Abstract
Objectives
Diabetes mellitus (DM) is a long-standing and non-transmissible endocrine disease that generates significant clinical issues and currently affects approximately 400 million people worldwide. The aim of the present review was to analyze the most relevant and recent studies that focused on the potential application of plant extracts and phytocompounds in nanotechnology for the treatment of T2DM.
Methods
Various databases were examined, including Springer Link, Google Scholar, PubMed, Wiley Online Library, and Science Direct. The search focused on discovering the potential application of nanoparticulate technologies in enhancing drug delivery of phytocompounds for the mentioned condition.
Results
Several drug delivery systems have been considered, that aimed to reduce adverse effects, while enhancing the efficiency of oral antidiabetic medications. Plant-based nanoformulations have been highlighted as an innovative approach for DM treatment due to their eco-friendly and cost-effective synthesis methods. Their benefits include targeted action, enhanced availability, stability, and reduced dosage frequency.
Conclusions
Nanomedicine has opened new opportunities for the diagnosis, treatment, and prevention of DM. The use of nanomaterials has demonstrated improved outcomes for both T1DM and T2DM. Notably, flavonoids, including substances such as quercetin, naringenin and myricitrin, have been recognized for their enhanced efficacy when delivered through novel nanotechnologies in preventing T2DM onset and associated complications. The perspectives on the addressed subject point to the development of more nanostructured phytocompounds with improved bioavailability and therapeutic efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term "diabetes mellitus" (DM) refers to a variety of long-term metabolic conditions characterized by hyperglycemia and an imbalance in the activity or levels of insulin, the only hormone known to lower blood glucose levels [1]. Although abnormalities in insulin production and/or activity can lead to hyperglycemia through pathogenetic routes, the failure or loss of pancreatic β-cells represents the underlying factor that connects all types of diabetes. Numerous endocrine cell types, including essential blood glucose-regulating cells that release glucagon and insulin, are found in the pancreatic islets of Langerhans. Genetic anomalies, epigenetic changes, insulin resistance, autoimmune diseases, inflammation, and environmental factors are some of the elements causing β-cell degeneration [2].
DM is a chronic metabolic disease characterized by its complex nature and major clinical concerns and can be correlated with patient risks and metabolic syndrome development. The condition is generally associated with a chronic increase in blood glucose and lipid levels, and oxidative stress, thus leading to chronic complications that mostly affect kidneys, eyes, nerves, and blood vessels, among other organs. According to the World Health Organization, DM is an outbreak that can cause serious illness and even death. Moreover, pre-diabetes, as a risk factor, also increases the overall number of fatalities linked to diabetes by 2%. Although this represents a rather slight increase, it illustrates the large risk that DM poses [3].
Etiology and classification of DM
DM, featuring complex pathophysiology and various types, can be classified according to both etiology and pathogenesis into: T1DM and latent autoimmune diabetes of adulthood, T2DM, gestational DM, and secondary DM which results as a consequence of other medications, endocrine or hereditary diseases, as shown in Table 1. An accurate diagnosis assessment of the diabetes type is critical for the selection of appropriate treatment [4].
Patients with DM may develop diabetic ketoacidosis, different infections, and other acute or chronic complications such as vascular diseases, diabetic nephropathy, retinopathy, diabetic foot, as well as other chronic issues due to long-term carbohydrate, lipid, and protein metabolism alterations [5].
Pathophysiology and risk factors
Environmental, metabolic, and genetic factors interact to raise the risk of developing T2DM. Although there is a strong genetic component to each patient's susceptibility to T2DM given inherent risk factors, such as race and genetic susceptibility, research has shown that various cases of TD2M can be prevented by addressing the key variable risk elements (obesity and poor diet) [6]. DeFronzo proposed in 2009 that incretin dysregulation, lipolysis, hyperglucagonemia, increased glucose reabsorption in the kidney, and central appetite dysregulation have major roles in the pathophysiology of T2DM. Later, this association was expanded to encompass impairments in 12 interconnected pathways, with β-cell dysfunction serving as the link between these pathways (Table 2) [7].
Treatment of DM
The management of diabetes enhances lifestyle and mitigates complications. Efficient glycemic control reduces the progression of microvascular issues (e.g.: retinopathy and nephropathy). A 1 to 2% reduction in HbA1c results in a 25% decrease in retinopathy and a 50% reduction in microvascular complications, respectively. The long-term advantages of intensive primary prevention methods outweigh those of glycemic control improvement following vascular injury [8].
Metformin is suggested as the first line of treatment in both the 2020 European Society of Cardiology/European Association for the Study of Diabetes (ESC/EASD) and the 2019 update of the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) guidelines [9]. A SGLT-2 inhibitor or GLP-1 agonist should be given before metformin in treatment-naive recently diagnosed T2DM patients with confirmed cardiovascular (CV) disease or very high-risk patients (i.e., with target organ damage or multiple CV risk factors), in accordance with the 2019 ESC/EASD guidelines and the 2020 American College of Cardiology (ACC) Expert Consensus. However, given that 75% of the patients in the key cardiovascular outcomes trials (CVOTs) were taking metformin at the beginning of their treatments, this advice is somewhat questionable. The 2022 ADA Standards of Medical Care guidelines close this gap and suggest that metformin should be used as first choice therapy for the majority of patients, but that GLP-1 agonists and SGLT2 inhibitors can also be used in patients with atherosclerotic CV disease, heart failure or chronic kidney disease, with or without albuminuria, regardless of baseline metformin use and glycemic control [10].
Clinical trials have investigated the decrease in the consequences of major adverse CV events, including nonfatal myocardial infarction, nonfatal stroke, and mortality. Regardless of the glycemic target, the American and European diabetes guidelines currently advise using SGLT-2 inhibitors or GLP-1 agonists for patients with confirmed atherosclerotic disease or who are at high risk for CV disease [11].
DM poses an escalating risk for both micro- and macrovascular complications, that contribute to growing concerns in global public health due to its increasing occurrence. While certain therapies have shown benefits for patients with DM, the existing treatments come with associated adverse effects. Therefore, novel approaches are essential to address the challenges of current antidiabetic medications [12].
Over the past two decades, the number of therapeutic drugs for managing hyperglycemia in T2DM has significantly increased. In 2008, the US Food and Drug Advisory Committee ordered that CVOTs be used to examine the safety of all new antihyperglycemic medications, which was prompted by concerns about rosiglitazone's impact on CV events, thus typically employing three-point major adverse events (non-fatal stroke, non-fatal myocardial infarction, and cardiovascular death) as their primary endpoint. Heart failure, a frequent consequence of T2DM that increases death rates, is frequently included, typically as a secondary endpoint. Recently, trials that exclusively looked at renal outcomes have shifted their focus [13].
Biguanides are a well-known class of drugs used to treat diabetes. The only readily available biguanide is metformin. It is the most widely used oral glucose-lowering drug and it is sometimes called the “foundation therapy” for those with newly diagnosed T2DM. Metformin improves insulin sensitivity and reduces gluconeogenesis. According to the Biopharmaceutical Classification System, metformin is classified as a class III medication, meaning it has high solubility and low permeability [14].
Thiazolidinediones, a third-line therapy, can also be used in the treatment of DM. Incretin analogues and DPP4-inhibitors, which target the incretin system, as well as SGLT2 inhibitors, which target the kidneys' reabsorption of glucose, represent newer treatments. If metformin is not tolerated or indicated for the patient, such new drugs can be used alone or as second or third-line treatments [15]. The primary characteristics of the most common antidiabetic medications are mentioned in Table 3 and describe their pharmacodynamics to control hyperglycemia.
Herbal medicines in diabetes mellitus—clinical trials
Numerous common herbs have the potential to lower blood glucose levels, making it possible to achieve improved glycemic control or reduce insulin injections, which is undeniably appealing. Nonetheless, the choice of plants can be influenced by factors such as the stage of diabetes progression, the presence of other medical conditions, as well by availability, cost, and safety [22].
Some herbal medicines demonstrated efficacy throughout various diabetes stages. For example, due to its known benefits and safety, curcumin is recommended as a potential pre-diabetes treatment to halt T2DM progression. Cinnamon may be more appropriate for diabetics with concurrent hypertension, while Aloe vera leaf ethanolic extract may increase insulin levels from regenerated pancreatic β-cells [22, 23].
In clinical trials that focus on diabetes treatment, there's a growing interest regarding the use of plants. However, the broad inconsistencies in adhering to regulatory standards in herbal medicine research complicate the regulation process for such drugs. This section provides a brief overview of plants or their phytocompounds that have been clinically tested for their role in diabetic management.
Cinnamomum zeylanicum has been used as a traditional remedy for diabetes. It contains compounds such as anthraquinones, coumarins, flavonoids, tannins and terpenoids. Given its accessibility, cost-effectiveness, and safety profile, this species is considered as a low-risk alternative for diabetic patients. Tangvarasittichai et al. conducted a study examining the influence of cinnamon supplementation (500 mg capsules) over a 60-day period on insulin sensitivity and resistance, malondialdehyde levels and total antioxidant capacity in diabetic patients. The primary active components in cinnamon are generally represented by polyphenolic polymers that are thought to act as antioxidants, enhance insulin function, and that could help manage glucose intolerance and diabetes. Consistent with prior research, it has also been shown that cinnamon could serve as a natural insulin sensitizer. The results demonstrated a significant decrease in insulin resistance, oxidative stress and inflammation while insulin sensitivity and total antioxidant capacity were notably increased [24]. In a separate study led by Anderson et al., the intake of dried aqueous extract of Cinnamomum cassia in patients with increased serum glucose resulted in reduced 2-h postprandial blood glucose and fasting insulin. The treatment group was administered a commercially available spray-dried cinnamon extract (CinSulin®) capsules twice per day. The control group received placebo capsules comprising of dark brown wheat flour, closely resembling the appearance of the cinnamon extract. The cinnamon treatment resulted in a decrease in total cholesterol levels [25].
Curcumin, a bioactive component derived from Curcuma longa, shows a wide range of therapeutic properties (e.g.: antioxidant, anti-inflammatory, anticancer, neuroprotective, antidiabetic) [26]. Curcumin is being considered as a potential approach in the treatment of pre-diabetes to hinder the progression of T2DM, given its established advantages and safety. According to a randomized, double-blinded, placebo-controlled clinical trial led by Chuengsamarn et al., none of the subjects treated with curcumin over a 9-month period developed T2DM, while 16.4% of those from the placebo group did. Moreover, during the final visit, subjects treated with curcumin displayed enhanced overall β-cell function, with lower C-peptide values and higher homeostasis model assessment for insulin resistance-β values compared to those in the placebo group. Moreover, subjects treated with curcumin exhibited notably elevated levels of the anti-inflammatory cytokine (adiponectin) in comparison to those in the placebo group. These increased levels could potentially have a beneficial impact on minimizing inflammation in β-cells, thus offering protection against their degradation [27].
During an 8-week randomized, double-blind, and placebo-controlled clinical trial, capsules infused with green cumin (Cuminum cyminum) significantly reduced insulin levels, which was also accompanied by enhanced insulin sensitivity. The primary constituents identified by gas chromatography were thymoquinone, cumin aldehyde, p-cymene, limonene, myrcene, terpinene, safranal, sterol compounds, and β-pinene. Researchers also noted that cumin supplementation helped regulate inflammatory markers, specifically Tumor Necrosis Factor-α and high-sensitivity C-reactive protein [28].
Resveratrol (RSV) is a polyphenolic constituent specifically belonging to the stilbenoid class. In diabetic patients, a 30-day dietary supplementation with RSV demonstrated suppressed postprandial glucagon responses. Another clinical trial involving 62 T2DM patients compared two groups: one treated solely with an oral hypoglycemic agent (metformin and/or glibenclamide) and the other combining this agent with 250 mg RSV administered daily for three months. The latter group exhibited improved HbA1c levels, indicating enhanced glycemic control in T2DM patients [29]. Furthermore, a separate study that administered 2 × 5 mg RSV over four weeks to patients with T2DM led to a reduction in insulin resistance, associated with a decrease in oxidative stress [30].
Many studies suggest Aloe vera as a potential medicine, which is attributed to its rich bioactive compounds such as alkaloids, anthraquinones, and enthrones. These compounds are associated with therapeutical properties, including antioxidant, anti-inflammatory, neuroprotective, and antidiabetic effects [31]. Consistent with demonstrating Aloe vera's antidiabetic properties in human subjects, several studies presented evidence of reduced fasting blood glucose as well as triglycerides levels in diabetic individuals after the administration of A. vera fractions containing acemannan and verectin, three times per day for 12 weeks [31].
Olive (Olea europaea) leaves are commonly used in Europe and Mediterranean areas as a natural remedy for diabetes, often chewed or consumed as tea by local people, containing phenolic compounds, such as tyrosol, hydroxytyrosol and oleuropein. In a controlled randomized clinical trial, diabetic patients who took a daily 500 mg tablet of olive leaf extract experienced notable reductions in HbA1c and fasting insulin levels (8.0 to 1.5% and 11.3 to 4.5%, respectively) over 14 weeks, compared to those from the placebo group (8.9 to 2.25% and 13.7 to 4.1% respectively) [32]. Additionally, rats with diabetes induced using streptozotocin (STZ) that were administered olive leaf extract exhibited a noteworthy decrease in starch digestion and absorption compared to the control group. This suggests that the potential mechanism of action may involve disaccharide digestion inhibition in the intestinal mucosa [33].
Extracts from Cornus officinalis and some of its active constituents, such as loganin, morroniside and ursolic acid could enhance the management of diabetes-related complications through various mechanisms. Ursolic acid primarily functions by neutralizing reactive oxygen species and inhibiting α-glucosidase, while loganin's blood glucose-reducing effects come from promoting glucose uptake. Notably, diabetic mice experienced minimal weight loss when treated with a combination of loganin and ursolic acid [34].
Several recent clinical trials have demonstrated the antidiabetic potential of various medicinal plants, including Cinnamomum cassia, Ficus racemosa, Portulaca oleracea and Scoparia dulcis. Subsequent laboratory investigations into herbal products have resulted in the development of several branded formulations such as Diabeta Plus®, Diabecon® and Glyoherb® for diabetic patients [35]. Consequently, plant supplements could be considered adjuvants or promising alternative therapies for diabetes management (Table 4).
Patients often prefer herbal medicines for diabetes, either as primary treatments or as adjunctive to conventional methods, given their trust in natural resources and cost-effectiveness. As a result, lab research has been translated into patient care through clinical trials and commercial products. The use of herbal remedies can serve as an alternative for managing diabetes as they contribute not only to lowering glucose levels but also to improving lipid profile. These remedies offer antioxidant benefits and assist in regulating blood pressure, among other positive effects [36].
However, with the rapid expansion of phytomedicines for diabetes management, there's a pressing need for validated testing methods to assess the consistency and potency of active ingredients in final products. These products must undergo rigorous clinical trials in humans, approved and recognized by the nation's regulatory authorities, in order to ensure their safety and effectiveness. This will support consumer confidence in such herbal solutions. Nonetheless, comprehensive toxicity studies are essential before introducing such extracts as supplementary treatments for diabetes [22].
Nanotechnology and diabetes – general aspects
A significant demand for antidiabetic drugs was determined by the increasing number of people with sedentary lifestyles and the high incidence of obesity, which has led pharmaceutical companies to increase their investment in research and development to obtain targeted formulations [37].
There are always certain limitations with conventional drug delivery systems, such as the inability to treat due to inefficient or incorrect dosage, decreased potency, or altered effects caused by drug metabolism, as well as the absence of target specificity. The FDA has approved several nanotherapeutics in the last 20 years for the treatment of conditions such as Parkinson's disease, hypercholesterolemia, diabetes, cancer, hepatitis, neurological, autoimmune, and CV diseases. Employing herbal remedies provides an alternative approach to diabetes management, aiding not just in reducing glucose levels but also in improving lipid profiles. These natural solutions come with antioxidant advantages and help maintain normal blood pressure, among other positive effects. Moreover, oral nanodelivery systems bring certain benefits, such as improved pharmacokinetic parameters, bypass of the first-pass effect, as well as reduced adverse reactions [38].
Given that nanotechnology is expected to lead to numerous truly innovative medical breakthroughs that will have an impact on our daily lives, nanodelivery methods are the subject of an increasing number of studies. Extensive research over the years into nanoformulations has led to substantial progress in the advancement of nanoparticulate drug delivery systems for antidiabetic medications [39].
Nanotechnology involves researching the characteristics and uses of materials with a size between 1 and 100 nm. When the material is reduced to nanoscale, its characteristics change, and therefore develops special features. The substance is made up of macroscopic components, molecules, and atoms. The major distinction between NPs and bulk materials is the proportionate increase in surface area; in particular, a ladder structure that stands in for unsettled atoms with high surface energy is found on the surface of ultrafine particles. Due to their small particle size, these atoms can readily attach to other atoms and provide increased amounts of surface-active atoms. Nanomedicine is mostly focused on altering the surface of NPs by employing their special qualities to provide targeted, regulated release, simple-to-detect drug transport carriers, and new techniques for treating local wounds. Nanotechnology is therefore essential for regenerative medicine [40].
Several plant extracts have been used to manage diabetes and its associated consequences. Due to improved pharmacokinetics and bioavailability, the effectiveness of herbal extracts can be enhanced by using nanoformulations. The use of nanotechnology in the treatment of diabetes has seen a significant rise in interest over the past couple of decades. Furthermore, natural polysaccharide nanocarriers have drawn a lot of attention given their enhanced physiological stability, biocompatibility and biodegradability, and improved safety compared to other types of hypoglycemic nanocarriers [41].
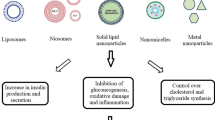
Natural nanocarriers in the treatment of T2DM and its complications
Finding effective diabetes therapies is the next step after a diagnosis has been made. Given that around 80–90% of diabetes cases are represented by T2DM and just 5–10% are T1DM, recent research has investigated the potential use of nanomaterials in both T1DM and T2DM therapies, with a focus on the latter one. Antidiabetic drugs can be used efficiently to promote insulin secretion or reduce glucose production [39]. Contrarily, in T1DM, where the pancreas produces very little or no insulin, treatment with insulin is necessary. Despite this, nanomaterials containing natural (e.g., Momordica charantia, Panax ginseng, Allium cepa, Euphorbia hirta) and synthetic (e.g., insulin, glibenclamide, metformin) antidiabetic drugs improved clinical outcomes in both T1DM and T2DM, due to the innovative properties of NPs, such as increased drug targeting, sustained hypoglycemic effect, and reduced risk of adverse reactions [42].
Several studies have indicated that natural polysaccharides exhibit noteworthy hypoglycemic effects through various mechanisms. These include the repair of pancreatic islet cells, enhancement of insulin sensitivity, regulation of related digestive enzyme activity, and modulation of key enzymes involved in the metabolism of liver glucose [39].
In the last twenty years, there has been a significant surge in employing nanotechnology for the development of medications targeting diabetes, with several normoglycemic nanocarriers being developed. The appropriate term for normoglycemic nanocarriers is hypoglycemic nanocarriers which are designed to deliver drugs that lower blood glucose levels in patients with diabetes. Systems using nanoencapsulation have many benefits and are frequently used in different industries. Both hydrophobic and hydrophilic molecules can be encapsulated, thus improving stability, decreasing toxicity, and protecting against degradation. Drugs can be attached to the surface of nanocarriers or enclosed within their matrix [41]. Apart from possessing the appropriate particle size, variable surface charge, and drug-loading capacity, nanocarriers also have various other advantages for drug delivery. Polysaccharide-based nanocarriers, for instance, demonstrate reduced toxicity, increased safety, enhanced encapsulation efficiency, targeted drug delivery, controlled release, and accelerated intestinal absorption [40].
Polysaccharides can be found in nature in a variety of sources, such as algae (e.g.: alginate), animals (e.g.: chitosan, chondroitin), microorganisms (e.g.: dextran, xanthan gum) and plants (e.g.: guar gum, pectin). The diversity of their structure and properties is a result of the abundance of reactive groups, a wide range of molecular weights, and varied chemical composition. Nanoemulsions, nanomicelles, nanoliposomes, nanoparticles (NPs) and nanohydrogels represent the primary categories of polysaccharide-based nanocarriers [42].
Polysaccharide-based nanoliposomes
Liposomes represent some of the most noteworthy potential carriers for numerous therapeutic drugs. Active molecules can be encapsulated either in the hydrophilic core or the hydrophobic lipid bilayer of liposomes, or they can attach to the vesicle's surface given their limited permeability. Liposomes offer several advantages, including biodegradability, lack of toxicity and non-immunogenicity. Polysaccharide-based nanoliposomes can serve as nanocarriers, thus enhancing drug stability and targeted delivery [43].
Maestrelli et al. developed two distinct metformin-loaded chitosomes (chitosan-coated niosomes) and niosomes (non-ionic surfactant vesicles). However, these formulations lacked a sustained release function. To address this, the two colloidal dispersions were coated with calcium alginate beads, resulting in a significant increase in gastric drug release. The amount of calcium alginate was then adjusted to optimize this effect. Research conducted on rats demonstrated that coating chitosomes and niosomes with alginate beads markedly enhanced the hypoglycemic impact of metformin. The niosome-alginate bead formulation proved better at sustaining glucose levels over time, thus enabling a reduction in dose and adverse effects, while also enhancing patient compliance [44].
Zhang et al. created a multi-layered insulin loading technique on an anionic nanoliposome surface by using insulin's electrostatic interaction with chitosan. In simulated gastric fluid and intestinal fluid, the layer-by-layer (LbL) coated nanoliposomes exhibited an insulin loading rate of 10.7% and demonstrated improved protection with limited release (only 6% in 1 h) [45]. Insulin, which has a negative charge depending on the pH, was retained by alternating cationic layers of chitosan. The coated liposome showed remarkable stability for 4 weeks in phosphate buffer saline at 37 °C. The outermost chitosan layer of the LbL-coated liposome enhanced absorption and transport in Caco-2 cells, and insulin-maintained bioactivity in a glucose uptake assay on 3T3 L1-MBX adipocytes. The capacity of these liposomes to protect insulin during intestinal penetration and to ensure its delivery into the systemic blood circulation suggests a potential application for oral protein delivery [45].
Polysaccharide-based nanoparticles
Metformin-loaded pectin NPs were created by Chinnaiyan et al. for the long-term management of T2DM [46]. The encapsulation rate was 68.4%, which demonstrated good and sustained release properties in vitro. The fact that the generated NPs remained mostly stable in the presence of bovine serum albumin provided additional proof of their stability in blood. Metformin and placebo-induced hemolysis rates were both less than 5%, demonstrating the safety and hemocompatibility of the NPs when taken orally. Consequently, this nanoparticle system might offer advantages in terms of extended release, fewer doses administered, and increased patient compliance [47].
Khan et al. created empagliflozin-loaded chitosan NPs that showed pharmacokinetic benefits. Improved release profile compared to pure empagliflozin and enhanced uptake suggest that the drug was distributed more effectively throughout the systemic circulation [47].
Moreover, Zhang et al. developed a novel nanoparticulate system designed for targeted delivery of insulin. The formulation consisted of cholic acid, hydroxypropyl methyl cellulose phthalate and quaternary ammonium-modified chitosan derivatives. It has been demonstrated that the cholic acid group significantly enhanced absorption in HepG-2 cells through the bile acid transporter mechanism and notably improved NP transport across the Caco-2 monolayer. The adhesive characteristics of chitosan derivatives in this system can enhance the intestinal absorption of insulin. Additionally, in the highly acidic stomach environment, hydroxypropyl methylcellulose phthalate can prevent the denaturation and degradation of insulin [48].
In their study, Alaa et al. synthesized nanoparticles comprising of melatonin loaded in chitosan/lecithin and evaluated their efficacy in treating STZ-induced diabetes in rats. These NPs exhibited remarkable anti-inflammatory, anticoagulant, and antioxidant properties and effectively reduced glucose levels, while also demonstrating a robust capacity to contribute to the regeneration of pancreatic β-cells. Furthermore, synthesized melatonin loaded chitosan/lecithin NPs increased insulin levels and reduced the high levels of cholesterol, creatinine, and urea [49].
Polysaccharide-based nanoemulsions
Nanoemulsions are highly malleable systems that can encapsulate drugs within the dispersed phase. They can improve the drug’s efficacy and minimize the side effects and toxic responses. The water–oil-water homogenization method was employed to produce nanoemulsions coated with alginate/chitosan, intended for oral insulin administration. Chitosan and calcium chloride were used for coating an alginate-containing nanoemulsion dispersion. The nanoemulsion was successfully maintained in simulated gastric liquid, according to an in vitro investigation. Moreover, compared to subcutaneous insulin, the nanoemulsion presented a hypoglycemic effect that lasted far longer. Consequently, the alginate/chitosan coating on nanoemulsions may enhance GI permeability and bioadhesion, while preserving insulin stability [50].
Kumar et al. created an emulsion cross-linking technique to formulate a metformin-loaded alginate nanoemulsion. This formulation not only enhanced the antidiabetic effect but also facilitated the intestinal permeability and GI absorption of the drug. The metformin loading and encapsulation efficiencies were 3.12% and 78%, respectively. The metformin-loaded alginate nanosystem performed better in in vivo efficacy tests and in vitro drug release studies, with its effectiveness being nearly three times higher compared to free metformin. The simple manufacture, low cost, biocompatibility, biodegradability, and high encapsulation efficacy were among the most important characteristics of this nanoformulation [51].
Similarly, Rani et al. successfully created NPs loaded with glycyrrhizin and metformin through the ionotropic gelation method, employing chitosan and gum arabic as polymers. The metformin-loaded NPs dose contained 9.2 mg of metformin while producing antihyperglycemic and antihyperlipidemic effects equivalent to 150 mg/kg of pure metformin for 21 days in diabetic rats [52].
Polysaccharide-based nanohydrogels
Nanohydrogels present a three-dimensional structure that can retain a large amount of liquids within their composition. Given their advantages, polysaccharide-based nanohydrogels are becoming more widely acknowledged as drug nanodelivery systems. Such advantages include biodegradability, cost-effectiveness, non-toxicity, biocompatibility, efficient drug release, and similarity to components of the extracellular matrix.
For the treatment of T2DM, Yang et al. designed a hydrogel for oral insulin delivery, using carboxymethyl-cyclodextrin-γ-carboxymethyl chitosan. The study carried out on diabetic mice demonstrated that oral administration of the insulin polysaccharide hydrogel over four weeks could greatly reduce the symptoms of polyuria, polyphagia, and weight loss. Moreover, in mice with T2DM, it demonstrates a notable reduction in fasting glucose levels, a decrease in insulin resistance, and an increase in insulin sensitivity. The lipid metabolism can be controlled by this hydrogenated insulin polysaccharide, and issues related to diabetes nephropathy can be avoided. Moreover, the insulin polysaccharide hydrogel has the potential to reverse histological kidney and pancreas degradation in diabetic mice as well as significantly increase their antioxidant capacity. These findings suggest that insulin polysaccharides hydrogels could constitute potential therapeutic options for T2DM [53].
In their research, Masood et al. introduced a hydrogel infused with silver NPs by incorporating chitosan and polyethylene glycol. This innovative hydrogel aimed to increase wound healing in diabetic patients [54]. In vitro tests revealed the antimicrobial and antioxidant properties of the silver nanoparticle-infused hydrogel. Notably, it exhibited a significant degree of swelling and a high-water vapor transition rate compared to a simple chitosan-PEG hydrogel. Moreover, the developed hydrogel presented a sustained release of silver NPs over a week, indicating a gradual biodegradation process. These findings suggested that chitosan-PEG hydrogels loaded with Ag NPs could represent an excellent method for treating chronic diabetic wounds [54].
Natural nanoformulations of plant compounds
Phytocompounds sourced from medicinal plants, including alkaloids, flavonoids, polyphenols, saponins, and terpenoids, have been thoroughly investigated to ascertain their potential as anti-diabetic agents. Despite the promising pharmacological properties of various substances, the practical translation of their advantageous effects when taken orally still presents significant challenges. In trials, higher phytocompound dosages frequently demonstrated greater therapeutic effectiveness, which may be connected to their limited bioavailability. Therefore, further clinical applications depend significantly on increasing their oral bioavailability [55].
Plants have the potential to assemble specific amounts of metals in some of their organs. As a result, plant extract-based biosynthetic approaches have drawn more attention as an easy, effective, affordable, and accessible way as well as a great substitute for traditional methods for NPs manufacturing. Various plants including Solanum nigrum, Lonicera japonica, Datura stramonium, Fritillaria cirrhosa have been used to synthesize Ag, Se, Zn, and Au NPs, as listed in Table 5 [72].
Naturally occurring secondary metabolites known as polyphenols are extracted from plant sources and have a variety of bioactivities that support good health. Polyphenols are abundant in plant-based foods, including fruits, vegetables, grains, cereals, coffee, chocolate, and tea. Typically, the classification of polyphenols generally relies on the number of phenol rings and the structural components that interconnect [73].
Flavonoid subclasses associated with diabetes include anthocyanins, flavonols, flavones and isoflavonoids, given that the consumption of foods containing such substances reduces the incidence of T2DM. For instance, flavonoids may reduce the risk of developing diabetes by regulating immunological response, regulating glucose absorption, blood glucose levels, and insulin secretion [74].
The major enzymes α-glucosidase and α-amylase, which are involved in the digestion of carbohydrates into glucose, are inhibited by polyphenols such as flavonoids and tannins, that play a vital role in carbohydrate metabolism. A few polyphenols, including RSV, epigallocatechin-3-gallate, and quercetin, increased the absorption of glucose in muscles and adipocytes by translocating glucose transporter type 4 (GLUT4) to the plasma membrane, mostly through activating the AMP-activated protein kinase pathway [75].
Polyphenolic compounds
A significant category of natural products is represented by flavonoids, which are one of the largest categories of secondary metabolites found in plants. This class contains over 6000 phenolic representatives, out of which some present beneficial health impacts in several diseases, such as diabetes, cancer, obesity, and CV conditions. Flavonoids are abundant in nature and can be classified into six subcategories according to their chemical structure. These include flavonols, flavan-3-ols, flavones, flavanones, isoflavones, and anthocyanidins. Flavonoids generally show low bioavailability, that could be increased using different nanotechnologies, with some potentially influencing the development of T2DM [75].
Some of the most frequently used natural polyphenolic substances with antioxidant and anti-inflammatory activities that could prove beneficial in diabetes, or its complications are discussed below.
Quercetin
Quercetin, a widely distributed flavonol, is renowned for several pharmacological effects, such as antioxidant, anti-diabetic, hepatoprotective, and nephroprotective. The phytocompound has received significant attention in recent years as one of the most effective antioxidants due to its important superoxide anion, peroxyl, and hydroxyl radicals scavenging activities. Despite numerous reports found in reputable scientific journals, there is still lack of information regarding quercetin's multimechanistic role [76].
Various encapsulation approaches have been developed to enhance the chemical stability and solubility of quercetin, which primarily consist of biopolymeric colloidal particles. In their study, Hussein et al. used a quercetin nanoemulsion to reduce cardiac toxicity and DNA damage in experimental diabetes [76]. Furthermore, Mandic et al. illustrated that by controlling the combination of stationary and alternating magnetic fields, the release kinetics of quercetin from superparamagnetic magnetite nanoparticles could be regulated. This control extends to the distribution of quercetin among particles and the suspension within the membrane. Given that the ratio of the material in the membrane's solution is adjusted more in favor of NPs, quercetin is released more frequently in non-sink conditions. These findings could be used to better understand the general release of insoluble flavonoids in aqueous solutions [77].
Additionally, Fei Tong et al. found that quercetin may reduce diabetic nephropathy and, consequently, could be an effective therapy for this complication. Recent studies confirm that using NPs to deliver drugs can increase effectiveness, while reducing negative side effects. When administered to Sprague Dawley rats, quercetin/PEG-β-(PELG-ɣ-PZLL) (quercetin/poly(ethylene glycol)-block-(poly(ethylenediamine l-glutamate)-graft-poly(ε-benzyloxycarbonyl-l-lysine) considerably raised the serum concentration of quercetin. Furthermore, it significantly reduced diabetic nephropathy and decreased hyperglycemia [78].
To lower the dosage and dosing frequency, Chitkara et al. produced quercetin-loaded PLGA NPs for the treatment of diabetes by the use of an emulsion diffusion evaporation method. In a pharmacokinetic investigation, nano quercetin’s relative oral bioavailability was shown to be 523% higher than that of the quercetin solution. The therapeutic impact on diabetic rats suggested that the same amount of quercetin nanoformulation administered every five days provided results comparable to that of a quercetin suspension administered daily [79].
Naringenin
Naringenin (NRG) is one of the most significant flavonoids from a medicinal standpoint. NRG exhibits several noteworthy properties, including anticancer and insulin-like activities, antioxidant, and anti-inflammatory properties under specific conditions related to hypertension, alongside various other activities, such as anti-mutagenic, antiproliferative, antifibrogenic, antibacterial, anti-atherosclerotic, neuroprotective, antidiabetic, immunomodulatory and hepatoprotective [80].
NRG has demonstrated anti-hyperglycemic effects by inhibiting the absorption of glucose in the GI tract. The anti-diabetic effect of NRG is believed to involve mechanisms such as improved insulin sensitivity, reduction in blood glucose levels, inhibition of macrophage migration into adipose tissue, blocking monocyte chemoattractant protein-1 and decreased insulin receptor substrate-2 levels. Nonetheless, oral administration is constrained by its lack of water solubility, quick biodegradation, and low bioavailability [81].
NRG’s hydrophobic nature results in an extremely poor in vivo bioavailability, which restricts its utility. It quickly transforms into its crystalline form and has a short half-life, resulting in poor absorption via the digestive system. Numerous initiatives to increase NRG’s bioavailability have been made as a response to such drawbacks. Implicitly, nanotechnological delivery methods offer a potential solution to surmount these limitations. By protecting NRG from enzymatic and hydrolytic degradation in the GI tract and encapsulating it within polymeric NPs, the drug's bioavailability can be enhanced [81].
So far, NRG has been delivered using a variety of nanocarriers that improve water solubility, bioavailability, and therapeutic efficacy. This, in turn, translates into potential dose reductions. Some examples are represented by lipid-based nanocarriers, hydrogels, dendrimers, protein-based NPs, carbon-based nanocarriers, micelles, nanoemulsions, nanocomposites and metal oxide NPs. In an alternative approach, these systems can incorporate NRG into their layouts and release it under controlled conditions [81].
For instance, a nanovehicle containing NRG was created using safe and economical polymers. The suggested alginate-coated chitosan core-shell nanocarrier system was safe concerning processing conditions, including the absence of dangerous chemicals. Its structural chemistry also produced NPs with notable encapsulation effectiveness (> 90%) and smaller size. Mucoadhesion analyses and release studies revealed that the synthesized nanoformulations produced gradual, sustained and pH-responsive release of NRG. Findings from in vivo studies indicated that administering the proposed system orally substantially decreased blood glucose levels in diabetic rats [82].
One other study performed by Wang et al. focused on incorporating NRG into a liposomal system intended for oral delivery. Thin-film hydration was used to create liposomes that were loaded with NRG. The in vitro release profiles were determined in three GI media. When liposomes were used, tissue distribution analyses revealed greater concentrations of NRG in many tissues, particularly the liver. In addition, after oral administration of the encapsulated medication to rats, a striking improvement in bioavailability and solubility was observed. Furthermore, oral administration of the polymeric NPs was safe, according to histopathological and blood parameters [83].
Moreover, Maity and Chakrabort used an emulsion-diffusion-evaporation method to create NRG PLGA NPs. The NPs attained a size of around 129 nm, and the NRG entrapment was about 70%. In addition, the anti-diabetic effects of free NRG and NRG-loaded PLGA NPs in STZ-induced diabetic rats were evaluated comparatively. According to in vivo tests, rats treated with NPs showed a significant reduction in HbA1c, an increase in insulin levels, and an improvement in dyslipidemia and oxidative stress parameters, while animals treated with free NRG did not [84].
Myricitrin
Myricitrin, a flavonol glycoside, was isolated from species such as Eugenia uniflora, Myrica rubra, Manilkara zapota, and Pouteria gender. The compound is believed to possess anti-inflammatory, anti-nociceptive, and antioxidant properties. This flavonoid glycoside is used as a significant addition in medications due to its great antioxidant action. It has been discovered that myricitrin prevents reactive oxygen species-induced venous endothelial cell dysfunction by lowering malondialdehyde, preventing H2O2-induced oxidative stress and controlling the activity of antioxidant enzymes [85].
The primary factors to be taken into consideration are the flavonoids’ bioavailability and metabolism. The size and high polarity of this molecule prevent it from passing through membranes with ease. Furthermore, glycosidases found in the cells of the GI mucosa, liver, and kidney extensively metabolize and hydrolyze flavonoids [86].
In a study conducted by Ahangarpour et al., SLN incorporating myricitrin exhibited both antidiabetic and antioxidant properties. These effects included the restoration of body and tissue weight, mitigation of oxidative stress and pancreatic apoptosis, reduction in hyperglycemia, maintenance of skeletal muscle glycogen content, improvement in insulin resistance and enhancement of GLUT-4 gene expression, all of which were affected by STZ-nicotinamide-induced T2DM. Myotube cells subjected to a hyperglycemic state showed better antioxidant protection, glycogen content, and cellular viability after in vitro testing with myricitrin SLNs. Additionally, certain outcomes were more prominent in the groups that were administered SLN compared to the one receiving metformin [87].
Myricitrin is sensitive to high temperatures, hence myricitrin SLNs have been prepared using the cold homogenization process. In mice with STZ-nicotinamide-induced diabetes and hyperglycemic myotubes, SLNs containing myricitrin demonstrated antioxidant, anti-diabetic, and antiapoptotic properties [88].
Curcumin
Curcumin has a wide range of biological properties that could be used for future treatments. Given its certain disadvantages, such as poor pharmacokinetics and bioavailability, rapid metabolization, low physico-chemical stability, low penetration and decreased absorption, curcumin has limited clinical efficacy [89].
In one study, Ganugula et al. developed biodegradable nanosystems encapsulating curcumin (nCUR), thus improving oral bioavailability by at least 9 times. They also described how nCUR could prevent STZ from inducing inflammation and death in rat pancreatic islet β-cells, which would result in lower glucose levels and less oxidative stress [89].
In pancreatic tissue homogenates, levels of inflammatory cytokines were dramatically reduced by both curcumin and nCUR, which was closely connected with a low histiocytic infiltration. 8-oxo-2’-deoxyguanosine, a known biomarker of reactive oxygen species-induced DNA damage in the pancreas, was inhibited by the proposed nanosystem rather than the curcumin pre-treatment. According to study results in healthy rodents, a 25 to 100 mg/kg nCUR daily dose for 28 days did not have any negative effects on the carrier’s health. Additionally, the obtained data shows that nCUR is risk-free and offers a certain degree of protection against diabetes in rats, since it reduces oxidative stress, inflammation, and pancreatic apoptosis [90].
Curcumin-loaded PLA-PEG NPs were successfully created by El-Naggar et al. using the emulsion-diffusion evaporation method. PLA and PEG represented the hydrophobic and hydrophilic portions of the copolymer, respectively, in this amphiphilic system. The section of the hydrophobic polymer that contained curcumin was enclosed and stabilized using a cationic surfactant (cetyltrimethylammonium bromide). The outcome proved that the encapsulated curcumin was superior to polymer NPs alone and free curcumin in raising plasma insulin levels, lowering plasma glucose levels, preventing liver inflammation, and improving the hepatic function in STZ-induced diabetic rats [91].
Liu et al. developed a safe and efficient method to accelerate the healing of skin wounds in diabetics. To increase the solubility and stability of curcumin, self-assembled NPs were created using the reprecipitation technique. These NPs were subsequently enclosed in gelatin microspheres, that can react to matrix metallopeptidase 9, which is typically overexpressed and exists in diabetic patients’ non-healing skin wounds. It was shown that the loaded gelatin microspheres can release curcumin to the wound bed and after being mixed with a thermoresponsive poloxamer hydrogel can coat the skin wound of diabetics [50].
Hu et al. reported a hyaluronic acid and chitosan-based hydrogel for loading and delivering nanotechnologically-modified curcumin and epidermal growth factor. This biomaterial’s tremendous therapeutic potential as a diabetic wound dressing is demonstrated by the significantly increased wound healing with optimal re-epithelialization, granulation tissue development, and skin appendage regeneration [92].
According to Chauhan et al., curcumin-loaded chitosan NPs exhibited a more promising effect on the relocation of GLUT4 to the cell surface in L6 rat skeletal muscle cells compared to free curcumin [93]. Moreover, Akbar et al. generated curcumin-loaded mixed polymeric micelles, using alginate, chitosan, maltodextrin, and tween 80. The obtained results indicated that the curcumin-loaded micelles exhibited a comparable antidiabetic effect to that of metformin in diabetic rats [94].
Resveratrol
Resveratrol, a very popular polyphenol found in various plants, particularly in red grapes, has been shown to offer multiple pharmacological properties, being a potent antioxidant, anti-inflammatory, analgesic, antiplatelet, cardioprotective, neuroprotective, and anti-aging agent. RSV significantly reduces oxidative damage and influences glucose metabolism, demonstrating its potential as an antidiabetic agent in pancreatic β cells. This is reflected in reduced blood glucose levels, enhanced insulin production, cellular protection, and antioxidant effects during RSV treatment [95].
In one study, Yucel et al. assessed the antidiabetic and antioxidative effects of nano-sized liposomal formulations containing RSV. The effects of RSV-loaded nanoliposomes against oxidative damage in diabetes were evaluated using the superoxide dismutase and glutathione peroxidase enzymes tests. The outcomes indicated that the formulated liposomes notably elevated insulin levels, decreased elevated glucose levels in groups of diabetic cells, and displayed extended antioxidant effects over 24-h, outperforming the effectiveness of the RSV solution. Consequently, these nanoliposomes might be beneficial in treating T2DM and related oxidative stress complications [96].
Polydatin is a resveratrol glycoside derived from the roots of Polygonum cuspidatum. Abdel et al. developed chitosan NPs loaded with polydatin for oral administration, aiming to optimize the therapeutic potential of polydatin in T2DM treatment. The newly developed NPs not only exhibited biocompatibility but also showcased significant effectiveness in combating diabetes compared to free polydatin. This enhanced efficacy is likely a result of prolonged-release characteristics and improved absorption, proving the potential of such NPs as efficient carriers for sustained polydatin delivery [97].
Rosmarinic acid
In a retinopathy mouse model, rosmarinic acid, known for its antioxidant properties, exhibited promising effects. Evidence showed a considerable dose-dependent decrease of retinal endothelial cell proliferation, as well as in vitro prevention of tube formation angiogenesis. Furthermore, rosmarinic acid did not cause any retinal damage. According to these findings, the compound could represent a powerful inhibitor of retinal neovascularization and could be used to treat vasoproliferative retinopathies [98].
In one other study, Da Silva et al. assessed the safety, permeability, mucoadhesiveness, and possible use of carriers containing rosmarinic acid in ocular cell-based models, employing chitosan NPs. A small size range and a proper particle size must be chosen for ophthalmic nano-drug delivery systems to guarantee low irritancy, good bioavailability, and biocompatibility. NPs exhibiting mucoadhesive properties had an extended retention duration over the ocular mucosa following delivery. Moreover, when used topically, such particles may develop into efficient medication delivery systems for use in oxidative eye disorders [99].
Gallic acid
Gallic acid’s bioavailability and therapeutic efficacy have been demonstrated to be improved by several types of nanostructured systems [100]. A promising antidiabetic nanoformulation was obtained by using chitosan NPs encapsulating gallic acid, that demonstrated enhanced inhibition of α-glucosidase activity [101].
An efficient oral formulation to reduce hyperglycemia has been reported to contain hydroxyapatite NPs delivering insulin and gallic acid. In vitro studies revealed that these particles enhanced hepatic glucose metabolism by stimulating PI3K/Akt/GLUT4 activation. It has been suggested that the proposed formulation is a stable, non-toxic, and efficient option for orally delivering a combination of insulin and gallic acid to address diabetes symptoms [102].
Anthocyanins
The consumption of fruits from the Vaccinium genus may significantly delay the onset and progression of T2DM, certain CV diseases, chronic neurological decline, and gradual weight gain considering their high anthocyanin (ACN) content.
An ACN-rich Vaccinium meridionale extract was included into non-ionic niosomes and its impact was assessed in a diet-induced obesity mice model to determine its ability to promote metabolic activity. ACNs may delay degenerative processes linked to T2DM and other chronic diseases, but there is still debate on their bioavailability [103, 104].
ACN-loaded particles had a 57% encapsulation effectiveness, a relatively monodisperse surface charge, and were negatively charged. In the diet-induced obesity mice model, insulin resistance and glucose intolerance were reduced by ACN-loaded niosomes. In addition, they decreased the weight of the animals and the levels of total cholesterol, leptin, glucose, and insulin in mice with obesity [103, 104].
Rutin
Rutin, a well-known flavonoid glycoside, is present in various plants, such as Ruta graveolens L., Sophora japonica L. and Eucalyptus spp. and possesses different clinical benefits such as antidiabetic, antioxidant, anti-inflammatory, and anticarcinogenic properties [105, 106].
Nevertheless, the use of this bioactive compound is constrained by its low bioavailability and stability. To address these issues, Amjadi et al. encapsulated rutin in nanophytosomes and assessed the therapeutic efficacy of this nanocarrier in diabetic rats. The in vivo evaluation of the formulation demonstrated managed weight loss and food consumption in diabetic rats. This therapeutic approach proved more effective than free rutin in lowering plasma glucose, alanine aminotransferase, aspartate aminotransferase, total hemoglobin, and HbA1c. Furthermore, it elevated insulin levels, ameliorated hyperlipidemia, and mitigated oxidative stress parameters [107].
Other natural compounds
Glycyrrhizic acid
Licorice is a traditional medicinal herb widely used in Europe and Asia. It is usually used as dried roots and rhizomes of certain Fabaceae family members (Glycyrrhiza glabra, G. inflata, and G. uralensis). The primary bioactive component of licorice is glycyrrhizic acid (GA), commonly known as glycyrrhizin [108].
Several pharmacological effects of GA, including anti-inflammatory, antioxidant, antiviral, immunoregulatory, anticancer, and antidiabetic activities, have been demonstrated in several studies. GA has therapeutic effects in T2DM, including lowering blood glucose levels, boosting insulin sensitivity, enhancing glucose tolerance and homeostasis, and controlling lipid metabolism [109].
Given that GA presents low bioavailability, it has been further used to generate NPs. In nicotinamide plus STZ-induced T2DM mice, GA-loaded NPs enhanced lipid profile and decreased fasting blood glucose levels [99]. Additionally, GA-loaded NPs and thymoquinone-loaded nanocapsules were combined, and the results demonstrated greater anti-diabetic action compared to when administered individually in nicotinamide and STZ-induced T2DM mice. Another biochemical measure for determining the extent of diabetes is glycosylated HbA1c; a high level of this marker indicates ineffective blood glucose regulation. GA-loaded NPs proved useful in lowering glycosylated HbA1c [109, 110].
Oleanolic acid
The oleanane group naturally produces oleanolic acid (3-hydroxy-olean-12-en-28-oic acid), which can be found in several plants either in its free acid form or as a triterpenoid saponin. Oleanolic acid, a naturally occurring substance found in many plant foods and medicinal herbs, possesses a variety of pharmacological activities, although their therapeutic potential has only been partially explored [110].
Oleanolic acid is frequently discovered in these plants' epicuticular waxes, which function as a line of defense against pathogens and water loss. In addition to its ecological functions in plants, the phytocompound has been shown to have some pharmacological benefits in various disease models, including antioxidant, antitumor, anti-inflammatory, antidiabetic, and antimicrobial properties [111].
Wang et al. discovered that oleanolic acid demonstrated antioxidant effects by upregulating the expression of thioredoxin peroxidase and catalase, and enhancing glutathione synthesis, by a direct chemical reaction with free radicals [111]. Zhang et al. converted a naturally occurring insulin sensitizer from plants into a biocompatible nano-transport system. Oleanolic acid was conjugated to polygalacturonic acid, a naturally occurring polymer, to create self-assembled micelles, which were then administered orally as nanomedicine for the treatment of insulin resistance in T2DM [112].
Oleanolic acid loaded polygalacturonic acid micelles have been shown through in vitro and in vivo research to have the proper stability to pass through GI barriers and to enhance drug intestinal absorption, maintaining plasma drug concentrations for a longer period. The formulation demonstrated long-term results in glucose level management even after drug elimination in the T2DM rat model of insulin resistance [112, 113].
Asiatic acid
Due to its pharmacological characteristics and therapeutic potential in treating a wide variety of disorders, asiatic acid, a pentacyclic triterpenoid, has received a large amount of attention. It has been discovered that the use of nanotechnology can help overcome pharmaceutical restrictions, improve compliance, and increase the beneficial effects of this substance [114].
After administering asiatic acid tromethamine salt-loaded SLN orally, the bioavailability in rats was reported to be 2.5 times higher. PEGylated asiatic acid-loaded nanostructured lipid carriers also enabled improved absorption and transportation of the compound within the small intestine of rats. The increase in the elimination half-life showed that the nanoformulation increased the oral bioavailability of asiatic acid. Nevertheless, a comprehensive investigation of the effects of asiatic acid NPs on diabetes is still pending [115].
Triterpenes
In their study, Escobar et al. developed a nanoformulation using PLGA with encapsulated triterpene-enriched fractions: oleanolic acid, ursolic acid, and an ursolic acid lactone extracted from the leaves of Eucalyptus tereticornis. The administration of the oral nanoformulation improved insulin sensitivity, glucose tolerance, and dyslipidemia, resulting in decreased fasting glucose levels in a diet-induced obesity mouse model. Moreover, the bioactive compounds induce alterations in hepatic gene expression and cellular events, impacting both carbohydrate and lipid metabolism. These findings emphasize the existence of another option to address obesity and certain associated metabolic issues [116].
The current section reviewed some of the most important and recent nanoformulations including polyphenols, as well as other natural compounds that could prove useful in the treatment of T2DM. A summary of such nanoformulations can be found in Table 6.
Conclusions and perspectives
More than 4 million people with DM die annually, which represents a major problem for modern society. Decisional efforts have been made to mitigate occurrence and management of DM. According to literature review, prolonged use of antidiabetic medications is responsible for altering the activity of the liver, kidney, digestive system, and of other organs. Consumption of natural food containing specific phytocompounds are frequently associated with health benefits for DM early management. Furthermore, nanomedicine has opened new opportunities for the diagnosis, treatment, and prevention of this condition. Nanotechnology is particularly crucial for emerging drug delivery systems. It should also be noted that the body's enzyme breakdown process and the pH difference in the digestive tract impose a number of restrictions on the oral administration of phytocompounds. To resolve such issues, nanosystems provide several benefits over conventional medication delivery. Such systems may be employed for sophisticated drug delivery applications, including targeted drug administration, controlled release, and increased permeability and retention effects, in addition to overcoming pharmacokinetic and pharmacodynamic restrictions of many potential active substances. Innovative delivery systems have been designed to improve production processes, pharmacokinetics, pharmacodynamics, biodistribution, biocompatibility, therapeutic efficacy, and long-term safety. Nevertheless, there are currently only a few effective nanomedicines available that considerably enhance the quality of life for diabetics. Therefore, the perspectives outlined by the current literature analysis can be associated with the development of more nanostructured phytocompounds with improved bioavailability.
Abbreviations
- DM:
-
Diabetes mellitus
- T1DM:
-
Type 1 Diabetes mellitus
- T2DM:
-
Type 2 Diabetes mellitus
- GI:
-
Gastrointestinal
- DPP-4:
-
Dipeptidyl peptidase 4
- ESC/EASD:
-
European Society of Cardiology/European Association for the Study of Diabetes
- ADA/EASD:
-
American Diabetes Association/European Association for the Study of Diabetes
- ACC:
-
American College of Cardiology
- CVOTs:
-
Cardiovascular outcomes trials
- GLUT4:
-
Glucose transporter type 4
- SLN:
-
Solid lipid nanoparticles
- STZ:
-
Streptozotocin
- NRG:
-
Naringenin
- nCUR:
-
Nanosystems encapsulating curcumin
- PLA-PEG:
-
Polylactide-poly (ethylene glycol)
- ACN:
-
Anthocyanin
- LbL:
-
Layer-by-layer
- NPs:
-
Nanoparticles
- RSV:
-
Resveratrol
- CV:
-
Cardiovascular
- NICE:
-
The National Institute of Health and Clinical Excellence
- PLGA:
-
Poly (lactic-co-glycolic acid)
References
Wang Y, Wang C, Li K, et al. Recent advances of nanomedicine-based strategies in diabetes and complications management: Diagnostics, monitoring, and therapeutics. J Control Release. 2021;330:618–40. https://doi.org/10.1016/j.jconrel.2021.01.002.
Aloke C, Egwu CO, Aja PM, et al. Current advances in the management of diabetes mellitus. Biomedicines. 2022;10:2436. https://doi.org/10.3390/biomedicines10102436.
Chan JCN, Lim L-L, Wareham NJ, et al. The lancet commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2020;396:2019–82. https://doi.org/10.1016/S0140-6736(20)32374-6.
Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a consensus report from the American diabetes association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:1617–35. https://doi.org/10.2337/dci20-0022.
Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21:6275. https://doi.org/10.3390/ijms21176275.
Dimas AS, Lagou V, Barker A, et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63:2158–71. https://doi.org/10.2337/db13-0949.
DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36:S127–38. https://doi.org/10.2337/dcS13-2011.
Chen C, Cohrs CM, Stertmann J, et al. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017;6:943–57. https://doi.org/10.1016/j.molmet.2017.06.019.
Libianto R, Davis TM, Ekinci EI. Advances in type 2 diabetes therapy: a focus on cardiovascular and renal outcomes. Med J Aust. 2020;212:133–9. https://doi.org/10.5694/mja2.50472.
DeMarsilis A, Reddy N, Boutari C, et al. Pharmacotherapy of type 2 diabetes: an update and future directions. Metabolism. 2022;137:155332. https://doi.org/10.1016/j.metabol.2022.155332.
Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes. J Am Coll Cardiol. 2020;76:1117–45. https://doi.org/10.1016/j.jacc.2020.05.037.
Tsoutsouki J, Wunna W, Chowdhury A, Chowdhury TA. Advances in the management of diabetes: therapies for type 2 diabetes. Postgrad Med J. 2020;96:610–8. https://doi.org/10.1136/postgradmedj-2019-137404.
Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60:1586–93. https://doi.org/10.1007/s00125-017-4336-x.
Simos YV, Spyrou K, Patila M, et al. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J Pharm Sci. 2021;16:62–76. https://doi.org/10.1016/j.ajps.2020.05.001.
Kalyani RR. Glucose-lowering drugs to reduce cardiovascular risk in type 2 diabetes. N Engl J Med. 2021;384:1248–60. https://doi.org/10.1056/NEJMcp2000280.
Baker C, Retzik-Stahr C, Singh V, et al. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther Adv Endocrinol Metab. 2021;12:204201882098022. https://doi.org/10.1177/2042018820980225.
Sola D, Rossi L, Schianca GPC, et al. State of the art paper Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840–8. https://doi.org/10.5114/aoms.2015.53304.
Greenfield JR, Chisholm DJ. Experimental and clinical pharmacology: Thiazolidinediones - mechanisms of action. Aust Prescr. 2004;27:67–70. https://doi.org/10.18773/austprescr.2004.059.
Kalra S, Baruah M, Sahay R, et al. Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes: Past, present, and future. Indian J Endocrinol Metab. 2016;20:254–67. https://doi.org/10.4103/2230-8210.176351.
Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab. 2009;23:479–86. https://doi.org/10.1016/j.beem.2009.03.004.
Thynne T, Doogue M. Experimental and Clinical Pharmacology: Sodium-glucose co-transporter inhibitors: Mechanisms of action. Aust Prescr. 2014;37:14–6. https://doi.org/10.18773/austprescr.2014.005.
Choudhury H, Pandey M, Hua CK, et al. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med. 2018;8:361–76. https://doi.org/10.1016/j.jtcme.2017.08.012.
Rajasekaran S, Sivagnanam K, Subramanian S. Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. J Pharm Pharmacol. 2010;57:241–6. https://doi.org/10.1211/0022357055416.
Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–80. https://doi.org/10.4239/wjd.v6.i3.456.
Anderson RA, Zhan Z, Luo R, et al. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J Tradit Complement Med. 2016;6:332–6. https://doi.org/10.1016/j.jtcme.2015.03.005.
Marton LT, Pescinini-e-Salzedas LM, Camargo MEC, et al. The effects of curcumin on diabetes mellitus: a systematic review. Front Endocrinol (Lausanne). 2021;12:669448. https://doi.org/10.3389/fendo.2021.669448.
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–7. https://doi.org/10.2337/dc12-0116.
Jafari S, Sattari R, Ghavamzadeh S. Evaluation the effect of 50 and 100 mg doses of Cuminum cyminum essential oil on glycemic indices, insulin resistance and serum inflammatory factors on patients with diabetes type II: A double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2017;7:332–8. https://doi.org/10.1016/j.jtcme.2016.08.004.
Knop FK, Konings E, Timmers S, et al. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses but suppresses postprandial glucagon in obese subjects. Diabetic Med. 2013;30:1214–8. https://doi.org/10.1111/dme.12231.
Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–41. https://doi.org/10.1016/j.nutres.2012.06.003.
Haghani F, Arabnezhad M-R, Mohammadi S, Ghaffarian-Bahraman A. Aloe vera and streptozotocin-induced diabetes mellitus. Rev Bras Farmacogn. 2022;32:174–87. https://doi.org/10.1007/s43450-022-00231-3.
Wainstein J, Ganz T, Boaz M, Bar Dayan Y, Dolev E, Kerem Z, Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012;15(7):605–10. https://doi.org/10.1089/jmf.2011.0243.
Konstantinidi M, Koutelidakis AE. Functional foods and bioactive compounds: a review of its possible role on weight management and obesity’s metabolic consequences. Medicines. 2019;6(3):94. https://doi.org/10.3390/medicines6030094.
He K, Song S, Zou Z, et al. The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother Res. 2016;30:283–91. https://doi.org/10.1002/ptr.5529.
Modak M, Dixit P, Londhe J, et al. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–73. https://doi.org/10.3164/jcbn.40.163.
Kaur M, Valecha V. Diabetes and Antidiabetic Herbal Formulations: An Alternative to Allopathy. Eur J Med. 2014;6:226–40. https://doi.org/10.13187/ejm.2014.6.226.
Prasad M, Lambe UP, Brar B, et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother. 2018;97:1521–37. https://doi.org/10.1016/j.biopha.2017.11.026.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–31. https://doi.org/10.1016/j.arabjc.2017.05.011.
Joseph TM, Kar Mahapatra D, Esmaeili A, et al. Nanoparticles: taking a unique position in medicine. Nanomaterials. 2023;13:574. https://doi.org/10.3390/nano13030574.
Bertsch T. An Introduction to Tirzepatide. Clin Diabetes. 2022;40:371–2. https://doi.org/10.2337/cd22-0038.
Qiu A, Wang Y, Zhang G, Wang H. Natural polysaccharide-based nanodrug delivery systems for treatment of diabetes. Polymers (Basel). 2022;14:3217. https://doi.org/10.3390/polym14153217.
Rehman A, Jafari SM, Tong Q, et al. Drug nanodelivery systems based on natural polysaccharides against different diseases. Adv Colloid Interface Sci. 2020;284:102251. https://doi.org/10.1016/j.cis.2020.102251.
Acevedo-Guevara L, Nieto-Suaza L, Sanchez LT, et al. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int J Biol Macromol. 2018;111:498–504. https://doi.org/10.1016/j.ijbiomac.2018.01.063.
Maestrelli F, Mura P, González-Rodríguez ML, et al. Calcium alginate microspheres containing metformin hydrochloride niosomes and chitosomes aimed for oral therapy of type 2 diabetes mellitus. Int J Pharm. 2017;530:430–9. https://doi.org/10.1016/j.ijpharm.2017.07.083.
Zhang Y, Xiong GM, Ali Y, et al. Layer-by-layer coated nanoliposomes for oral delivery of insulin. Nanoscale. 2021;13:776–89. https://doi.org/10.1039/D0NR06104B.
Chinnaiyan SK, Deivasigamani K, Gadela VR. Combined synergetic potential of metformin loaded pectin-chitosan biohybrids nanoparticle for NIDDM. Int J Biol Macromol. 2019;125:278–89. https://doi.org/10.1016/j.ijbiomac.2018.12.009.
Khan T, Nabi B, Rehman S, et al. Quality by design approach to formulate empagliflozin-loaded chitosan nanoparticles: in vitro, in vivo and pharmacokinetic evaluation of anti-diabetic drugs. Nano. 2021;16:2150149.
Zhang Z, Li H, Xu G, Yao P. Liver-targeted delivery of insulin-loaded nanoparticles via enterohepatic circulation of bile acids. Drug Delivery. 2018;25(1):1224–33. https://doi.org/10.1080/10717544.2018.1469685.
Alaa H, Abdelaziz M, Mustafa M, et al. Therapeutic effect of melatonin-loaded chitosan/lecithin nanoparticles on hyperglycemia and pancreatic beta cells regeneration in streptozotocin-induced diabetic rats. Sci Rep. 2023;13:10617. https://doi.org/10.1038/s41598-023-36929-0.
Liu J, Chen Z, Wang J, et al. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl Mater Interfaces. 2018;10:16315–26. https://doi.org/10.1021/acsami.8b03868.
Kumar S, Bhanjana G, Verma RK, et al. Metformin-loaded alginate nanoparticles as an effective antidiabetic agent for controlled drug release. J Pharm Pharmacol. 2017;69:143–50. https://doi.org/10.1111/jphp.12672.
Rani R, Dahiya S, Dhingra D, et al. Evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2017;106:220–30. https://doi.org/10.1016/j.ejps.2017.05.068.
Yang Y, Chen S, Liu Y, et al. Long-term treatment of polysaccharides-based hydrogel microparticles as oral insulin delivery in streptozotocin-induced type 2 diabetic mice. Biomed Pharmacother. 2021;133:110941. https://doi.org/10.1016/j.biopha.2020.110941.
Masood N, Ahmed R, Tariq M, Ahmed Z, Masoud MS, Ali I, Asghar R, Andleeb A, Hasan A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23–36. https://doi.org/10.1016/j.ijpharm.2019.01.019.
Wang T, Zheng Y, Shi Y, Zhao L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv Transl Res. 2019;9:227–39. https://doi.org/10.1007/s13346-018-00609-8.
He J-H, Chen L-X, Li H. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia. 2019;134:270–89. https://doi.org/10.1016/j.fitote.2019.02.033.
Malapermal V, Botha I, Krishna SBN, Mbatha JN. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J Biol Sci. 2017;24:1294–305. https://doi.org/10.1016/j.sjbs.2015.06.026.
Elekofehinti OO. Momordica charantia nanoparticles potentiate insulin release and modulate antioxidant gene expression in pancreas of diabetic rats. Egypt J Med Hum Genet. 2022;23:63. https://doi.org/10.1186/s43042-022-00282-0.
Balan K, Qing W, Wang Y, et al. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016;6:40162–8. https://doi.org/10.1039/C5RA24391B.
Fan D, Li L, Li Z, et al. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci Technol Adv Mater. 2020;21:505–14. https://doi.org/10.1080/14686996.2020.1788907.
Deepa T, Mohan S, Manimaran P. A crucial role of selenium nanoparticles for future perspectives. Results Chem. 2022;4:100367. https://doi.org/10.1016/j.rechem.2022.100367.
Pérez Gutiérrez RM, Soto Contreras JG, Martínez Jerónimo FF, et al. Assessing the ameliorative effect of selenium Cinnamomum verum, Origanum majorana, and Origanum vulgare nanoparticles in diabetic zebrafish (Danio rerio). Plants. 2022;11:893. https://doi.org/10.3390/plants11070893.
Vinotha V, Iswarya A, Thaya R, et al. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J Photochem Photobiol B. 2019;197:111541. https://doi.org/10.1016/j.jphotobiol.2019.111541.
Bayrami A, Haghgooie S, Rahim Pouran S, et al. Synergistic antidiabetic activity of ZnO nanoparticles encompassed by Urtica dioica extract. Adv Powder Technol. 2020;31:2110–8. https://doi.org/10.1016/j.apt.2020.03.004.
Sharma A, Nagraik R, Sharma S, et al. Green synthesis of ZnO nanoparticles using Ficus palmata: Antioxidant, antibacterial and antidiabetic studies. Results Chem. 2022;4:100509. https://doi.org/10.1016/j.rechem.2022.100509.
Mohammadi Arvanag F, Bayrami A, Habibi-Yangjeh A, Rahim PS. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Mater Sci Eng C Mater Biol Appl. 2019;97:397–405. https://doi.org/10.1016/j.msec.2018.12.058.
Guo Y, Jiang N, Zhang L, Yin M. Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on streptozotocin induced rats. Arab J Chem. 2020;13:5096–106. https://doi.org/10.1016/j.arabjc.2020.02.009.
Ayyoub S, Al-Trad B, Aljabali AAA, et al. Biosynthesis of gold nanoparticles using leaf extract of Dittrichia viscosa and in vivo assessment of its anti-diabetic efficacy. Drug Deliv Transl Res. 2022;12:2993–9. https://doi.org/10.1007/s13346-022-01163-0.
Oladipo IC, Lateef A, Azeez MA, et al. Antidiabetic properties of phytosynthesized gold nanoparticles (AuNPs) from Datura stramonium seed. IOP Conf Ser Mater Sci Eng. 2020;805:012035. https://doi.org/10.1088/1757-899X/805/1/012035.
Aljabali A, Akkam Y, Zoubi M, Al-Batayneh K, Al-Trad B, Abo Alrob O, Alkilany A, Benamara M, Evans D. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus zizyphus and their Antimicrobial Activity. Nanomaterials. 2018;8(3):174. https://doi.org/10.3390/nano8030174.
Sekar V, Al-Ansari MM, Narenkumar J, et al. Synthesis of gold nanoparticles (AuNPs) with improved anti-diabetic, antioxidant and anti-microbial activity from Physalis minima. J King Saud Univ Sci. 2022;34:102197. https://doi.org/10.1016/j.jksus.2022.102197.
Bacanli M, Dilsiz SA, Başaran N, Başaran AA. Effects of phytochemicals against diabetes. Adv Food Nutr Res. 2019;89:209–38. https://doi.org/10.1016/bs.afnr.2019.02.006.
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. https://doi.org/10.4161/oxim.2.5.9498.
Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. https://doi.org/10.1093/ajcn/79.5.727.
Shahwan M, Alhumaydhi F, Ashraf GMd, et al. Role of polyphenols in combating type 2 diabetes and insulin resistance. Int J Biol Macromol. 2022;206:567–79. https://doi.org/10.1016/j.ijbiomac.2022.03.004.
Hussein J, El-Naggar ME. Synthesis of an environmentally quercetin nanoemulsion to ameliorate diabetic-induced cardiotoxicity. Biocatal Agric Biotechnol. 2021;33:101983. https://doi.org/10.1016/j.bcab.2021.101983.
Mandić L, Matković M, Baranović G, Šegota S. The increased release kinetics of quercetin from superparamagnetic nanocarriers in dialysis. Antioxidants. 2023;12:732. https://doi.org/10.3390/antiox12030732.
Tong F, Liu S, Yan B, et al. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Int J Nanomedicine. 2017;12:7799–813. https://doi.org/10.2147/IJN.S146978.
Chitkara D, Nikalaje SK, Mittal A, et al. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Deliv Transl Res. 2012;2:112–23. https://doi.org/10.1007/s13346-012-0063-5.
Salehi B, Fokou P, Sharifi-Rad M, et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:11. https://doi.org/10.3390/ph12010011.
Bhia M, Motallebi M, Abadi B, et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics. 2021;13:291. https://doi.org/10.3390/pharmaceutics13020291.
Maity S, Mukhopadhyay P, Kundu PP, Chakraborti AS. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—an in vitro and in vivo approach. Carbohydr Polym. 2017;170:124–32. https://doi.org/10.1016/j.carbpol.2017.04.066.
Wang Y, Wang S, Firempong CK, Zhang H, Wang M, Zhang Y, Zhu Y, Yu J, Xu X. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: preparation and in vitro and in vivo evaluations. AAPS PharmSciTech. 2017;18(3):586–94. https://doi.org/10.1208/s12249-016-0537-8.
Maity S, Chakraborti AS. Formulation, physico-chemical characterization and antidiabetic potential of naringenin-loaded poly D, L lactide-co-glycolide (N-PLGA) nanoparticles. Eur Polym J. 2020;134:109818. https://doi.org/10.1016/j.eurpolymj.2020.109818.
Najmanová I, Vopršalová M, Saso L, Mladěnka P. The pharmacokinetics of flavanones. Crit Rev Food Sci Nutr. 2020;60:3155–71. https://doi.org/10.1080/10408398.2019.1679085.
Nagula RL, Wairkar S. Recent advances in topical delivery of flavonoids: A review. J Control Release. 2019;296:190–201. https://doi.org/10.1016/j.jconrel.2019.01.029.
Ahangarpour A, Oroojan AA, Khorsandi L, et al. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid Med Cell Longev. 2018;2018:1–18. https://doi.org/10.1155/2018/7496936.
Pereira M, Siba IP, Chioca LR, et al. Myricitrin, a nitric oxide and protein kinase C inhibitor, exerts antipsychotic-like effects in animal models. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1636–44. https://doi.org/10.1016/j.pnpbp.2011.06.002.
Ganugula R, Arora M, Jaisamut P, et al. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of Type 1 diabetes mellitus. Br J Pharmacol. 2017;174:2074–84. https://doi.org/10.1111/bph.13816.
Chauhan P, Tamrakar AK, Mahajan S, Prasad GBKS. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018;213:226–35. https://doi.org/10.1016/j.lfs.2018.10.027.
El-Naggar ME, Al-Joufi F, Anwar M, et al. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf B Biointerfaces. 2019;177:389–98. https://doi.org/10.1016/j.colsurfb.2019.02.024.
Hu B, Gao M, Boakye-Yiadom KO, et al. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact Mater. 2021;6:4592–606. https://doi.org/10.1016/j.bioactmat.2021.04.040.
Chauhan P, Tamrakar AK, Mahajan S, Prasad GBKS. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018;13:226–35. https://doi.org/10.1016/j.lfs.2018.10.027.
Akbar MU, Zia KM, Akash MSH, et al. In-vivo anti-diabetic and wound healing potential of chitosan/alginate/maltodextrin/pluronic-based mixed polymeric micelles: Curcumin therapeutic potential. Int J Biol Macromol. 2018;120:2418–30. https://doi.org/10.1016/j.ijbiomac.2018.09.010.
Su M, Zhao W, Xu S, Weng J. Resveratrol in treating diabetes and its cardiovascular complications: a review of its mechanisms of action. Antioxidants. 2022;11:1085. https://doi.org/10.3390/antiox11061085.
Yücel Ç, Karatoprak GŞ, Aktaş Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J Nanosci Nanotechnol. 2018;18(6):3856–64. https://doi.org/10.1166/jnn.2018.15247.
Abdel-Moneim A, El-Shahawy A, Yousef AI, et al. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int J Biol Macromol. 2020;154:1496–504. https://doi.org/10.1016/j.ijbiomac.2019.11.031.
Kim JH, Lee BJ, Kim JH, et al. Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21WAF1 expression. Eur J Pharmacol. 2009;615:150–4. https://doi.org/10.1016/j.ejphar.2009.05.015.
Da Silva SB, Ferreira D, Pintado M, Sarmento B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int J Biol Macromol. 2016;84:112–20. https://doi.org/10.1016/j.ijbiomac.2015.11.070.
Martakov IS, Shevchenko OG, Torlopov MA, et al. Formation of gallic acid layer on γ-AlOOH nanoparticles surface and their antioxidant and membrane-protective activity. J Inorg Biochem. 2019;199:110782. https://doi.org/10.1016/j.jinorgbio.2019.110782.
Purbowatiningrum, Ngadiwiyana, Ismiyarto, et al. Antidiabetic activity from Gallic acid encapsulated nanochitosan. IOP Conf Ser Mater Sci Eng. 2017;172:012042. https://doi.org/10.1088/1757-899X/172/1/012042
Zhang Y, Zhang L, Ban Q, et al. Preparation and characterization of hydroxyapatite nanoparticles carrying insulin and gallic acid for insulin oral delivery. Nanomedicine. 2018;14:353–64. https://doi.org/10.1016/j.nano.2017.11.012.
Guo X, Yang B, Tan J, et al. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70:1360–7. https://doi.org/10.1038/ejcn.2016.142.
Colorado D, Fernandez M, Orozco J, Lopera Y, Muñoz DL, Acín S, et al. Metabolic activity of anthocyanin extracts loaded into non-ionic niosomes in diet-induced obese mice. Pharm Res. 2020;37:152. https://doi.org/10.1007/s11095-020-02883-z.
Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol. 2017;67:220–35. https://doi.org/10.1016/j.tifs.2017.07.008.
Negahdari R, Bohlouli S, Sharifi S, et al. Therapeutic benefits of rutin and its nanoformulations. Phytother Res. 2021;35:1719–38. https://doi.org/10.1002/ptr.6904.
Amjadi S, Shahnaz F, Shokouhi B, et al. Nanophytosomes for enhancement of rutin efficacy in oral administration for diabetes treatment in streptozotocin-induced diabetic rats. Int J Pharm. 2021;610:121208. https://doi.org/10.1016/j.ijpharm.2021.121208.
Sharma V, Katiyar A, Agrawal RC. Glycyrrhiza glabra: chemistry and pharmacological activity. Sweeteners. 2018;2018:87–100. https://doi.org/10.1007/978-3-319-27027-2_21.
Tan D, Tseng HHL, Zhong Z, et al. Glycyrrhizic acid and its derivatives: promising candidates for the management of type 2 diabetes mellitus and its complications. Int J Mol Sci. 2022;23:10988. https://doi.org/10.3390/ijms231910988.
Sherwani SI, Khan HA, Ekhzaimy A, et al. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95–104. https://doi.org/10.4137/BMI.S38440.
Wang X, Liu R, Zhang W, et al. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol Cell Endocrinol. 2013;376:70–80. https://doi.org/10.1016/j.mce.2013.06.014.
Zhang Y, Li J, Wang Z, et al. Natural plant-derived polygalacturonic acid-oleanolic acid assemblies as oral-delivered nanomedicine for insulin resistance treatment. Chem Eng J. 2020;390:124630. https://doi.org/10.1016/j.cej.2020.124630.
Castellano JM, Guinda A, Delgado T, et al. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62:1791–9. https://doi.org/10.2337/db12-1215.
Nagoor Meeran MF, Goyal SN, Suchal K, et al. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: a pentacyclic triterpenoid of therapeutic promise. Front Pharmacol. 2018;9:892. https://doi.org/10.3389/fphar.2018.00892.
Lingling G, Yuan Z, Weigen L. Preparation, optimization, characterization and in vivo pharmacokinetic study of asiatic acid tromethamine salt-loaded solid lipid nanoparticles. Drug Dev Ind Pharm. 2016;42:1325–33. https://doi.org/10.3109/03639045.2015.1135934.
Escobar-Chaves E, Acin S, Muñoz DL, et al. Polymeric nanoformulation prototype based on a natural extract for the potential treatment of type 2 diabetes mellitus. J Drug Deliv Sci Technol. 2023;81:104264. https://doi.org/10.1016/j.jddst.2023.104264.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stoleru, O.A., Burlec, A.F., Mircea, C. et al. Multiple nanotechnological approaches using natural compounds for diabetes management. J Diabetes Metab Disord 23, 267–287 (2024). https://doi.org/10.1007/s40200-023-01376-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01376-1