei X, Seldin MM, Little HC, Choy N, Klonisch T, Wong GW. C1q/TNF-related protein 6 (CTRP6) links obesity to adipose tissue inflammation and insulin resistance. J Biol Chem. 2017;292(36):14836–50. https://doi.org/10.1074/jbc.M116.766808.
Wang M, Tang X, Li L, Liu D, Liu H, Zheng H, et al. C1q/TNF-related protein-6 is associated with insulin resistance and the development of diabetes in Chinese population. Acta Diabetol. 2018;55(12):1221–9. https://doi.org/10.1007/s00592-018-1203-2.
Zheng WF, Zhang SY, Ma HF, Chang XW, Wang H. C1qTNF-related protein-6 protects against doxorubicin-induced cardiac injury. J Cell Biochem. 2019;120(6):10748–55. https://doi.org/10.1002/jcb.28366.
Murayama MA, Kakuta S, Inoue A, Umeda N, Yonezawa T, Maruhashi T, et al. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat Commun. 2015;6(1):1–12.
Kim MJ, Lee W, Park EJ, Park SY. C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol Cells. 2010;30(1):59–64. https://doi.org/10.1007/s10059-010-0088-x.
Lei H, Wu D, Wang J-Y, Li L, Zhang C-L, Feng H, et al. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol. 2015;110(4):35. https://doi.org/10.1007/s00395-015-0492-7.
Wei C, Liu Y, Xing E, Ding Z, Tian Y, Zhao Z, et al. Association between Novel Pro- and Anti- Inflammatory adipocytokines in patients with Acute Coronary Syndrome. Clin Appl Thromb Hemost. 2022;28:10760296221128021. https://doi.org/10.1177/10760296221128021.
Emamgholipour S, Moradi N, Beigy M, Shabani P, Fadaei R, Poustchi H, et al. The association of circulating levels of complement-C1q TNF-related protein 5 (CTRP5) with nonalcoholic fatty liver disease and type 2 diabetes: a case-control study. Diabetol Metab Syndr. 2015;7:108. https://doi.org/10.1186/s13098-015-0099-z.
Fadaei R, Moradi N, Baratchian M, Aghajani H, Malek M, Fazaeli AA, et al. Association of C1q/TNF-Related Protein-3 (CTRP3) and CTRP13 serum levels with coronary artery disease in subjects with and without type 2 diabetes Mellitus. PLoS ONE. 2016;11(12):e0168773. https://doi.org/10.1371/journal.pone.0168773.
Bai B, Ban B, Liu Z, Zhang MM, Tan BK, Chen J. Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in type 2 diabetes mellitus: in vivo regulation by glucose. PLoS ONE. 2017;12(2):e0172271.
Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Reviews Endocr Metabolic Disorders. 2014;15(2):111–23. https://doi.org/10.1007/s11154-013-9255-7.
Lu L, Zhang RY, Wang XQ, Liu ZH, Shen Y, Ding FH, et al. C1q/TNF-related protein-1: an adipokine marking and promoting atherosclerosis. Eur Heart J. 2016;37(22):1762–71.
Moradi N, Fadaei R, Emamgholipour S, Kazemian E, Panahi G, Vahedi S, et al. Association of circulating CTRP9 with soluble adhesion molecules and inflammatory markers in patients with type 2 diabetes mellitus and coronary artery disease. PLoS ONE. 2018;13(1):e0192159.
Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416(2):161–77. https://doi.org/10.1042/bj20081240.
Xu E, Yin C, Yi X, Liu Y. Knockdown of CTRP6 inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells through regulating the Akt/NF‐κB pathway. Clin Exp Pharmacol Physiol. 2020;47(7):1203–11.
Lee W, Kim MJ, Park EJ, Choi YJ, Park SY. C1qTNF-related protein-6 mediates fatty acid oxidation via the activation of the AMP-activated protein kinase. FEBS Lett. 2010;584(5):968–72. https://doi.org/10.1016/j.febslet.2010.01.040.
Sadeghi A, Fadaei R, Moradi N, Fouani FZ, Roozbehkia M, Zandieh Z et al. Circulating levels of C1q/TNF-α-related protein 6 (CTRP6) in polycystic ovary syndrome. 2020;72(7):1449–59; https://doi.org/10.1002/iub.2272.
Liao X, Liu S, Tang X, Yang D, Liu H, Gao L, et al. Circulating CTRP6 levels are increased in overweight or obese Chinese individuals and Associated with insulin resistance parameters: a pilot study. Experimental and clinical endocrinology & diabetes: official journal. German Soc Endocrinol [and] German Diabetes Association. 2021;129(7):535–41. https://doi.org/10.1055/a-0929-6072.
Wu WJ, Mo DL, Zhao CZ, Zhao C, Chen YS, Pang WJ, et al. Knockdown of CTRP6 inhibits adipogenesis via lipogenic marker genes and Erk1/2 signalling pathway. Cell Biol Int. 2015;39(5):554–62. https://doi.org/10.1002/cbin.10422.
Wu W, Sun Y, Zhao C, Zhao C, Chen X, Wang G, et al. Lipogenesis in myoblasts and its regulation of CTRP6 by AdipoR1/Erk/PPARγ signaling pathway. Acta Biochim Biophys Sin. 2016;48(6):509–19.
Li Y, Sun J, Gu L, Gao X. Protective effect of CTRP6 on cerebral ischemia/reperfusion injury by attenuating inflammation, oxidative stress and apoptosis in PC12 cells. Mol Med Rep. 2020;22(1):344–52. https://doi.org/10.3892/mmr.2020.11108.
Xu E, Yin C, Yi X, Liu Y. Knockdown of CTRP6 inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells through regulating the Akt/NF-κB pathway. Clin Exp Pharmacol Physiol. 2020;47(7):1203–11. https://doi.org/10.1111/1440-1681.13289.
Aronson D, Edelman ER. Coronary artery disease and diabetes mellitus. Cardiol Clin. 2014;32(3):439–55.
Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–86. https://doi.org/10.1161/circulationaha.108.835223.
Chi L, Hu X, Zhang W, Bai T, Zhang L, Zeng H, et al. Adipokine CTRP6 improves PPARγ activation to alleviate angiotensin II-induced hypertension and vascular endothelial dysfunction in spontaneously hypertensive rats. Biochem Biophys Res Commun. 2017;482(4):727–34. https://doi.org/10.1016/j.bbrc.2016.11.102.
Acknowledgements
Tehran University of Medical Sciences (TUMS), Tehran, Iran, (grant number: 59099) supported this project. We thank all the patients and individuals participating in the study.
Funding
Tehran University of Medical Sciences (TUMS), Tehran, Iran, (grant number: 59099) supported this project.
Author information
Authors and Affiliations
Contributions
Design: Nariman Moradi, Reza Fadaei. Data curation: Ali Abbasi, Seyed abdolhossein Tabatabaei. Formal analysis and Validation: Reza Fadaei. Investigation: Nariman Moradi. Frood Malekshahi, Arash Falahat. Methodology: Nariman Moradi, Akram Vatannejad, Maryam Mirahmad. Project administration: Ali Abbasi, Seyed abdolhosein Tabatabaei. Supervision and Resources: Ali Abbasi. Writing? original draft: Vida Farrokhi, Maryam Mirahmad, Reza Afrisham, Writing? review & editing: Reza Afrisham, Reza Fadaei, Ali Abbasi, Nariman Moradi. Frood Malekshahi, Arash Falahat.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This investigation was performed according to the Helsinki Declaration as well as was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SHARIATI.REC.1401.017). All study contributors signed forms of written and informed consent.
Consent for publication
Not applicable.
Competing interests
The authors announce that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tabatabaei, S.a., Fadaei, R., Moradi, N. et al. Circulating levels of C1q/TNF-α‐related protein 6 (CTRP6) in coronary artery disease and its correlation with inflammatory markers. J Diabetes Metab Disord 23, 1233–1241 (2024). https://doi.org/10.1007/s40200-024-01415-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-024-01415-5