Abstract
Background
This study aims to assess the possible relationship between frailty and anthropometric indices in older adults using data from the first phase of the Birjand Longitudinal Aging Study (BLAS).
Methods
In this cross-sectional study, we assessed the association between frailty (Frailty index (FI) and Fried frailty phenotype) and body composition indices in 1364 participants aged ≥ 60 years (September 2018 to April 2019). Analysis was conducted using one-way ANOVA and ordinal logistic regression.
Results
Participants were categorized as frail (n = 164), non-frail (n = 415), and pre-frail (n = 785) based on FI. A significant positive association was observed between the frailty and body mass index (BMI) (OR: 1.04, 95% CI:1.01- 1.07), waist circumference (WC) (OR: 1.02, 95% CI: 1.01- 1.03), waist-to-hip ratio (WHR) (OR: 2.36, 95% CI 1.05- 5.27) and waist-to-height ratio (WHtR) (OR: 1.27, 95%CI: 1.09- 1.47). Body shape index, body roundness index, and body adiposity index showed no significant association with frailty. Moreover, a BMI greater than 29 kg/m2 increased the odds of frailty and prefrailty by 79% (OR = 1.79, 95%CI = 1.30- 2.46, P < 0.001).
Conclusion
Results of this study showed that the risk of frailty increases as BMI and abdominal obesity indices increase. Therefore, BMI and abdominal obesity indices (WC, WHR, and WHtR) could serve as suitable tools for evaluating frailty in the elderly. However, additional studies are needed to evaluate the utility of the newly developed anthropometric indices in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Over the past century, life expectancy has witnessed significant growth worldwide, owing to advancements in public health [1]. This notable demographic shift has contributed to a rise in the population of older adults across developed, developing, and low-income countries [2]. The World Population Report projects that the global population of individuals aged 60 years and older will reach 2 billion by the year 2050 [1]. According to a meta-analysis of population-level studies encompassing 62 countries, the prevalence of frailty was found to be 24% and pre-frailty 49%, as determined by the deficit accumulation model [3]. Also, Different regions in Iran have reported a varied prevalence of frailty, ranging from 14.3% to 46.7% [4,5,6,7,8].
Frailty is a clinical condition that is defined as a decline in an individual’s physical and mental capacities (Intrinsic Capacity) and increased vulnerability to cope with everyday or acute stressors which leads to adverse outcomes [9, 10]. Frailty syndrome depends on several factors including genetic, cumulative environmental impact, nutrition, lifestyle choices, physiological changes in aging, psychological factors, chronic disease, etc. [11,12,13,14].
The two most commonly used measurement tools for frailty are the Fried phenotype and The Frailty Index. The Fried phenotype [15] consists of five components including unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity. The Frailty Index is based on the accumulative health deficits model. In contrast with the phenotype model, The Frailty Index considers the cognitive condition of the older adult, and is also more sensitive and multidimensional to adverse health outcomes [5, 16, 17].
Various pathological conditions are accompanied by frailty such as weight loss, sarcopenia, anorexia, and low protein intake [18, 19]. As well as low body weight, obese older people are at risk of frailty [12]. Several articles have reported on the relationship between general and central obesity and frailty in the older population [20,21,22]. They have shown a positive association between abdominal obesity and increased risk of frailty. Moreover, findings indicated a direct association between the simultaneous presence of general and abdominal obesity with frailty and pre-frailty. The study underscored the importance of evaluating Body Mass Index (BMI) and Waist Circumference (WC) together in older adults [22]. Anthropometric indices including weight, BMI, WC, Waist-to-Hip Ratio (WHR), Hip Circumference (HC), and Waist-to-Height Ratio (WHtR) constitute essential components in the assessment of body composition. Recently, new anthropometric indices have been developed to more accurately reflect the composition of body fat and visceral fat by combining traditional indices such as WC, HP, and BMI.
Body Roundness Index (BRI) is an obesity-related index that represents body shape and some studies have demonstrated that BRI is associated with diabetes mellitus, and cardiovascular diseases [23,24,25,26]. A Body Shape Index (ABSI) is another novel anthropometric tool, based on BMI, WC, and height. ABSI was developed in 2012 by Krakauer et al. and was offered as a risk factor for premature death [27]. Moreover, the Body Adiposity Index (BAI) is suggested as a simple index to reflect on obesity, with a high correlation with body fat measured by Dual-energy X-ray Absorption (DXA) [28].
Due to demographic growth, the concept of “Frailty Syndrome” has been the center of attention by the public health policy-makers. Frailty has a dynamic nature [29], thus, knowing the associated factors of frailty can make it possible to preserve or improve physical and cognitive impairment, to prevent disability, dependency, hospitalization, and death [9]. To our knowledge, literature regarding the association between novel anthropometric indices and both frailty index and frailty phenotype is absent. We aimed to investigate the existence and extent of the relationship between frailty and various anthropometric indices in older adults, using data from the Birjand Longitudinal Aging Study (BLAS).
Methods
Design and Population
This cross-sectional study was conducted using data from the enrolment phase of the Birjand longitudinal aging study (BLAS) (total sample size = 1420) which was conducted from September 2018 to April 2019 in Birjand, Iran. BLAS is an ongoing prospective cohort study and the participants of this research are community-dwelling older adults over 60 years of age, residents of Birjand (excluding the rural areas). details of the study method have already been published [30]. Anthropometric indices, dietary habits, socio-demographic characteristics, and history of chronic illnesses and medications were obtained through pretested valid questionnaires, which were completed by trained interviewers. This study was approved by the research ethics committee of the Endocrinology Metabolism Research Institute of Tehran University of Medical Sciences (code: IR.TUMS.EMRI.REC.1396.00158) and written informed consent was obtained from all participants before participation.
Exclusion criteria
Exclusion criteria were as follows: anatomical defects or decreased strength that could affect performance on tests, being a chair or bed-ridden even if transiently, advanced Parkinson’s disease and missing anthropometric data. Out of 1420 BLAS participants, 56 elderly were excluded from the current research and analysis was done on 1364 participants.
Anthropometric Assessment
Weight was measured with the least amount of clothing by calibrated SECA digital scale at the nearest 0.1 kg (SECA, Germany). Height was measured with participants standing straight, at the nearest 0.1 cm (SECA, Germany). Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Waist circumference (WC) was recorded to the nearest 0.5 cm using non-stretch tape placed midway between the iliac crest and lowest rib while participants were in the standing position. Hip circumference (HC) was measured at the distance around the largest part of the hips (the widest part of the buttocks). Waist-to-height ratio (WHtR) and waist-to-hip ratio (WHR) were measured by dividing WC into height and hip circumference, respectively.
Body roundness index (BRI), body adiposity index (BAI), and body shape index (ABSI) were calculated using the following formulas [31, 32]:
As the values were too small, the Z-score of WHtR and ABSI was used for analysis ((value-mean)/SD). Based on a recent meta-analysis, BMI was categorized as a risk factor(≤ 24 and ≥ 29) or normal (24 < BMI < 29), according to the cut-off points determined for the elderly population [33].
Assessment of frailty
A frailty index counts deficits in health. Restricted activity, disability in Activities of Daily Living (ADL), impairments in general cognition (including Mini–mental state examination (MMSE) and Six-item Cognitive Impairment Test (6-CIT)), physical performance (including impaired grip strength, impaired walking, and low skeletal muscle index), co-morbidity, self-rated depression/mood disorder were evaluated as health deficits. For each individual, The Frailty Index (FI) was calculated by summing all health deficits and dividing it by the total number of health deficits. We classified the continuous frailty index into non-frail (FI < 0.20), pre-frail (0.20 ≤ FI < 0.45), and frail (FI ≥ 0.45) [34].
The frailty phenotype is based on five criteria testing the presence or absence of signs and symptoms of frailty (including involuntary weight loss, exhaustion, slow gait speed, poor handgrip strength, and sedentary behavior). The number of criteria is categorized into a 3-level variable depicting robustness (none of the criteria), pre-frailty (one or two criteria), and frailty (three or more criteria) [15].
Other variables
Socio-demographic information was obtained including age, sex, smoking status, marital status, education status, physical activity, and chronic disorders (cardiovascular, hypertension, hyperlipidemia, osteoporosis, diabetes mellitus, stroke, seizure, arthritis, heart failure, cancer, gout, thyroid disorders, and surgical history). The Patient Health Questionnaire-9 (PHQ-9) was used to assess depression [35]. Polypharmacy (using 3 drugs or more) and multimorbidity (having more than 3 diseases) were assessed in the participants. The forward selection method was used to choose confounders and covariates [36]. Variables including age, sex, duration of education, physical activity, job, depression, and smoking remained in the model as covariates.
Statistical Analysis
Continuous variables were presented as mean (standard deviation (SD)), and categorical variables were presented as frequency (%). All results were reported separately for frail, pre-frail, and non-frail individuals. The one-way ANOVA was used to compare demographic and anthropometric indices between the three groups of frailty. For the categorical variables, the difference between the two groups was assessed by Chi-square or Fisher's exact test. The association of body composition indices and frailty was assessed by ordinal logistic regression and results were expressed as Odds Ratio (OR) (95% Confidence Interval (CI)). The BLAS data was initially provided in SPSS format, we proceeded to clean and analyze the basic data (general participant characteristics) in SPSS. Subsequently, the main analyses were carried out in STATA version 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.), and a P-value ≤ 0.05 was considered statistically significant.
Results
Table 1 summarizes the anthropometric and demographic characteristics of the 1364 participants. The study sample was comprised of 707 women (51.8%) and 657 men (48.2%) with a mean age of 69.77 ± 7.66 years old. Few participants had a university education (17.7% of men and 12.7% of women). Most participants were married (81.3%) and illiterate (45.7%), and they were mostly housewives or freelancers (55.2%). Only a minor group of all participants were smokers (7%) and had low physical activity (13.2%). Polypharmacy was common among the studied population (65%) and 55.1% of them were suffering from multiple chronic diseases.
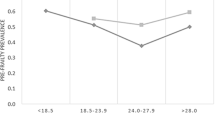
Comparing the three groups of frail (n = 164), non-frail (n = 415), and pre-frail (n = 785) participants based on FI, it was observed that all the socio-demographic characteristics of the participants were significantly different (P ≤ 0.05), except for multimorbidity (P = 0.09). The score of the PHQ-9 questionnaire had an ascending trend in non-frail (mean (SD) = 1.92 (2.66)), pre-frail (6.12 (4.85)), and frail (10.55 (5.48)) participants (P < 0.001). Regarding the body composition indices, weight, BMI, WHtR, BAI, ABSI (P < 0.001), and WC (P = 0.002) were significantly different between the three groups, while WHR (P = 0.08), HC (P = 0.85), and BRI (P = 0.88) had no remarkable difference. Except for BRI and weight, all the anthropometric variables had a descending trend as the severity of frailty increased.
As shown in Table 2, a significant direct association between all body composition indices and FI was observed in the crude model (P < 0.05), except for BRI (P > 0.05). After adjustment for confounders, BMI, WC, WHR, and WHtR were still recognized as risk factors for frailty and pre-frailty, while the significance of the relationship disappeared regarding BAI and ABSI.
Regarding the frailty phenotype, BMI was the only index that was significantly related to frailty in both the crude and the adjusted models (OR = 0.97; 95%CI = 0.95- 0.99; P = 0.02). the association between BMI and frailty phenotype was inverse (OR < 1.00), while this association was direct when considering FI (OR > 1.00).
Considering the specific cut-off points of BMI among older adults, 24 ≤ BMI ≤ 29 kg/m2 was considered as the reference category. Analysis showed that both low (OR = 1.29; 95%CI = 1.01- 1.65; P = 0.04) and high BMI (OR = 1.98; 95%CI = 1.52- 2.57; P < 0.001) increased the odds of frailty and pre-frailty in the crude model (Table 3). The association was still significant in the adjusted models only for the group with BMI > 29 kg/m2. In other words, BMI > 29 kg/m2 increased the odds of frailty and prefrailty by 79% (OR = 1.79, 95%CI = 1.30- 2.46, P < 0.001) while no significant relationship was observed for frailty phenotype and categories of BMI.
Discussion
The current study was performed on 1364 older adults over 60 years old to evaluate the relationship between frailty and anthropometric indices, using data from the Birjand longitudinal aging study (BLAS). Overall, our study analysis illustrated a significant positive association between FI and BMI, WC, WHR and, WHtR in the final model, which was adjusted for age, sex, education, physical activity, depression, job and, smoking. On the other hand, only BMI was significantly associated with frailty phenotype as a protective factor (OR < 1).
The prevalence of frailty and pre-frailty were 12% and 57% in this study using FI, respectively. The prevalence of frailty is dependent on the used measurement tool. The prevalence of frailty in the elderly population of different countries varies from 1.5% to 43.41%. It can be partly explained by different measurement tools in each study, such as FI, Fried's phenotype, or Kihon's checklist [37,38,39,40]. Numerous tools are used to appraise frailty, but there has been no consensus on an international gold-standard measurement tool.
The socio-demographic findings of the present study were almost in line with previous studies. The majority of the frail elderly in this study were physically inactive (77.4%). A review article reported that increasing physical activity could improve frailty status and recommends increasing physical activity interventions to prevent and reverse frailty [41]. However, considering the nature of cross-sectional studies, it may be possible that being frail resulted in physical inactivity in elderly people [38]. The frail participants in our study were mostly female (82.3%) and married (61.6%), as well as previous studies [42, 43]. The predominance of frailty in females was observed in several studies and women’s longer life expectancy can explain it [38, 44,45,46]. Moreover, due to the onset of menopause in women, physiological changes like increasing weight can trigger systemic inflammation and speed up the frailty process [47, 48]. We found 76.2% of frail people in our study were illiterate and 86% were housewives or freelancers. These results are also consistent with a large number of studies that revealed that lower socioeconomic status, such as individual and neighborhood deprivation [49], lower education [50, 51], and job status [52] are strongly associated with a higher risk of frailty. As well as frailty syndrome, depression is associated with disabilities in older adults, and their overlapping will be challenging. In the present study, PHQ-9 progressively increased among non-frail, pre-frail, and frail subjects and, it was significantly associated with frailty in the elderly population its score was 10.55 in frail individuals, which was more than two-fold of non-frails. A systematic review reported that prevalence of the depression among frail individuals increases with increasing age [53].
Previously published studies proposed that anthropometric indices are strong predictors of frailty in old populations. It is assumed that weight loss and sarcopenia are the main manifestations of frailty and obese/overweight persons are not at risk of being frail [12, 54]. However, according to our results, a high BMI increases the odds of frailty according to the FI score. Evidence showed divergent results regarding the correlation between BMI and frailty. Some studies including a recent large cohort study with 29,937 participants have shown a positive association between a higher level of BMI and a higher risk of frailty [55,56,57,58,59]. On the other hand, a cohort study on 6662 women in France showed that higher BMI is a protective factor against adverse outcomes in frail elderly women [60]. Another study showed a higher risk of frailty and mortality among those who have a lower BMI than 25 kg/m2 [61]. So we evaluated the association between frailty (both FI and phenotype) and BMI categories (> 29 and < 24 kg/m2). While results showed both high and low BMI increased FI in a crude model, after adjustment for confounding factors, a positive association between BMI > 29 and frailty (according to FI) remained. Contrary to the BMI > 29 and FI association, BMI was a protective factor for the frailty phenotype (OR < 1). This finding could be explained by one of the Fried frailty phenotype criteria which is unintentional weight loss, It means lower weight could be regarded as a risk factor in the phenotype assessment tool. Another difference between these two instruments is that the frailty index measures cognitive function. Evidence demonstrated that losing weight improves cognitive performance especially executive function in obese and overweight subjects via mechanisms including systemic inflammation, insulin resistance, and high lipid profile [62,63,64]. So the effect of obesity and cognitive impairment, one of the frailty syndrome’s components according to FI, might be another explanation for this observation. Two cohort studies in Taiwan and England reported a U shape relationship between BMI and frailty risk which means both wasting and obesity are correlated with frailty, and maintaining a normal BMI would be better for retaining physical ability in the elderly [65, 66]. In our analysis, a similar correlation between BMI categories and frailty was seen in our crude model, but after adjustment for confounding factors, the relationship for BMI < 24 disappeared. Despite BMI being known as a good obesity indicator globally, it can not discriminate between muscle mass and fat mass. The term “sarcopenic obesity” is defined as decreased muscle mass and increased fat mass [45] and, individuals with sarcopenic obesity tend to be more physically frail and poor [67]. In summary, adipose tissue releases a group of hormones and pro-inflammatory cytokines that contribute to the elevation of inflammatory markers, leading to systemic inflammation in the body [68,69,70]. Inflammation has been understood as one of the potential pathophysiological mechanisms linked with frailty [70] which affects muscle function and mobility [71]. It is hypothesized that insulin resistance and hyperinsulinemia impair muscle protein production and breakdown [72, 73]. Impaired muscle quality subsequently brings low muscle strength and energy dysregulation and decreases in performance, respectively, as seen in frailty [74].
WC and WHtR are good indicators of abdominal obesity [59, 75], and abdominal fat is associated with an increased risk of cardiovascular and metabolic disease in late adulthood [75]. Most of the studies showed a significant relationship between frailty and central obesity [20, 21]. According to previously published articles, abdominal obesity is more closely associated with a higher risk of frailty than general obesity [58]; even a study showed that subjects with low BMI but higher WC were frailer [58, 66]. WHtR is another anthropometric tool that is more accurate than WC in predicting central obesity [59]. Thus we decided to investigate the association between WHtR with frailty in this study. One key finding of the current study is that the frail group has greater waist-to-height circumference than the non-frail group. These results are consistent with previous studies [40, 45, 58]. Findings confirmed that WHtR could be a good tool to discriminate abdominal fat, which is highly associated with visceral fat [76,77,78] and serves cardiovascular and metabolic risks that cause co-morbidity and might increase the risk of frailty in older adults [58].
Most previously published studies reported a significant positive relationship between higher WHR and frailty risk [79,80,81]. A cohort study on the older population of England reported a significant relationship between high WHR and frailty risk [79, 81]. our findings showed the exact correlation between WHR and frailty, such that WHR increases the risk of frailty approximately 2.5-fold.
Newly developed obesity-related indices are proposed to reflect body composition and body fat better than traditional indices. In this study, BRI, BAI, and ABSI were calculated for each participant. Some studies have asserted that these novel anthropometric indices are associated with metabolic syndrome, osteoporosis, peripheral artery occlusive disease, and fatty liver [82,83,84,85]. According to final logistic regression models, none of the aforementioned indices were associated with FI and frailty phenotype. A systematic review investigated the validity of the BAI in predicting body fat and reported that evidence showed that BAI is not a satisfying indicator of body fat percentage in adults, which supports our results [86].
The strength of this study is that it has appraised vast types of body composition indices in the elderly, especially novel obesity-related indices such as BRI, BAI, and ABSI, and evaluated their association with frailty. Also, frailty index and frailty phenotype, the two most commonly used tools, were considered and compared together in our study. The results of this study help to recognize the anthropometric risk factors for being frail in the elderly. In addition, our investigations illustrated the prevalence of frailty and socio-demographic characteristics in a larger sample size in comparison with other studies in Iran and the Middle East [87]. This study had some limitations; first of all, some of the disabled frail subjects were unable to collaborate in our research. Moreover, it should be noted that although we adjusted the analysis for many variables, there may still be other confounding factors. Another limitation is the design of the study which could assess the cross-sectional relationships, which makes it impossible to draw causal interferences.
Overall, this study showed that the risk of frailty increases as body anthropometric indices increase and the elderly progress towards overweight and obesity. This study could help policy-makers to design interventional preventive plans to reduce frailty. Moreover, abdominal obesity indices (WC, WHR, and, WHtR) could be an appropriate tool for the evaluation of frailty in the elderly, while further studies are needed to evaluate the utility of the newly developed anthropometric indices in older adults.
Data availability
The datasets generated during the current study are not publicly available due to ethical concerns but are available from the corresponding author on reasonable request.
References
Organization WH. Are you ready? What you need to know about ageing. World health day. 2012.
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing. 2017.
O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104.
Jafaripour S, Omidvar N, Eini-Zinab H, Rezaei M, Rezazadeh A. Prevalence of Frailty and Its Relationships with Malnutrition in Elderly Living in Tehran City, 2021. Iran J Nutrition Sci Food Technol. 2023;17(4):19–29.
Delbari A, Zanjari N, Momtaz YA, Rahim F, Saeidimehr S. Prevalence of frailty and associated socio-demographic factors among community-dwelling older people in southwestern Iran: a cross-sectional study. J Diabetes Metab Disord. 2021;20(1):601–10.
Mousavisisi M, Shamshirgaran SM, Rezaeipandari H, Matlabi H. Multidimensional approach to frailty among rural older people: Applying the tilburg frailty indicator. Elderly Health J. 2019;5(2):92–101.
Shohani M, Mohammadi I, Seidkhani H, Mohamadnejad S. The prevalence of frailty and its associated factors among Iranian hospitalized older adults. Nursing and Midwifery Studies. 2022;11(3):215.
Abdi M, Dabiran S. Validity and reliability of Tilburg frailty indicatr in assess of prevalence and risk factors of this syndrome in Iranian elderly. In: The First Iranian Congress of Social Medicine. 2017. p. 14–18.
Organization WH. WHO clinical consortium on healthy ageing: topic focus: frailty and intrinsic capacity: report of consortium meeting, 1–2 December 2016 in Geneva. Switzerland: World Health Organization; 2017.
Boyd CM, Xue Q-L, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118(11):1225–31.
Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. In search of an integral conceptual definition of frailty: opinions of experts. J Am Med Dir Assoc. 2010;11(5):338–43.
Heuberger RA. The frailty syndrome: a comprehensive review. J Nutrition Gerontol Geriatrics. 2011;30(4):315–68.
Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2(1):1–8.
Haapanen M, Perälä M, Salonen M, Kajantie E, Simonen M, Pohjolainen P, et al. Early life determinants of frailty in old age: the Helsinki Birth Cohort Study. Age Ageing. 2018;47(4):569–75.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–57.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. The Scientific World J. 2001;1:323–36.
Andrew MK. Frailty and social vulnerability. Frailty in Aging. 2015;41:186–95.
van Kan GA, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–2.
Carmeli E. Frailty and primary sarcopenia: a review. In: Pokorski M, editor. Clinical Research and Practice. Advances in Experimental Medicine and Biology, vol 1020. Cham: Springer; 2017. https://doi.org/10.1007/5584_2017_18.
Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(4):1118–28.
Hawkins KL, Zhang L, Althoff KN, Palella FJ, Kingsley LA, Jacobson LP, et al. Abdominal obesity, sarcopenia, and osteoporosis are strongly associated with frailty in the MACS. AIDS (London, England). 2018;32(10):1257.
Afonso C, Sousa-Santos AR, Santos A, Borges N, Padrão P, Moreira P, et al. Frailty status is related to general and abdominal obesity in older adults. Nutr Res. 2021;85:21–30.
Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–71.
Zhao Q, Zhang K, Li Y, Zhen Q, Shi J, Yu Y, et al. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross-sectional study. Diabet Med. 2018;35(11):1580–7.
Geraci G, Zammuto M, Gaetani R, Mattina A, D’Ignoto F, Geraci C, et al. Relationship of a Body Shape Index and Body Roundness Index with carotid atherosclerosis in arterial hypertension. Nutr Metab Cardiovasc Dis. 2019;29(8):822–9.
Yalcin G, Ozsoy E, Karabag T. The relationship of body composition indices with the significance, extension and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2020;30(12):2279–85.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7): e39504.
Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, et al. A better index of body adiposity. Obesity. 2011;19(5):1083–9.
Faller JW, Pereira DdN, de Souza S, Nampo FK, Orlandi FdS, Matumoto S. Instruments for the detection of frailty syndrome in older adults: a systematic review. PloS one. 2019;14(4):e0216166.
Moodi M, Firoozabadi MD, Kazemi T, Payab M, Ghaemi K, Miri MR, et al. Birjand longitudinal aging study (BLAS): the objectives, study protocol and design (wave I: baseline data gathering). J Diabetes Metab Disord. 2020;19:551–9.
Nkwana MR, Monyeki KD, Lebelo SL. Body roundness index, a body shape index, conicity index, and their association with nutritional status and cardiovascular risk factors in South African rural young adults. Int J Environ Res Public Health. 2021;18(1):281.
Yeşil E, Köse B, Özdemir M. Is body adiposity index a better and easily applicable measure for determination of body fat? J Am Coll Nutr. 2020;39(8):700–5.
Jiang M, Zou Y, Xin Q, Cai Y, Wang Y, Qin X, et al. Dose–response relationship between body mass index and risks of all-cause mortality and disability among the elderly: a systematic review and meta-analysis. Clin Nutr. 2019;38(4):1511–23.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):1–10.
Chen I-P, Liu S-I, Huang H-C, Sun F-J, Huang C-R, Sung M-R, et al. Validation of the Patient Health Questionnaire for depression screening among the elderly patients in Taiwan. Int J Gerontol. 2016;10(4):193–7.
VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34:211–9.
Wanaratna K, Muangpaisan W, Kuptniratsaikul V, Chalermsri C, Nuttamonwarakul A. Prevalence and factors associated with frailty and cognitive frailty among community-dwelling elderly with knee osteoarthritis. J Community Health. 2019;44(3):587–95.
Llano PMPd, Lange C, Sequeira CAdC, Jardim VMdR, Castro DSP, Santos F. Factors associated with frailty syndrome in the rural elderly. Revista Brasileira de Enfermagem. 2019;72:14–21.
Nunes DP, Duarte YA, Santos JL, Lebrão ML. Screening for frailty in older adults using a self-reported instrument. Revista de saude publica. 2015;49.
Martins BA, Visvanathan R, Barrie H, Huang CH, Matsushita E, Okada K, et al. Frailty prevalence using Frailty Index, associated factors and level of agreement among frailty tools in a cohort of Japanese older adults. Arch Gerontol Geriatr. 2019;84:103908.
O’Connell ML, Coppinger T, McCarthy AL. The role of nutrition and physical activity in frailty: A review. Clin Nutrition ESPEN. 2020;35:1–11.
Runzer-Colmenares FM, Samper-Ternent R, Al Snih S, Ottenbacher KJ, Parodi JF, Wong R. Prevalence and factors associated with frailty among Peruvian older adults. Arch Gerontol Geriatr. 2014;58(1):69–73.
Sánchez-García S, Sánchez-Arenas R, García-Peña C, Rosas-Carrasco O, Ávila-Funes JA, Ruiz-Arregui L, et al. Frailty among community-dwelling elderly M exican people: Prevalence and association with sociodemographic characteristics, health state and the use of health services. Geriatr Gerontol Int. 2014;14(2):395–402.
Muszalik M, Gurtowski M, Doroszkiewicz H, Gobbens RJ, Kędziora-Kornatowska K. Assessment of the relationship between frailty syndrome and the nutritional status of older patients. Clin Interv Aging. 2019;14:773.
Rodrigues RP, Fhon JS, Huayta VR, Neira WF, Pontes M, Silva A, et al. Frailty syndrome and anthropometric measurements in the elderly living at home. J Aging Res Clin Pract. 2017;6:133–8.
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92.
Buzzachera CF, Krause MP, Elsangedy HM, Hallage T, Granato P, Krinski K, et al. Prevalence of overweight, general and central obesity in elderly women from Curitiba, Paraná. Brazil Revista de Nutrição. 2008;21:525–33.
Espinoza SE, Fried LP. Risk factors for frailty in the older adult. Clin Geriatr. 2007;15(6):37.
Lang IA, Hubbard RE, Andrew MK, Llewellyn DJ, Melzer D, Rockwood K. Neighborhood deprivation, individual socioeconomic status, and frailty in older adults. J Am Geriatr Soc. 2009;57(10):1776–80.
Franse CB, van Grieken A, Qin L, Melis RJ, Rietjens JA, Raat H. Socioeconomic inequalities in frailty and frailty components among community-dwelling older citizens. PLoS ONE. 2017;12(11):e0187946.
Szanton SL, Seplaki CL, Thorpe RJ, Allen JK, Fried LP. Socioeconomic status is associated with frailty: the Women’s Health and Aging Studies. J Epidemiol Community Health. 2010;64(01):63–7.
Alvarado BE, Zunzunegui M-V, Béland F, Bamvita J-M. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63(12):1399–406.
Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–72.
MohdHamidin FA, Adznam SNA, Ibrahim Z, Chan YM, Abdul Aziz NH. Prevalence of frailty syndrome and its associated factors among community-dwelling elderly in East Coast of Peninsular Malaysia. SAGE open medicine. 2018;6:2050312118775581.
Fugate Woods N, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–30.
Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53(6):927–34.
Jayanama K, Theou O, Godin J, Mayo A, Cahill L, Rockwood K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022;20(1):1–12.
Liao Q, Zheng Z, Xiu S, Chan P. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res. 2018;30(11):1319–25.
Eslami M, Pourghazi F, Khazdouz M, Tian J, Pourrostami K, Esmaeili-Abdar Z, Ejtahed HS, Qorbani M. Optimal cut-off value of waist circumference-to-height ratio to predict central obesity in children and adolescents: a systematic review and meta-analysis of diagnostic studies. Front Nutrit 2023;9:985319.
Boutin E, Natella P-A, Schott A-M, Bastuji-Garin S, David J-P, Paillaud E, et al. Interrelations between body mass index, frailty, and clinical adverse events in older community-dwelling women: The EPIDOS cohort study. Clin Nutr. 2018;37(5):1638–44.
Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53(1):40–7.
Veronese N, Facchini S, Stubbs B, Luchini C, Solmi M, Manzato E, et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;72:87–94.
Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–55.
Siervo M, Arnold R, Wells J, Tagliabue A, Colantuoni A, Albanese E, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011;12(11):968–83.
Ho H-E, Yeh C-J, Chu W-M, Lee M-C. Midlife body mass index trajectory and risk of frailty 8 years later in Taiwan. J Nutr Health Aging. 2019;23(9):849–55.
Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol Series: Biomed Sci Med Sci. 2010;65(4):377–81.
Ozkok S, Aydin CO, Sacar DE, Catikkas NM, Erdogan T, Bozkurt ME, et al. Sarcopenic obesity versus sarcopenia alone with the use of probable sarcopenia definition for sarcopenia: associations with frailty and physical performance. Clin Nutr. 2022;41(11):2509–16.
Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232–44.
García-Esquinas E, José García-García F, León-Muñoz LM, Carnicero JA, Guallar-Castillón P, Gonzalez-ColaçoHarmand M, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in S pain. Obesity. 2015;23(4):847–55.
Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8.
Addison O, Drummond M, LaStayo P, Dibble L, Wende A, McClain D, et al. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18(5):532–8.
Boirie Y, Gachon P, Cordat N, Ritz P, Beaufrère B. Differential insulin sensitivities of glucose, amino acid, and albumin metabolism in elderly men and women. J Clin Endocrinol Metab. 2001;86(2):638–44.
Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90.
Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813–8.
Benedetti TRB, Meurer ST, Morini S. Anthropometric indices related to cardiovascular and metabolic diseases in older adults. Revista da Educação Física/UEM. 2012;23:123–30.
Kim JE, Choi J, Kim M, Won CW. Assessment of existing anthropometric indices for screening sarcopenic obesity in older adults. Brit J Nutrit. 2023;129(5):875–87.
Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–8.
Chen W, Xu-Hong H, Zhang M-L, Yu-Qian B, Yu-Hua Z, Zhong W-H, et al. Comparison of body mass index with body fat percentage in the evaluation of obesity in Chinese. Biomed Environ Sci. 2010;23(3):173–9.
Niederstrasser NG, Rogers NT, Bandelow S. Determinants of frailty development and progression using a multidimensional frailty index: Evidence from the English Longitudinal Study of Ageing. PLoS ONE. 2019;14(10):e0223799.
Verheij E, Wit FW, Verboeket SO, van der Loeff MF, Nellen JF, Reiss P, Kirk GD. Frequency, risk factors, and mediators of frailty transitions during long-term follow-up among people with HIV and HIV-negative AGEhIV cohort participants. JAIDS (1999). 2021;86(1):110.
Zaslavsky O, Rillamas-Sun E, LaCroix AZ, Woods NF, Tinker LF, Zisberg A, et al. Association between anthropometric measures and long-term survival in frail older women: Observations from the Women’s Health Initiative Study. J Am Geriatr Soc. 2016;64(2):277–84.
Wu L, Zhu W, Qiao Q, Huang L, Li Y, Chen L. Novel and traditional anthropometric indices for identifying metabolic syndrome in non-overweight/obese adults. Nutr Metab. 2021;18:1–10.
Wung C-H, Chung C-Y, Wu P-Y, Huang J-C, Tsai Y-C, Chen S-C, et al. Associations between metabolic syndrome and obesity-related indices and bone mineral density T-Score in hemodialysis patients. J Personalized Med. 2021;11(8):775.
Wung C-H, Lee M-Y, Wu P-Y, Huang J-C, Chen S-C. Obesity-related indices are associated with peripheral artery occlusive disease in patients with type 2 diabetes mellitus. J Personalized Med. 2021;11(6):533.
Lin I-T, Lee M-Y, Wang C-W, Wu D-W, Chen S-C. Gender differences in the relationships among metabolic syndrome and various obesity-related indices with nonalcoholic fatty liver disease in a Taiwanese population. Int J Environ Res Public Health. 2021;18(3):857.
Cerqueira MS, Santos CAd, Silva DAS, Amorim PRdS, Marins JCB, Franceschini SdCC. Validity of the body adiposity index in predicting body fat in adults: a systematic review. Advances Nutrition. 2018;9(5):617–24.
Alqahtani BA, Alshehri MM, Elnaggar RK, Alsaad SM, Alsayer AA, Almadani N, Alhowimel A, Alqahtani M, Alenazi AM. Prevalence of frailty in the middle east: systematic review and metaanalysis. Healthcare. 2022;10(1):108
Acknowledgements
We are grateful to the participants in this study and the people who had any cooperation
Funding
Endocrinology and Metabolism Research Institute funded this study.
Author information
Authors and Affiliations
Contributions
FS, MM, and HSE came up with the idea and designed the study. ZSH, SMA, MK, and HK contributed to collecting data. FS was the biostatistics advisor of this study. ME, FP, and HF drafted and revised the final manuscript, All of the authors read the final manuscript and approved it.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the research ethical committee of Endocrinology and Metabolism Research Institute (EMRI), Tehran University of Medical Sciences ( Ethical code: IR.TUMS.EMRI.REC.1396.00158) and ethical committee of Birjand University of Medical Sciences (Ethical code: IR.BUMS.Rec.1397.282) written informed consent was obtained from all participants before participation. All methods were carried out in accordance with relevant institutional guidelines and regulations.
Consent for publication
Not applicable.
Competing Interests
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eslami, M., Fakhrzadeh, H., Pourghazi, F. et al. The association between frailty and body composition among the elderly: Birjand Longitudinal Aging Study (BLAS). J Diabetes Metab Disord 23, 967–976 (2024). https://doi.org/10.1007/s40200-023-01373-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01373-4