Abstract
Purpose
The triglyceride-to-high-density lipoprotein-cholesterol (TG/HDL-C) ratio is considered an alternative marker for insulin resistance. This longitudinal retrospective study investigated the relationship between TG/HDL-C ratio and the risk of progression to prediabetes.
Methods
We investigated 24,604 Japanese participants (14,609 men and 9,995 women) who underwent annual medical health checkups in 2017 (baseline) and 2022. All participants had no diabetes and prediabetes at baseline. No lipid-lowering medications were taken during the follow-up period. Participants were divided into four groups according to the quartiles of TG/HDL-C ratio at baseline. Multivariable-adjusted Cox regression analysis was conducted to examine hazard ratios (HRs) of progression to prediabetes. Receiver operating characteristic curves were used to determine the optimal cutoff value of TG/HDL-C ratio for prediction of prediabetes.
Results
Compared with the lowest TG/HDL-C ratio quartile (Q1) group, the adjusted HRs (95% confidence intervals (CI)) of progression to prediabetes in the Q2, Q3, and Q4 groups, respectively, were 1.17 (0.92–1.47), 1.26 (1.01–1.56), and 1.77 (1.41–2.23) for men and 1.07 (0.60–1.11), 1.19 (1.08–1.29), and 1.58 (1.18–2.31) for women. For every 1 unit increase in TG/HDL-C ratio, the adjusted HRs (95% CI) for progression to prediabetes was 1.09 (1.04–1.13) in men and 1.10 (1.04–1.15) in women. The optimal TG/HDL-C ratio cutoffs were 1.71 and 0.97 in men and women, respectively, but the area under the curve was > 0.70 in both sexes.
Conclusion
High TG/HDL-C ratio is a risk factor for progression to prediabetes in Japanese men and women, but it had low discriminative ability in predicting prediabetes risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prediabetes refers to an intermediate stage of metabolic disorder characterized by moderate hyperglycemia below the diagnostic threshold for diabetes. In Japanese population, the actual 5-year rate of progression from prediabetes to diabetes is estimated to be 8.5% [1]. Despite not meeting the criteria for diabetes, individuals with prediabetes may still have concomitant damage in vital organs, including the eyes, nerve fibers, kidneys, and blood vessels, which are traditionally considered as complications of diabetes [2]. Therefore, early detection and intervention for prediabetes may potentially contribute to the prevention of diabetic complications, leading to better health outcomes for affected individuals.
Hyperglycemia often coexists with dyslipidemia, characterized by elevated serum triglyceride (TG) and low-density lipoprotein-cholesterol (LDL-C), as well as decreased high-density lipoprotein-cholesterol (HDL-C). Furthermore, dyslipidemia often precedes the manifestation of hyperglycemia, as demonstrated by epidemiological cohort studies, which have identified higher TG and lower HDL-C as independent risk factors for the development of type 2 diabetes mellitus (T2DM) [3, 4].
The TG/HDL-C ratio, considered an atherogenic dyslipidemia parameter, has been recently believed to be a potential surrogate marker of insulin resistance in patients with T2DM [5] and healthy subjects [6]. Moreover, the TG/HDL-C ratio has been closely associated with the occurrence and progression of T2DM [7,8,9]. A study conducted in Chinese patients with prediabetes revealed a positive correlation between the TG/HDL-C ratio and the incidence of T2DM [9]. Among nondiabetic populations, longitudinal studies in Japan (follow-up period of 6 years) [10] and Iran (follow-up period of 12 years) [11] identified the TG/HDL-C ratio as an independent risk factor for T2DM.
Prediabetes is widely recognized for its hallmark features of metabolic dysregulation and inflammatory burden increments [12]. Likewise, the TG/HDL-C ratio has established associations with chronic conditions characterized by inflammatory burdens, including hypertension [13], cardiovascular disease [14], hepatic steatosis [15], and renal dysfunction [16]. Hence, ascertaining the connection between prediabetes and the TG/HDL-C ratio holds the potential to interrupt the cascade of metabolic dysregulation and thus, mitigate the risk of severe disease.
Despite the established association between the TG/HDL-C ratio and progression to T2DM, there is a paucity of studies addressing the relation between the TG/HDL-C ratio and progression to prediabetes [11, 17,18,19]. Some studies have reported a significant association between the TG/HDL-C ratio and prediabetes [11, 17, 19], whereas others have yielded conflicting results [18]. Furthermore, some of these previous studies had relatively small sample sizes [11, 18]. Moreover, one was a cross-sectional study with a normal range of LDL-C [19], whereas a few used a longitudinal cohort study design [11, 18]. Even when the aforementioned studies were conducted as longitudinal analyses, they may contain participants who were on lipid-lowering medications (i.e., statins) [11, 17, 18], which can potentially affect glycosylated hemoglobin (HbA1c) levels in individuals with normal glucose tolerance [20].
Prediabetes is a condition with a multifaceted pathophysiology, and its association with the TG/HDL-C ratio may vary depending on factors, such as age, gender, race/ethnicity, study design, and data processing methods. Therefore, to examine this relationship, we conducted a 5-year retrospective cohort study aimed at investigating the association between serum TG/HDL-C ratio and the risk of progression to prediabetes in Japanese individuals who were not taking statins. In addition, we aimed to determine the optimal cutoff point of the TG/HDL-C ratio for predicting the progression to prediabetes and evaluated its performance as a predictive marker.
Methods
Study participants
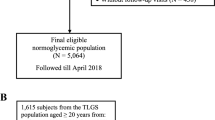
This retrospective, longitudinal cohort study was conducted in a rural city located in Nagano Prefecture, Japan. The database used for the study was obtained from the Chubu Public Health Center. A total of 35,491 Japanese individuals who underwent annual medical examinations at the center in 2017 (baseline) were analyzed and were re-evaluated 5 years later. Then, individuals with diabetes or prediabetes (n = 8,244) and/or taking lipid-lowering agents (n = 1,525) at baseline were excluded from the study. Additionally, individuals who progressed to diabetes (n = 173) and those who newly started lipid-lowering agents (n = 945) during the 5-year follow-up were also excluded. Finally, the analysis was performed on a cohort comprising 21,301 individuals with normoglycemia (12,587 men and 8,714 women) and 3,303 individuals with prediabetes (2,022 men and 1,281 women) who met the inclusion criteria for the study (Fig. 1).
Clinical and anthropometric measurements
Waist circumference (WC) was measured between the lower rib margin and iliac crest after a normal expiratory breath. The body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters. Blood pressure (BP) was measured using an automated sphygmomanometer on the right arm of the seated participants. Blood samples were obtained after an overnight fast. Serum total cholesterol (TC), TG, LDL-C, and HDL-C were analyzed enzymatically. Serum uric acid (UA), fasting plasma glucose (FPG), HbA1c, and creatinine levels were measured using standard methods (Hitachi 47 automatic analyzer, Hitachi Ltd., Japan). The estimated glomerular filtration rate (eGFR) was calculated using the formula of the Japanese Society of Nephrology according to the equation: 194 × serum creatinine− 1.094 × age− 0.287 mL/min/1.73 m2, further multiplied by 0.739 for women participants [21].
Definition criteria
In this study, prediabetes was defined as an FPG of 100–125 mg/dL and/or HbA1c of 5.7–6.4%. Diabetes was defined as FPG of ≥ 126 mg/dL, HbA1c of ≥ 6.5%, self-reported history, and/or the use of antidiabetic medications, while normoglycemia was defined as FPG of < 100 mg/dL and HbA1c of < 5.7%, according to the American Diabetes Association definitions [22]. Smoking status was considered; participants were grouped into current smokers (daily and occasional smoking) and nonsmokers (never and former smoking). For alcohol consumption, participants were classified as drinkers (who drank > 12 drinks a year least once per week) and nondrinkers (who drank ≤ 12 drinks a year) [23]. A familial history of diabetes was defined as having one or more relatives (i.e., either a parent or sibling) with diabetes. Information regarding medical history, smoking status, alcohol consumption, medications (e.g., lipid-lowering and antidiabetic medications), and familial history of diabetes were obtained via interview using a structured questionnaire.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows version 21.0 (IBM Corp., Armonk, NY, USA). Data are expressed as mean ± standard derivation or as percent frequency, unless otherwise specified. The Kolmogorov–Smirnov test was used to assess the data for normality. The baseline characteristics of the participants grouped by TG/HDL-C ratio quartiles were compared using analysis of variance, Mann–Whitney U test, and chi-square test for continuous data, skewed continuous data, and categorical data, respectively. Univariate and multivariate Cox proportional hazards regression analyses were used to determine factors related to prediabetes. After collinearity screening, significant variables from univariate analysis were included on multivariate regressions, with prediabetes as outcome. Then, with the lowest TG/HDL-C ratio quartile group (Q1) as the reference, multivariate Cox regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) between the baseline TG/HDL-C ratio and risk of progression to prediabetes. Furthermore, the receiver operating characteristic (ROC) curve was applied to determine the optimal cutoff value of TG/HDL-C ratio. The optimal threshold of TG/HDL-C ratio was obtained according to the highest Youden’s index. To determine the diagnostic efficacy in the prediction of progression to prediabetes, the area of under curve (AUC), sensitivity, and specificity were calculated. All analyses were stratified by sex. Statistical significance was set at probability (P) of < 0.05 (two-sided).
Results
Baseline characteristics of the study population by TG/HDL-C ratio quartiles
The baseline characteristics of men and women are presented in Tables 1 and 2, respectively. The study participants were classified into quartiles (Q1, Q2, Q3, and Q4) according to their baseline TG/HDL-C levels (men: <0.97, 0.97–1.49, 1.50–2.47, and > 2.47, respectively; women: <0.64, 0.64–0.89, 0.90–1.34, and > 1.34 mg/dL, respectively). There was a large disparity in the TG/HDL-C ratio quartile among men compared with women. In both sexes, as the quartile of the TG/HDL-C ratio increases, the levels of TG tend to increase and the HDL-C levels tend to decrease. Among men, age was not significantly different across the TG/HDL-C ratio quartile groups. In contrast, in women, age significantly decreased as the TG/HDL-C ratio quartile increased. In both men and women, individuals in higher TG/HDL-C ratio quartiles were more likely to have a higher BMI, WC, systolic and diastolic BPs, TC, TG, LDL-C, UA, HbA1c, and FPG levels, as well as lower HDL-C levels.
Risk factors for progression to prediabetes
In men and women, univariate Cox regression analyses indicated that the following variables at baseline were significantly and positively associated with the risk of progression to prediabetes: age, BMI, systolic and diastolic BPs, TG/HDL-C ratio, FPG, HbA1c, and familial history of diabetes (Table 3). Notably, high HRs (95% CIs) were observed in HbA1c, i.e., 1.83 (1.78–1.88) for men and 1.64 (1.59–1.69) for women. Subsequently, multivariate Cox regression analysis was conducted to determine the independent risk factors for progression to prediabetes, and the following factors were identified as independent risk factors: age, BMI, TG/HDL-C ratio, FPG, HbA1c, and familial history of diabetes. This analysis revealed that a one-unit increase in the TG/HDL-C ratio was significantly associated with an 8.5% and 9.7% increased incidence of prediabetes in men and women, respectively.
Independent association between TG/HDL-C ratio and progression to prediabetes
Table 4 presents the risk of progression to prediabetes based on the quartiles of the TG/HDL-C ratio at baseline, adjusted for various factors in different models. Model 1 was adjusted for age and BMI at baseline. Model 2 was further adjusted for FPG, HbA1c, systolic and diastolic BPs, UA, and eGFR at baseline. Model 3 was further adjusted for smoking and alcohol drinking at baseline and familial history of diabetes. In both men and women, the HRs (95% CIs) in model 3 showed a positive and independent association between the TG/HDL-C ratio and the risk of progression to prediabetes. Compared with individuals in the lowest quartile (Q1) of the TG/HDL-C ratio, the HRs in the Q3 and Q4 were 1.26 (1.01–1.56) and 1.77 (1.41–2.23) in men and 1.19 (1.08–1.29) and 1.58 (1.18–2.31) in women, respectively.
The predictive value of TG/HDL-C ratio for progression to prediabetes
The ROC curve analysis demonstrated a significant association between the TG/HDL-C ratio and progression to prediabetes (Fig. 2). The optimal cutoff values of the TG/HDL-C ratio for predicting prediabetes were found to be 1.71 for men and 0.79 for women. However, the sensitivity and specificity in the optimal cutoff values were less than 0.6 in both sexes, and the model yielded low performance (AUC < 0.7) for the discrimination of prediabetes, with AUCs of 0.582 and 0.606 in men and women, respectively.
Discussion
This retrospective, longitudinal cohort study conducted in a Japanese population demonstrated that high TG/HDL-C ratio is a significant risk factor for progression to prediabetes from normoglycemia (i.e., normal FPG and normal HbA1c), even after adjusting for potential confounding factors. Our findings are consistent with previous cohort studies indicating a positive relationship between the TG/HDL-C ratio and the incidence of prediabetes [11, 18]. Notably, this study is novel as it was conducted among participants who were not taking lipid-lowering medications during the follow-up period, enhancing the reliability of our results. In addition, we conducted a sex-stratified analysis, offering novel insights and identifying the optimal cutoff point of the TG/HDL-C ratio for predicting the progression to prediabetes.
In a large cross-sectional study of 153,163 non-obese Chinese individuals with normal LDL-C revels, the TG/HDL-C ratio was positively associated with the incidence of prediabetes after adjusting for covariates (odds ratio = 1.185, 95%CI 1.145–1.226) [17]. In a retrospective cohort study conducted in the Chinese rural population, a one-unit increase in TG/HDL-C ratio was associated with a 4% increased risk of prediabetes during a 3.1-years follow-up [18]. Similarly, in a 11-year follow-up study in a Tehranian urban population, a one-unit increase in TG/HDL-C ratio was significantly associated with a 2% increased incidence of prediabetes, and the significance persisted even after adjusting for insulin resistance [11]. However, it’s important to note that these studies measured only FPG for defining prediabetes and did not include measurements of HbA1c, which is another criterion for diagnosing prediabetes [11, 17, 18]. In the current study, we employed both HbA1c and FPG to define prediabetes. The results showed that a one-unit increase in TG/HDL-C ratio corresponded to an 8.5% and 9.7% increased risk of prediabetes in men and women, respectively; this had relatively high HRs compared with previous reports. This difference in HRs may be attributed to variations in the definition of prediabetes used in different studies. Furthermore, there are notable heterogeneities between previous studies and our current study, including differences in (1) follow-up duration, (2) analysis based on the entire population versus sex-stratified analysis, (3) level of adjustment for confounding factors, (4) age of the study population, and (5) race/ethnicity.
Regarding the cutoff value of TG/HDL-C ratio for the diagnosis of prediabetes, limited data was available. A cross-sectional study of 2680 Chinese participants found an optimal cutoff TG/HDL-C ratio of 1.13, with an acceptable diagnostic performance and high AUC (95% CI); 0.796 (0.777–0.816). The current longitudinal study indicated that the optimal cutoff points of TG/HDL-C ratio were 1.71 for men and 0.97 for women. However, the AUC (95% CI) values were relatively small; 0.582 (0.569–0.596) for men and 0.606 (0.590–0.623) for women. Additionally, and the sensitivity and specificity in the cutoff values were less than 0.6 in both sexes. Similar to our results, another cross-sectional study of 7667 Chinese participants reported that the cutoff point was 1.00 with a small AUC (95% CI) of 0.590 (0.579–0.602) [24]. While the TG/HDL-C ratio showed a significant association with the risk of progression to prediabetes, its utility as a stand-alone marker for prediction of progression to prediabetes appears to be limited.
Our findings revealed an inverse relationship between the TG/HDL-C ratio and women’s age. There have been limited reports on the association between the TG/HDL-C ratio and age. In a previous study involving healthy Chinese individuals in their forties, no significant age differences were observed among quartile groups in men [25], which aligns with our observations. In contrast, the above mentioned study reported a positive correlation between TG/HDL quartile and the age in women [25], a finding that contradicts our results. While the exact cause of these discrepancies remains unclear, they could be attributed to differences in study populations, geographic locations, lifestyles, or genetic factors. Because aging is a major risk factor in the development of prediabetes, it’s noteworthy that even among younger women, a higher TG/HDL ratio is associated with an increased risk of prediabetes in Japanese women.
There are several pathophysiologic mechanisms that could potentially explain the link between an elevated TG/HDL-C ratio and progression to prediabetes. Serum HDL-C is involved in regulating the function of pancreatic β-cells and may provide direct β-cell protection against apoptosis induced by cytokines or glucose [26]. Conversely, elevated TG levels have been shown to affect pancreatic β-cell function and survival through the inflammatory free fatty acid metabolic pathway. Additionally, serum TG regulates the expression of genes that play a crucial role in insulin secretion and insulin signaling pathways [27]. A prospective observational study of 1016 individuals without diabetes revealed that decreased HDL-C and increased TG levels may contribute to sustain the abnormalities in insulin secretion [28]. Similarly, we have previously reported a negative correlation between the insulinogenic index, a measure of early-phase insulin secretion in response to glucose load, and both TG levels and the TG/HDL-C ratio in a Japanese population [29]. Therefore, elevated TG/HDL-C ratio directly leads to β-cell dysfunction, thereby impairing glucose tolerance and affecting insulin secretion.
This cohort study has several strengths. First, this was a relatively large-scale study with a community-based population. Second, none of the participants used lipid-lowering drugs during follow-up period. Third, although the accuracy of prediction for the progression to prediabetes was limited, a sex-specific cutoff point for TG/HDL-C was identified. However, this study also has some limitations. First, as it was a retrospective observational study, the results only suggest an association rather than establishing a causal relationship between the TG/HDL-C ratio and progression to prediabetes. Second, the serum sample variables were measured only once, but in reality, TG and HDL-C can fluctuate due to diet and physical activity. Finally, the study population consisted only of Japanese patients; thus, the results may not be generalizable to other populations.
In conclusion, the TG/HDL-C ratio may be independently and positively associated with the risk of progression to prediabetes in the Japanese population. Participants with elevated TG/HDL-C ratios should be considered more predisposed to prediabetes. Therefore, clinicians can use the TG/HDL-C ratio, along with other traditional risk factors, to conduct more individualized risk assessments. This can help identify patients who may benefit from closer monitoring and early intervention. Furthermore, our results support the connection between TG/HDL-C ratio and progression of glucose intolerance, which can provide a reference for the management of dyslipidemia. Thus, aggressive interventions for lowering TG and/or increasing HDL-C levels may contribute to reduce progression to prediabetes.
Abbreviations
- TG:
-
triglyceride
- HDL-C:
-
high-density lipoprotein-cholesterol
- AUC:
-
area of under the curve
References
Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, et al. HbA1c 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to Diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378:147–55. https://doi.org/10.1016/S0140-6736(11)60472-8.
Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77. https://doi.org/10.1146/annurev-publhealth-090419-102644.
Abbasi A, Corpeleijn E, Gansevoort RT, Gans RO, Hillege HL, Stolk RP, Navis G, Bakker SJ, Dullaart RP. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 Diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab. 2013;98:E1352–9. https://doi.org/10.1210/jc.2013-1680.
Beshara A, Cohen E, Goldberg E, Lilos P, Garty M, Krause I. Triglyceride levels and risk of type 2 Diabetes Mellitus: a longitudinal large study. J Investig Med. 2016;64:383–7. https://doi.org/10.1136/jim-2015-000025.
Lin D, Qi Y, Huang C, Wu M, Wang C, Li F, Yang C, Yan L, Ren M, Sun K. Associations of lipid parameters with insulin resistance and Diabetes: a population-based study. Clin Nutr. 2018;37:1423–9. https://doi.org/10.1016/j.clnu.2017.06.018.
Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism. 2010;59:299–304. https://doi.org/10.1016/j.metabol.2009.07.027.
Hadaegh F, Hatami M, Tohidi M, Sarbakhsh P, Saadat N, Azizi F. Lipid ratios and appropriate cut off values for prediction of Diabetes: a cohort of Iranian men and women. Lipids Health Dis. 2010;9:85. https://doi.org/10.1186/1476-511X-9-85.
Lim TK, Lee HS, Lee YJ. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 Diabetes in community dwelling adults: a longitudinal 12-year analysis of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. 2020;163:108150. https://doi.org/10.1016/j.diabres.2020.108150.
Sun Y, Wang Z, Huang Z, Hu H, Han Y. The association between the triglyceride-to-high-density lipoprotein cholesterol ratio and the risk of progression to Diabetes from prediabetes: a 5-year cohort study in Chinese adults. Front Endocrinol (Lausanne). 2022;13:947157. https://doi.org/10.3389/fendo.2022.947157.
Sheng G, Kuang M, Yang R, Zhong Y, Zhang S, Zou Y. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of Diabetes in a non-diabetic population. J Transl Med. 2022;20:266. https://doi.org/10.1186/s12967-022-03470-z.
Tohidi M, Asgari S, Chary A, Safiee S, Azizi F, Hadaegh F. Association of triglycerides to high-density lipoprotein cholesterol ratio to identify future prediabetes and type 2 Diabetes Mellitus: over one-decade follow-up in the Iranian population. Diabetol Metab Syndr. 2023;15:13. https://doi.org/10.1186/s13098-023-00988-0.
Vandercappellen EJ, Koster A, Savelberg H, Eussen S, Dagnelie PC, Schaper NC, Schram MT, van der Kallen CJ, van Greevenbroek MM, Wesselius A, Schalkwijk CG. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low-grade inflammation-relevance for (pre)Diabetes: the Maastricht Study. Diabetologia. 2022;65:777–89. https://doi.org/10.1007/s00125-022-05651-3.
Yi Q, Hu H, Zeng Q. Association of triglycerides to high density lipoprotein cholesterol ratio with Hypertension in Chinese adults: a cross-sectional study. Clin Exp Hypertens. 2023;45:2195996. https://doi.org/10.1080/10641963.2023.2195996.
Che B, Zhong C, Zhang R, Pu L, Zhao T, Zhang Y, Han L. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential Cardiovascular Disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol. 2023;22:1–11. https://doi.org/10.1186/s12933-023-01762-2.
Lu S, Kuang M, Yue J, Hu C, Sheng G, Zou Y. Utility of traditional and non-traditional lipid indicators in the diagnosis of nonalcoholic fatty Liver Disease in a Japanese population. Lipids Health Dis. 2022;21:95. https://doi.org/10.1186/s12944-022-01712-z.
Lv S, Zhang H, Chen J, Shen Z, Zhu C, Gu Y, Yu X, Zhang D, Wang Y, Ding X, Zhang X. The effect of triglycerides to high-density lipoprotein cholesterol ratio on the reduction of renal function: findings from China health and retirement longitudinal study (CHARLS). Lipids Health Dis. 2021;20:1–9. https://doi.org/10.1186/s12944-021-01542-5.
Gong R, Liu Y, Luo G, Liu W, Jin Z, Xu Z, Li Z, Yang L, Wei X. Associations of TG/HDL ratio with the risk of prediabetes and Diabetes in Chinese adults: a Chinese population cohort study based on open data. Int J Endocrinol. 2021;2021:9949579. https://doi.org/10.1155/2021/9949579.
Li X, Xue Y, Dang Y, Liu W, Wang Q, Zhao Y, Zhang Y. Association of non-insulin-based insulin resistance indices with risk of incident prediabetes and Diabetes in a Chinese rural population: a 12-year prospective study. Diabetes Metab Syndr Obes. 2022;15:3809–19. https://doi.org/10.2147/DMSO.S385906.
Wu L, Wu X, Hu H, Wan Q. Association between triglyceride-to-high-density lipoprotein cholesterol ratio and prediabetes: a cross-sectional study in Chinese non-obese people with a normal range of low-density lipoprotein cholesterol. J Transl Med. 2022;20:484. https://doi.org/10.1186/s12967-022-03684-1.
Alvarez-Jimenez L, Morales-Palomo F, Moreno-Cabanas A, Ortega JF, Mora-Rodriguez R. Effects of statin therapy on glycemic control and insulin resistance: a systematic review and meta-analysis. Eur J Pharmacol. 2023;947:175672. https://doi.org/10.1016/j.ejphar.2023.175672.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. https://doi.org/10.1053/j.ajkd.2008.12.034.
American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Supp 1):S15–33. https://doi.org/10.2337/dc21-S002.
Ricci C, Schutte AE, Schutte R, Smuts CM, Pieters M. Trends in alcohol consumption in relation to cause-specific and all-cause mortality in the United States: a report from the NHANES linked to the US mortality registry. Am J Clin Nutr. 2020;111:580–89. https://doi.org/10.1093/ajcn/nqaa008.
Zhou Y, Yang G, Qu C, Chen J, Qian Y, Yuan L, Mao T, Xu Y, Li X, Zhen S, Liu S. Predictive performance of lipid parameters in identifying undiagnosed Diabetes and prediabetes: a cross-sectional study in eastern China. BMC Endocr Disord. 2022;22:1–9. https://doi.org/10.1186/s12902-022-00984-x.
Wen JH, Zhong YY, Wen ZG, Kuang CQ, Liao JR, Chen LH, Wang PS, Wu YX, Ouyang CJ, Chen ZJ. Triglyceride to HDL-C ratio and increased arterial stiffness in apparently healthy individuals. Int J Clin Exp Med. 2015;8:4342–8.
Rutti S, Ehses JA, Sibler RA, Prazak R, Rohrer L, Georgopoulos S, Meier DT, Niclauss N, Berney T, Donath MY, von Eckardstein A. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 2009;150:4521–30. https://doi.org/10.1210/en.2009-0252.
Sanchez-Archidona AR, Cruciani-Guglielmacci C, Roujeau C, Wigger L, Lallement J, Denom J, Barovic M, Kassis N, Mehl F, Weitz J, Distler M. Plasma triacylglycerols are biomarkers of beta-cell function in mice and humans. Mol Metab. 2021;54:101355. https://doi.org/10.1016/j.molmet.2021.101355.
Natali A, Baldi S, Bonnet F, Petrie J, Trifiro S, Trico D, Mari A. Plasma HDL-cholesterol and triglycerides, but not LDL-cholesterol, are associated with insulin secretion in non-diabetic subjects. Metabolism. 2017;69:33–42. https://doi.org/10.1016/j.metabol.2017.01.001.
Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Impact of serum triglyceride and high density lipoprotein cholesterol levels on early-phase insulin secretion in normoglycemic and prediabetic subjects. Diabetes Metab J. 2014;38:294–301. https://doi.org/10.4093/dmj.2014.38.4.294.
Acknowledgements
The authors gratefully acknowledge Nobuhiro Tsutsui, a medical data technologist in the Department of Health Control Center, Chubu Public Health Clinic for interpretation of the study data.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
MS collected the data, did the analysis, interpreted the data, and drafted the manuscript. YM and TN participated in the interpretation of the study data and provided comments for the revision of the manuscript. All authors contributed to the article and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
This research conformed to the provisions of the Declaration of Helsinki (revised in Fortaleza, Brazil, October 2013) and was approved by the ethics committee of the Takara Clinic (approval No. 2023-002).
Consent
All participants provided informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimodaira, M., Minemura, Y. & Nakayama, T. Elevated triglyceride/high-density lipoprotein-cholesterol ratio as a risk factor for progression to prediabetes: a 5-year retrospective cohort study in Japan. J Diabetes Metab Disord 23, 655–664 (2024). https://doi.org/10.1007/s40200-023-01329-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01329-8