Abstract
Objective
Type 2 diabetes mellitus (T2DM) has emerged as one of the greatest global health challenges of twenty-first century. Visceral obesity is one of the most important determinant of insulin resistance (IR) as well as T2DM complications. Therefore this review focuses on the molecular mechanism of obesity induced inflammation, signaling pathways contributing to diabetes, as well as role of lifestyle interventions and medical therapies in the prevention and management of T2DM.
Method
Articles were searched on digital data base PubMed, Cochrane Library, and Web of Science. The key words used for search included Type 2 diabetes mellitus, obesity, insulin resistance, vascular inflammation and peripheral arterial disease.
Result
Visceral obesity is associated with chronic low grade inflammation and activation of immune systems which are involved in pathogenesis of obesity related IR and T2DM.
Conclusion
Metabolic dysregulation of adipose tissue leads to local hypoxia, misfolded/unfolded protein response and increased circulating free fatty acids, which in turn initiate inflammatory signaling cascades in the population of infiltrating cells. Mechanism that relates the role of adipocytokines with insulin sensitivity and glucose homeostasis might throw a light on the development of therapeutic interventions and subsequently might result in the reduction of vascular complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a clinical syndrome, characterized by hyperglycemia, polyuria, and polydipsia. It is due to absolute or relative deficiency of insulin or both. On the basis of pathogenesis there are four classes of diabetes mellitus namely type1diabetes, type 2 diabetes, gestational diabetes, and other specific types [1]. Insulin resistance and abnormal insulin secretion are the two most important factors that are responsible for the development of type 2 diabetes. In T2DM beta cells of pancreas are able to produce insulin in excess but insulin is not able to carry out its function [2]. Even though the prevalence of both type 1 and type 2 DM are increasing worldwide, the prevalence of type 2 is expected to increase with a rapid rate in the future due to changing life style. Currently India (31.7 million) has the highest number of people with diabetes mellitus followed by China (20.8 million) and United States of America (17.7 million) [3]. According to international diabetes foundation (IDF) 415 million people worldwide have been diagnosed with diabetes. It is estimated to increase over 600 million people by 2040 [4]. Studies suggest that the developed countries represents around 87% to 91% of the diagnosed patients with type 2 diabetes and 7% to 12% with type 1 diabetes, while 1% to 3% have other types of diabetes. Reportedly, in developed countries around 87% to 91% of the diagnosed diabetic people have type 2 diabetes, 7% to 12% have type 1 diabetes while 1% to 3% have other types of diabetes [2]. There are various risk factors which are associated with the development of DM viz genetic susceptibility, age, ethnic background, HDL cholesterol level ≤ 35 mg/dl or triglycerides level ≥ 250 mg/dL. Besides this, variety of life style factors like physical inactivity, sedentary lifestyle, smoking, and alcohol consumption are also associated with the development of T2DM [5]. Studies have shown that overweight or obesity is the most important modifiable risk factor for the progression of T2DM [6]. According to the World Health Organization, 2011 about 90% of the diabetic patients develop T2DM mostly relating to the overweight.
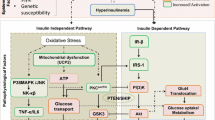
Impaired insulin secretion, peripheral insulin resistance, and excessive hepatic gluconeogenesis are the three pathophysiological abnormalities that characterize T2DM. To carry out the function properly every cell within the human body, requires a regular source of energy and glucose is the primary source of energy, which circulates in the blood as a mobilizable fuel source for cells [7], and the regulation of the blood glucose level is done by insulin [1].The elevated levels of blood glucose stimulate the secretion of insulin by the pancreatic beta cells. Insulin receptors are found primarily on hepatic cells, muscles, and adipocytes. The binding of insulin to its receptor triggers a conformational change that leads to the autophosphorylation of tyrosine residue in the carboxy- terminal domain of the receptor. The phosphorylated insulin receptor in turn phosphorylates insulin receptor substrates (IRS) on tyrosine residue. Phosphorylated IRS molecule act as docking site for enzyme PI-3 kinase. PI-3 kinase then phosphorylates PI 3, 4 bisphosphate (PIP2) leading to the formation of PI3, 4, 5- trisphosphate (PIP3) [8]. Binding of PIP3 with PKB leads to activation of PKB [9]. The activated PKB stimulates recruitment of glucose transporter (GLUT4) from internal membrane vesicles to the plasma membrane, causing rapid uptake of glucose from bloodstream to the peripheral tissues [10, 12]. In T2DM, these signaling pathways may get dysfunctional leading to insulin resistance, so even in presence of insulin there is decreased peripheral uptake of glucose which may lead to hyperglycemia. High glucose level causes further release of insulin from beta cells of pancreas by feedback mechanism which may lead to compensatory hyperinsulinemia. Insulin hypersecretion is followed by beta cells exhaustion resulting in beta cells failure and in long term T2DM is associated with reduced insulin secretion along with insulin resistance.
Obesity is characterized by increased fat deposition in adipocytes which may induce chronic low grade inflammation of adipocytes and other cells. This chronic low grade inflammation plays an important role in pathogenesis of T2DM. In addition many studies have showed that increased free fatty acids in obesity can induce ER stress in many cells, including adipocytes. Increased ER stress has been observed in the subcutaneous and visceral adipose tissues of the patients who are either overweight or obese. The ER stress markers have also been shown to be upregulated in the obese diabetic mice. In this review we have discussed how obesity contributes to development of T2DM and how altered AT macrophage phenotype impact on inflammation and trigger IR. We also discussed the complications that arise due to diabetes such as peripheral artery disease (PAD) and its pathophysiology. Further the role of lifestyle modifications and medical therapies in the management of diabetes has been discussed.
Obesity: risk factor for diabetes
Obese subjects show a high risk of developing T2DM because 80–90% of T2DM patients are either overweight or obese [6]. The term “diabesity “has been coined considering the role of obesity in pathogenesis of T2DM [13]. Prevalence of obesity particularly abdominal obesity has been on rise in subsequent years. Prevalence of both forms of obesity (generalized as well as abdominal obesity) is higher in urban population compared to rural population [14]. According to WHO in 2016, more than 1.9 billion adults aged 18 years and older were overweight and over 650 million adults among these were obese. In 2016, 39% of the adults aged 18 years and over (39% men and 40% of women) were overweight.Overall about 13% of the world´s adult population (11% of men and 15% of women) were obese in 2016. The prevalence of overweight and obesity among children and adolescents aged 5–19 years has risen dramatically from just 4% in 1975 to over 18% in 2016. This rise has been observed similarly among both boys and girls: in 2016, 18% of girls and 19% of boys were overweight [15]. Globally there are more obese people than underweight irrespective of region except some parts of sub-Saharan Africa and Asia. According to WHO the BMI cutoff for obesity is 25 kg/m2, while in Indian population there is revision of BMI criteria i.e. it has been fixed to 23 kg/m2[16].
Obesity induced changes in adipose tissue microenvironment:
Obesity is the accumulation of abnormal or excessive fat that may interfere with maintenance of an optimal state of health. It is the result of chronic imbalance between energy intake and energy expenditure [17]. There are two distinct types of adipose tissue, brown adipose tissue (BAT) and white adipose tissue (WAT). BAT mainly specialized in heat production, earlier it was thought, in humans, soon after birth BAT disappear, but now there are evidences to suggest that BAT is present in adult humans in the supraclavical and paraspinal regions [18]. BAT level and activity are inversely correlated with BMI, and loss of BAT activity may be associated with deposition of WAT [19,20,21]. Lipid storage is the primary functions of WAT. Depending on its anatomical location, WAT is subdivided into two categories: Subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). All fat depots are not equally deleterious for health, like subcutaneous adipose tissue (SAT) may not be a risk factor for metabolic disorder, while visceral adipose tissue (VAT) and ectopic fat deposition in or around the heart, muscles or liver is related to insulin resistance, impaired glucose homeostasis and vascular complications [22,23,24]. Visceral adiposity is strongly correlated with metabolic abnormalities and vascular diseases than subcutaneous adipose tissue [25,26,27]. Normal weight individuals with increased level of abdominal obesity could genetically predispose to the development of DM as well as vascular diseases [28]. Vascular complications are particularly common with obesity, but there are group of individuals who are phenotypically obese but may not be at an increased risk for vascular complications, are said to have metabolically healthy obese (MHO) [29, 30]. On the other side metabolically unhealthy obese individuals (MUO) are at increased risk of vascular complications irrespective of BMI, this group may include individuals within normal weight category with a BMI of 18.5- 24.9 kg/m2 [31, 32]. MHO individuals have less VAT and more SAT and low fat deposition in peripheral tissues as compared to MUO individuals [33,34,35]. During energy excess, free fatty acids (FFA) are stored in the form of triglycerides (TG) and during the nutrients deprivation, these stored fat are released from TG by the process of lipolysis [36]. The enzymatic control of rate limiting step for TG mobilization is hormonally regulated [37] and insulin is the main hormone which is involved in this regulatory process [38]. In case of obesity and insulin resistance, the adipocytes sensitivity to insulin is lost that results in increased FFA [39, 40], which is a characteristics feature of type 2 DM.
Adipose tissue stays in dynamic state, in both physiological as well as in pathological conditions. WAT has ability to change its characteristics, which directly influences its functionality [41]. The hyperplasia and hypertrophy of adipocytes causes WAT to expand which may lead to obesity [42]. In humans adipocyte size is positively correlated with glucose intolerance and hyper insulinemia [43]. During the advancement from normal weight to obese and then to overt diabetes, adipocytes derived factors contribute to the occurrence and development of β cell dysfunction and type 2diabetes [44]. Macrophages are the innate immune cells, constitute 5 to 10% of stromal cells of lean steady state adipose tissue, regulating tissue homeostasis. During nutritional overload because of increased FFA, the numbers of macrophages increase and reach upto < 40–50% of stromal cells in human and mouse [45]. On the basis of functions there are two subtypes of macrophages, namely M1 macrophages and M2 macrophages. Normal weight and MHO tissues are rich in M2 macrophages that are anti-inflammatory in nature and known to secrete anti – inflammatory cytokines such as IL-10, IL-4, IL-13, and IL-33 which assist in regulating physiological processes, and controlling insulin sensitivity. Obese tissues are rich in M1 macrophages which are induced by proinflammatory factors, and secrete inflammatory cytokines such as IL- 6, TNF-α, IL-1β, IL-12, IL-23, and monocyte chemoattractant protein 1 (MCP-1) [46] (Fig. 1). In a study no macrophage accumulation and inflammatory response has been observed in mice, deficient in MCP-1 and MCP-I receptor CCR-2, and also these mice were found to have resistance against diet induced insulin resistance [47]. For rapid ATP formation M1 macrophages use the glycolysis pathway whereas M2 macrophages use oxidative phosphorylation. Furthermore M1 macrophages also utilize oxidative pentose phosphate pathway for ATP as well as NADPH generation, leading to ROS generation and glutathione reduction [48]. These macrophages can be distinguished on the basis of the expression of their surface markers. F4/80 + CD206 + CD301 + CD11c- expressed by M2 macrophages while M1 macrophages express F4/80 + CD11c + [47].
The M1 macrophages activation, stimulated by lipopolysaccharides and interferon gamma (IFN-γ), that is largely expressed in T helper-1 and CD8 T cells. In the AT of obese individuals increased amount of these cells were observed [46, 48]. Rupture of hypertrophied adipocytes leads to release of FFA, which in turn interacts with Toll like receptor ( TLR) 4 and 2, and activate proinflammatory response and increased accumulation of local inflammatory cells [5, 49]). This condition leads to the release of various active molecules by adipocytes (Table 1). The interactions of FFA with TLR-2 stimulate the cyclo-oxygenase 2 and iNOS expression by macrophages. According to a study inflammatory response and obesity induced insulin resistance was prevented in TLR-4 and TLR-2 knockout mice [45]. Increased expression of inflammatory mediators has also been observed in visceral fat of obese individuals. Proteomic analysis showed mitochondrial impairment and decreased adipocyte differentiation in the visceral adipose tissue of metabolically unhealthy obese T2DM patients, which augment the M1 macrophages [49, 52]. Increased M1 macrophages also, cause the release of nitric oxide which may lead to the AT fibrosis by mitochondrial dysfunction of preadipocytes and excess collagen deposition [53]. Compared to healthy lean subjects, the adipose matrix metalloprotease-9 expression was found to be increased in metabolically unhealthy obese subjects [54, 55]. In obese individuals the increased AT MMP-9 positively correlates with homeostatis model assessment for insulin resistance (HOMA-IR) [54]. Also the ATM produces a metalloelastase MMP-12 and its activation leads to lipolysis [53]. Individuals having poor glycemic control, showed excess AT accumulation of collagen I, III, IV, VI [49, 56]. With proinflammatory accumulation and increased visceral fat mass collagen VI gene expression was found to be increased [57, 58].
Pathway linking obesity induced inflammation and insulin resistance:
The link between the obesity and diabetes has been attributed to the inflammatory pathways [78]. In obesity, due to adipocyte hypertrophy and excess release of free fatty acids (FFA), increased ROS generation has been observed in AT of mice. This excess FFA further deteriorate the local adipose tissue inflammation, also the constant demand for lipid processing and storage leads to organellar stress, with maximum stress to endoplasmic reticulum (ER) [79,80,81]. In addition, the poor oxygenation of AT in obesity causes AT hypoxia, which also results in ER stress. ER stress is accompanied by accumulation of unfolded and misfolded proteins which lead to activation of specific cellular process called unfolded protein response (UPR) [82]. Transmembrane proteins namely inositol requiring enzyme 1(IRE1), PKR – like ER kinase (PERK), and activating transcription factor 6 (ATF6) are involved in mediating UPR. These proteins remain inactive by binding with ER chaperons at basal state. During stress IRE1 becomes active and interacts with tumor necrosis factor receptor-associated factor 2 (TRAF2) which leads to activation of c-jun N-termnal kinase (JNK) and nuclear factor kappa β (NF-Kβ) [83]. It disrupts the insulin signaling by phosphorylating IRS1 on serine residue. JNK phosphorylates S307 in murine IRS1 and S312 in human IRS1. In a study JNK activation are reported to increase in AT of patients with obesity and T2DM [75]. In absence of any stimulus inhibitory protein named inhibitor of Kβ (IKβ) remain associated with NF-Kβ inside the cytosol. In the presence of proinflammatory stimulus, IKβ is phosphorylated at two serine residue by inhibitory kinase Kβ (IKKβ). After phosphorylation IKβ is rapidly degraded by proteasomes, releasing NF-Kβ for translocation to the nucleus where it activates transcription of target gene; this will lead to increased production of various cytokines including IL-6 and decrease in adiponectin [84] (Table 2). The increased production of these inflammatory cytokines leads to chronic low grade inflammation (Fig. 2). According to a study in a mouse model of obesity deletion of IKβ or neutralization of TNF-α [88] or IL-1β[89], leads to improvement in insulin sensitivity and glucose tolerance. Chronic inflammation decreases the lipid storage capacity of the adipose tissue by inhibiting preadipocytes differentiation and increases lipolysis. Increased FFA reduces the IRS-1 and PI3-K-AKT expression in the liver as well as in skeletal muscles which in turn causes IR in liver and muscles. In a study it was observed that the FFA induced expression of IL-6 and TNF-α was prevented in adipocytes by inhibition of IKKβ [50].
Obesity and environmental factors such as viruses and pathogens cause ER stress, which lead to activation of NF-kB, which in turn initiates a cascade of signaling activities, which leads to chronic low grade inflammation, and development of insulin resistance, eventually resulting in type 2 diabetes mellitus
Every type 2 diabetic patient does not show similar IR and vascular risk factor profile. There is significantly decreased insulin stimulated glucose disposal and insulin sensitivity index in obese type 2 diabetic, confirming that IR is the major contributor to the pathogenesis of hyperglycemia in obese diabetic, whereas normal weight type 2 diabetic are characterized primarily by defect in insulin secretion [90, 91].
Complications of diabetes mellitus:
Chronic hyperglycemia that arises from DM causes long term damage, dysfunction and failure of various organs, mainly the eyes, kidneys, nerves, heart and blood vessels [92]. This high accumulation of glucose inside the bloodstream not only damage the cells, but also leads to the several other complications, such as development of atherosclerosis, where artery gets narrowed and blocked due to deposition of fat inside it, which further increase the risk of MI or stroke. Peripheral artery disease (PAD) is also one of the major atherosclerotic complications of diabetes.
Diabetes mellitus greatly increases the risk of PAD, as well as it accelerates its course [93]. Patient with PAD therefore have an increased risk of MI, stroke and death [94]. It is a risk factors for foot ulceration and amputation [95] and increases the risk of coronary artery disease as well [96].The prevalence of PAD is estimated to be 10%- 25% in people aged ≥ 55 years [97]. After coronary artery disease and stroke, PAD is the third most common clinical manifestation of the atherosclerosis [98]. Diabetes is not only qualitative risk factors, but also a quantitative risk factor, as each 1% increase in glycosylated hemoglobin is associated with a 25% increase in the risk for PAD [99].
According to Beckman et al. glucose intolerance is associated with a more than 20% prevalence of an abnormal ankle—brachial pressure index (ABI) relative to 7% in those with normal glucose tolerance [100]. Atherosclerosis induced chronic limb ischemia (CLI) has been associated with a mortality rate of 20%- 25% in the first year after presentation and a survival rate of less than 30% at five years [97]. In year 2015, in individuals aged 25 years and older the global prevalence of PAD was 5.56% [101].
Pathophysiology of PAD in diabetes mellitus:
PAD is characterized by reduction of blood flow to the lower limb [102, 103]. It can be asymptomatic or symptomatic like intermittent claudication, a typical leg pain and critical limb ischemia [104,105,106]. Hypertension, smoking, age, obesity are important risk factors for the PAD [107]. Hyperglycemia, insulin resistance, and dyslipidemia are mainly responsible for the progression of PAD in diabetic patients [108,109,110]. Prolonged hyperglycemia favors atherosclerosis and thrombosis by increasing platelet activity and modifications in the vascular endothelium and coagulating factors [111,112,113,114].
The concentrations of C – reactive protein (CRP), an acute phase protein synthesize by liver increases in response to inflammation and it is strongly associated with the progression of PAD [115]. It has been reported that in patients with impaired glucose tolerance or diabetes there is increased plasma concentration of CRP [116]. Endothelial cell nitric oxide synthase (eNOS) is inhibited by CRP that results in the abnormalities in the regulation of vascular tone [117]. CRP also augments the release of plasminogen activator inhibitor (PAI)-1, that vitiates fibrinolysis [96]. Oxidative stress increases vascular endothelial cells permeability and promote leukocyte adhesion, these molecules cause internalization of monocyte,that change into macrophages, which stimulate the production of CRP by releasing IL-6, which further lead to reduction of nitric oxide (NO) [114]. NO is a signaling molecule that diffuses from endothelial cells to neighboring smooth muscles cells in the wall of the blood vessels, where it interact with iron bound to the active site of the enzyme guanylyl cyclase (NO receptor), resulting in the synthesis of cGMP, which leads to blood vessel dilation and relaxation of smooth muscles cells. NO also inhibits the aggregation of platelets within the vessels and prevents thrombotic events [96]. The elevated levels of CRP inhibits the NO mediated vasodilatation as well as enhances the platelet aggregation and therefore results in thrombotic events (Fig. 3).
Pathophysiology of peripheral artery disease in type 2 diabetes mellitus. CRP: c reactive protein, IL-6: Interleukin-6, TNF-α: Tumor necrosis factor alpha, ROS: Reactive oxygen species, PAI-1: Plasminogen activator inhibitor – 1, eNOS: Endothelial nitric oxide synthase, NO: Nitric oxide, PAD: Peripheral artery disease
In comparison to non diabetic individuals severity and prevalence of PAD is higher in diabetic individuals [118,119,120,121]. According to a study obese diabetic patients had higher chances of vascular events, as well as hypertension and target organ lesions than normal weight diabetic patients [122]. There are some studies according to which there is no any association between BMI and PAD [123, 124], while other studies have shown that a high BMI [125, 126] or low BMI [127, 128] are associated with PAD. According to Tseng et al. lower BMI (≤ 23 kg/m2) is a major risk factor for PAD [128]. In a study researcher noted a J- shaped association of BMI with prevalent PAD. This relationship was most robust in women with only a slight association of increasing BMI and PAD in men. Underweight and normal BMI men exhibited a higher prevalence of PAD than overweight and obese men, with a positive association of prevalent PAD with increasing BMI evident only in severe class III obesity (BMI ≥ 40 kg/m2) [129].
There are various invasive and non- invasive methods for the assessment of PAD substantiated with clinical findings. Ankle brachial pressure index (ABPI) is a non- invasive test for detection of earlier occurrence of PAD. It claims 98% overall accuracy (sensitivity 97%, accuracy 100%) against angiography using an ABPI threshold of 1.0 as being normal. The most important utility of ABPI is at primary health care setting for the early detection and cost effective treatment of PAD. The ABPI is used to diagnose patients for PAD as a fall in blood pressure in an artery at the ankle relative to the central blood pressure would suggest a stenosis in the arterial conduits somewhere between the aorta and the ankle [130]. It is calculated as-ABPI = Ankle systolic blood pressure/ Brachial systolic blood pressure.
Peripheral artery disease and risk of cardiovascular events and mortality:
Clinical studies describing PAD is a strong prognostic marker of future cardiovascular events. In a study conducted in our lab we found there was an inverse correlation between anthropometric parameters and obesity indices in healthy obese subjects [131]. In a prospective cohort study cardiovascular events were recorded in 1049 subjects divided into four groups: 558 with neither diabetes nor PAD, 192 with PAD but without T2DM, 153 with T2DM but without PAD, and 146 with T2DM and PAD. It was observed that the cardiovascular event rate was lowest in subjects with neither PAD nor T2DM. Non diabetic PAD patients were at higher cardiovascular risk than T2DM patients without PAD [132]. Furthermore, a total 289 diabetic subjects, 27 of whom had already been diagnosed with PAD, and remaining 262 asymptomatic patients without Known PAD were followed up for 10 years. In patients with abnormal ABI the cardiovascular mortality was higher (p < 0.05). A significantly higher survival was observed among patients with normal baseline ABI as compared to patients with an abnormal baseline ABI (P < 0.001) [133]. In a community based study, in White and African American men and women (N = 12,186) of age range 45–65 years ABI was measured and were followed up for median of 13.1 years. It was observed that risk of coronary heart disease increased exponentially with decreasing ABI in African American, White men and women [134].
The prevalence of coronary artery disease varies from 10.5% to 71% among PAD patients while it is 5.3% to 45.4% among subjects without PAD. Decreased ABI is also associated with prevalent cerebrovascular disease with odd ratios in the range of 1.3 to 4.2 [135]. These findings clearly point out that PAD is strongly associated with cardiovascular and cerebrovascular disease which contributes to a high risk of morbidity and mortality.
Management of diabetes:
Lifestyle modifications:
Most of the people with diabetes are either overweight or obese, therefore weight reduction is seen as the key therapeutic goal in the prevention and management of type 2 diabetes. Lifestyle modification is the first line intervention for weight loss [136,137,138,139,140]. Reduction in weight due to physical activity and decreased calorie intake or both, involve a significant decrease in VAT in obese individuals [141,142,143]. Evidences suggest that with regular exercise person can reduce the risk of cardiovascular disease and also improve the whole body blood glucose levels [144]. Reduced mortality risk was found in physically active individuals with type 2 DM as compared to physically inactive groups. According to studies, intra-abdominal fat mass significantly increase after reduction in daily foot-steps from 10,000 to 1500 even without a change in total fat mass [145]. Physical exercise helps to control risk factors for vascular complications [146]. Mechanistically, lifestyle might modulate whole body energy metabolism, as evidences suggest that during intake of high calorie diet concurrent physical activity increases fatty acid oxidation [42]. Various forms of physical activities like aerobic exercises (swimming, bicycling, walking or jogging), resistance exercises (push – ups), and flexibility exercises can help individuals to improve their metabolic status as well as prevention of chronic diseases. In a study cardiorespiratory fitness was found to be increased in both type 1 and type 2DM by aerobic exercise training [147]. Aerobic exercise can increase the heart rate upto 65%—90% of maximum, known to be associated with substantial reduction in cardiovascular mortality in T2DM patients [148]. In a meta- analysis HbA1c level was greatly reduced in subjects performing aerobic exercise with greater intensity compared to those with lower intensity [149]. Overweight T2DM treated with 6 month aerobic exercise programme, it was observed that compared with baseline and control group, patients treated with exercise have improved glycemic control, decreased insulin resistance and blood pressure, with reduced level of CRP and IL-18 [150]. A significant reduction in plasma P-selectin and ICAM-1 levels as well as increased plasma HDL levels were observed in sedentary older T2 DM participated in aerobic exercise [151]. Resistance training also has beneficial effect on glycemic control also it increases muscular strength. Patients with peripheral neuropathy or severely obese for whom aerobic exercise may be physically challenging, resistance training may be helpful. Erikson et al. observed that improvement in glycemic control was strongly correlated with muscles size after 3 month of resistance training programme in type 2 DM patients [152]. The effects of resistance exercise and aerobic exercise on glycemic control are additive as 40% reduction in HbA1c level was observed in the combined resistance and aerobic exercise group in comparison to non- exercise group [153]. Exercise also have beneficial role in improving metabolic homeostasis of adipose tissue. Exercise may improve metabolic function and cellularity of adipose tissue by promoting lipolysis and decreasing lipogenic gene expression in AT. According to study endurance exercise increases the triglycerides lipase activity in AT [154]. Exercise may also inhibit the hypoxic condition of AT by stimulating angiogenesis [155]. Exercise induced weight reduction in obese elderly men and women resulted in decreased insulin resistance, which was correlated with change in VAT [156]. Studies have suggested that exercise may improve adipocyte function by reducing inflammatory cytokines production. The increment of CD8 + T cells in AT reduces with physical exercise in obesity and thus exercise could be helpful in changing the M1 phenotype of AT to M2 phenotype [157]. In addition, cigarette smoking and alcohol consumption can also increase the risk of vascular complications in T2DM patients. Regular consumption of alcohol can interfere with blood sugar control in diabetic person [158]. According to studies risk of peripheral artery disease was double in diabetic patients who smoke than the non- smokers [159]. Therefore quitting smoking and alcohol consumption person may reduce the risk of T2DM as well as its complications.
Pharmacological therapy:
Metformin: It works by inhibiting glucose production from liver [160]. A large meta-analysis [161] supports the use of metformin monotherapy as first-line therapy. Within adipose tissues positive effect of metformin has also been observed [162]. With the use of metformin, the modest reduction in body weight [163], is associated with redistribution of fat from visceral depots to subcutaneous depots, which carry lesser cardiovascular risk [164].
Sulphonylureas: work by triggering insulin release from pancreatic β – cell [165]. Gliclazide, glipizide, glibenclamide and glimepiride are second generation sulphonylureas currently used, while first generation drugs are no longer used. It reduces glycosylated hemoglobin HbA1C level by 0.8 to 2.0% and fasting plasma glucose concentration (FPG) by 60 to 70 mg/dl, with the greatest reductions observed in patients with the high FPG concentration at the initiation of therapy [166,167,168].
Meglitinides: Like sulfonylureas increases insulin secretion from pancreatic β cells. Studies have suggested that meglitinides reduces hemoglobin A1C by 0.5% to 1.5% [169]. Alpha – glucosidase inhibitors: Acarbose and miglitol are the two drugs available in this class. It works by inhibiting the enzyme alpha—glucosidase, found on the brush border cells that line the small intestine [165].
Glucagon like peptide – 1 (GLP-1) receptor agonists: GLP-1 is a hormone that plays an important role in regulating appetite and blood sugar levels. GLP-1 receptor agonists mimic the action of this hormone. Its administration stimulates GLP-1 receptors, thereby increasing insulin secretion in response to oral and intravenous glucose to similar extent [170]. It includes exenatide and liraglutide and can reduce HbA1C level by 0.8% to 1.5% (171]. Dipeptidyl peptidase (DPP) – 4 inhibitors: DPP- 4 is a ubiquitous enzyme found in soluble form in plasma, or as a membrane component of many cells [172], including endothelial cell [173]. It inhibits the activity of incretin hormones, glucose – dependent insulinotropic polypeptide (GIP) and GLP-1. DPP-4 inhibitors can improve the activity of endogenously active GLP-1 and GIP by blocking its degradation by enzyme DPP – 4.
The biological half-life of circulating GLP-1 and GIP is very short hence the therapeutic use of native GLP-1 is very limited but DPP-4 inhibition offers a novel way to increase endogenous incretin concentration as a treatment.
Orlistat and sibutramine are the two drugs approved by Food and Drug Administration (FDA) for long term treatment of obesity. Orlistat via inhibition of lipase activity prevent the absorption of 30% dietary fat [174]. A study shows that orlistat causes weight loss of 9 to 10% compared with a 4 to 6% in placebo group [175, 176]. Sibutramine is a norepinephrine and serotonin reuptake inhibitor that acts as an appetite suppressant and increases thermogenesis [177]. It also improves lipid profile and glucose control, with a reduction of cardiovascular risk factors [178]. A clinical study demonstrated that there is significant reduction in weight and waist circumference with the use of sibutramine compared with placebo in obese type 2 diabetic patients, and it was associated with significant improvement in metabolic control such as reduction in HbA1C, fasting glucose and triglycerides levels [178].
Conclusion:
Diabetes is the fastest growing disease which affects millions of people worldwide. Behavioral changes that lead to increased body weight is a major contributing factor to the rising incidence of diabetes. Apart from genetic factor sedentary life style and unhealthy diet pattern play a major role in causation of obesity and T2DM. Adopting healthy lifestyle which includes increased physical activity and healthy diet can result in preventing diabetes. Excessive fat accumulation and subsequently might result in the reduction of vascular complications in T2DM patients. can trigger pro-inflammatory state, as well as increases the substrate load in the adipocytes which may further lead to ER stress. Suppression of ER stress may reduce the risk of obesity and its complications. Further the mechanism that can relate the role of adipocytokines with insulin sensitivity and glucose homeostasis might throw a light on the development of therapeutic interventions.
Data Availability
Data supporting the findings of the study were derived from resources which are in public domain (https://www.ncbi.nlm.nih.gov/pmc/, https://scholar.google.com/) and included with doi along with references.
References
Sicree R, Shaw J, Zimmet P. The global burden. Diabetes and impaired glucose tolerance. Prevalance and projections. International Diabetes Federation. 2006; 16–103.
Okur ME, Karantas ID, Siafaka PI. Diabetes mellitus: A review on pathophysiology, current status of oral medications and future perspectives. Acta Pharmaceutica Sciencia. 2017; Doi.https://doi.org/10.23893/1307-2080.APS.0555
Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australasian Med J. 2014;7(1):45–8.
Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: Epidemiology, perspective. Biochemica et Biophysica Acta. 2017;1863:1026–36.
Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Intl J Med Sci. 2014;11(11):1185–200.
Kumanyika S, Jeffery RW, Morabia A, Ritenbaugh C, Antipatis VJ. Public Health Approaches to the Prevention of Obesity (PHAPO) Working Group of the International Obesity Task Force (IOTF). Obesity prevention: the case for action. Int J Obes Relat Metab Disord. 2002;26(3):425–36. doi:https://doi.org/10.1038/sj.ijo.0801938.
Piero MN. Hypoglycemic effects of some Kenyan plants traditionally used in management of diabetes mellitus in eastern province, Msc thesis, Kenyatta University 2006.
Alessi DR, Cohen P. Mechanism of activation and function of protein Kinase B. Current Opin Genet Dev. 1998;8:55–62.
Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, et al. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18(12):6971–82. https://doi.org/10.1128/MCB.18.12.6971.
Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin- stimulated GLUT4 vesicle trafficking. J Bio Chem. 1999;274:2593–6.
Kupriyanova TA, Kandror KV. Akt-2 binds to Glut-4 containing vesicles and phosphorylates their component proteins in response to insulin. J Bio Chem. 1999;274:1458–64.
Martin S, Millar CA, Lyttle CT, Meerloo T, Marsh BJ, Gould GW, et al. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J Cell Sci. 2000;19:3427–38. https://doi.org/10.1242/jcs.113.19.3427.
Astrup A, Finer N. Redefining type 2 diabetes: “diabesity” or obesity dependent diabetes mellitus? Obes Rev. 2000;12:57–9.
Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, et al. Prevalance of generalized and abdominal obesity in urban and rular India- the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-3]. Indian J Med Res. 2015;142(2):139–50.
World Health Organization. Obesity and overweight. WHO. 2018.
Misra A, Chawbey P, Makkar BM, Vikram Nk, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70.
Krzysztoszek J, Wierzejska E, Zielinska A. Obesity: An analysis of epidemiological and prognostic research. Arch Med Sci. 2015;11:24–33.
Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (SilverSpring). 2011;19:1755–60.
SaitoM, OkamatsuoguraY, Matsushita M, Watanabe K, YoneshiroT, Niokobayashi J, et al. High incidence of metabolically active brown adipose effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531.
Van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. https://doi.org/10.1056/NEJMoa0808718.
Vijgen GHEJ, Bouvy ND, Teule GJJ, Brans B, Schrauwen P, Van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS ONE. 2011;6:e17247.
Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544–60. https://doi.org/10.3390/nu5051544.
Stanley WC, Recchia FA. Lipotoxicity and the development of heart failure: moving from mouse to man. Cell Metab. 2010;12:555–6.
Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–76. https://doi.org/10.1016/j.ijcard.2013.08.077.
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. 2007;116(1):39–48.
Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson heart study. J Clin Endocrinol Metab. 2010;95(12):5419–26.
Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham heart study. Circulation. 2007;116(11):1234–2124.
Lukich A, Gavish D, Shargorodsky M. Normal weight diabetic patients verses obese diabetics: Relation of overall and abdominal adiposity to vascular health. Cardiovascular Diabetology. 2014;13(141).
Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii,1–253.
Freedman DS, Horlick M, Berenson GS. A comparison of the Slaughter skinfold-thickness equations and BMI in predicting body fatness and cardiovascular disease risk factor levels in children. Am J Clin Nutr. 2013;98:1417–24.
Stefan N, Schick F, Haring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292–300.
Rubin R. What’s the best way to treat normal-weight people with metabolic abnormalities? JAMA. 2018;320(3):223–5.
Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1(2):152–62. https://doi.org/10.1016/S2213-8587(13)70062-7.
Bluher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol. 2014;171:R209–19.
Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes. 2011;35(7):971–81. https://doi.org/10.1038/ijo.2010.216.
Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809.
Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. https://doi.org/10.1146/annurev.nutr.27.061406.093734.
Large V, Arner P. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diabetes Metab. 1998;24:409–418.
Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Gen Clin Implic Diab. 1995;44:863–70.
Chen Y-DI, Golay A, Swislocki AL M, Reaven GM. Resistance to insulin suppression of plasma free fatty acid concentrations and insulin stimulation of glucose uptake in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:17–21.
Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075–88.
Iacobini Carla, Pugliese G, Fantauzzi CB, Federici M, Menini Stefano. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2018;92:51–60.
Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–506. https://doi.org/10.1007/s001250051560.
Zhao YF, Feng DD, Chen C. Contribution of adipocyte-derived factors to beta-cell dysfunction in diabetes. Int J Biochem Cell Biol. 2006;38:804–19.
Chakarov S, Blériot C, Ginhoux F. Role of adipose tissue macrophages in obesity-related disorders. J Exp Med. 2022;219(7):e20211948. doi:https://doi.org/10.1084/jem.20211948
Castoldi A, Naffah DSC, Câmara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol. 2016;6:637. https://doi.org/10.3389/fimmu.2015.00637.
Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance:cells, cytokines, and chemokines. ISRN Inflamm. 2013;139239. doi: https://doi.org/10.1155/2013/139239.
Appari M, Channon KM, McNeill E. Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes. Antioxid Redox Signal. 2018;29(3):297–312. https://doi.org/10.1089/ars.2017.7060.
Gómez-Serrano M, Camafeita E, García-Santos E, López JA, Rubio MA, Sánchez-Pernaute A, et al. Proteome-wide alterations on adipose tissue from obese patients as age-, diabetes- and gender-specific hallmarks. Sci Rep. 2016;6:25756. https://doi.org/10.1038/srep25756.
Ruggiero AD, Key CC, Kavanagh K. Adipose Tissue Macrophage Polarization in Healthy and Unhealthy Obesity. Front Nutr. 2021;8:625331. doi: https://doi.org/10.3389/fnut.2021.625331.
Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. https://doi.org/10.1016/j.mam.2012.10.001.
Brockman D, Chen X. Proteomics in the characterization of adipose dysfunction in obesity. Adipocyte. 2012;1(1):25–37. https://doi.org/10.4161/adip.19129.
Ruiz-Ojeda FJ, Méndez-Gutiérrez A, Aguilera CM, Plaza-Díaz J. Extracellular Matrix Remodeling ofAdipose Tissue in Obesity and Metabolic Diseases. Int J Mol Sci. 2019;20(19):4888. https://doi.org/10.3390/ijms20194888.
Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485–95. https://doi.org/10.1016/j.cmet.2008.04.002.
Tinahones FJ, Coín-Aragüez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J, et al. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12:4. https://doi.org/10.1186/1472-6793-12-4.
Belligoli A, Compagnin C, Sanna M, Favaretto F, Fabris R, Busetto L, et al. Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci Rep. 2019;9:11333. https://doi.org/10.1038/s41598-019-47719-y.
Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–62. https://doi.org/10.1210/jc.2009-0947.
Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–27. https://doi.org/10.1152/ajpendo.00329.2010.
Catrysse L, van Loo G. Adipose tissue macrophages and their polarization in health and obesity. Cell Immunol. 2018;330:114–9. https://doi.org/10.1016/j.cellimm.2018.03.001.
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7. https://doi.org/10.1126/science.1201475.
McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity associated metabolic disease. J Clin Invest. 2017;127(1):1–13. https://doi.org/10.1172/jci88876.
Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103(5):467–76. https://doi.org/10.1161/CIRCRESAHA.108.177105.
Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154(5):691–7. https://doi.org/10.1530/eje.1.02138.
Cinkajzlová A, Mráz M, Haluzík M. Adipose tissue immune cells in obesity, type 2 diabetes mellitus and cardiovascular diseases. J Endocrinol. 2021;252(1):R1–22. https://doi.org/10.1530/JOE-21-0159.
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberlé D, Shimada T, et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016;23(4):685–98. https://doi.org/10.1016/j.cmet.2016.03.002.
Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37(3):574–87. https://doi.org/10.1016/j.immuni.2012.06.016.
Soedono S, Cho KW. Adipose Tissue Dendritic Cells: Critical Regulators of Obesity-Induced Inflammation and Insulin Resistance. Int J Mol Sci. 2021;22(16):8666. https://doi.org/10.3390/ijms22168666.
Muir LA, Kiridena S, Griffin C, Del Proposto JB, Geletka L, Martinez-Santibañez G, et al. Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol. 2018;103:615–28.
Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–7. https://doi.org/10.1038/nm.2353.
Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes and endothelial dysfunction. Int J Mol Sci. 2017;18:1321.
Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Canadian Journal of Diabetes. 2017;1–12.
Schaffler A, Neumeier M, Herfarth H, Furst A, Scholmerich J, Buchler C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732:96–102.
Biscetti F, Nardella E, Bonadia N, Angelini F, Pitocco D, Santoliquido A, et al. Association between plasma omentin-1 level in type 2 diabetic patients and peripheral artery disease. Cardiovascular Diabetology. 2019;18(74).
Wannamethee SG, Tchernova J, Whincup P, Lowe GD, Kelly A, Rumley A, et al. Plasma leptin:associations with metabolic, inflammatory and haemostatic risk factors for cardiovascular disease. Atherosclerosis. 2007;191:418–26.
Kowalska I. Role of adipose tissue in the development of vascular complications in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2007;78:14–22.
Brema I. The relationship between plasma visfatin/Nampt and type 2 diabetes, obesity insulin resistance and cardiovascular disease. Endocrinol Metab Intl J. 2016;3(6):157–63.
Priya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216:T1–15.
Stepien H, Stepien A, Wlazet RN, Paradowski M, Banach M, Rysz J. Obesity indices and inflammatory markers in obese non-diabetics normo and hypertensive patients: A comparative pilot study. Lipids Health Dis. 2014;13(29).
Sarvottam K, Yadav RK. Obesity related inflammation & cardiovascular disease: Efficacy of a yoga- based lifestyle intervention. Indian J Med Res. 2014;139(6):822–34.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Draznin B. Molecular mechanism of insulin resistance: Serine phosphorylation of insulin receptor substrate-1 and increased expression of P85α. Diabetes. 2006;55(8):2392–7.
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90.
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8.
Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet induced insulin resistance with salicylates or targeted disruption of ikkbeta. Science. 2001;293:1673–7.
Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17.
Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine, interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–18.
Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. https://doi.org/10.1038/srep00799.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61.
Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. https://doi.org/10.2337/db08-1220.
Suraamornkul S, Kwancharoen R, Ovartlarnporn M, Rawdaree P, Bajaj M. Insulin clamp-derived measurements of insulin sensitivity and insulin secretion in lean and obese asian type 2 diabetic patients. Metab Syndr Relat Disord. 2010;8(2):113–8.
Meneilly GS, Elliott T. Metabolic alterations in middle-aged and elderly obese patients with type 2 diabetes. Diabetes Care. 1999;22:112–8.
Piero MN, Nzaro GM, Njagi JM. Diabetes mellitus- A devastating metabolic disorder. Asian Journal of Biomedical and Pharmaceutical Sciences. 2014;4(40):1–7.
Thiruvoipati T, Kielhorn CE, Armstrong EJ. Epidemiology, mechanism, and outcomes. World J Diabetes. 2015;6(7):961–9.
American Diabetes Foundation. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–41.
Agboghoroma OF, AkemoKwe FM, Puepet FH. Peripheral arterial disease and its correlates in patients with type 2 diabetes mellitus in a teaching hospital in northern Nigeria: a cross sectional study. BMC Cardiovascular Disorder. 2020;20:102.
Norman PE, Davis WA, Bruce DG, Davis TM. Peripheral arterial disease and risk of cardiac death in type 2 diabetes: the Fremantle diabetes study. Diabetes Care. 2006;29(3):575–80.
Krishna SM, Moxana JV, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16:11294–322.
Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40. https://doi.org/10.1016/S0140-6736(13)61249-0.
Hernando FJS, Conjero AM. Peripheral artery disease: Pathophysiology, diagnosis and treatment. Rev Esp Cardiol. 2007;60(9):969–82.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology and management. JAMA. 2002;287(19):2570–81.
Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, et al. Global regional and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–30.
Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag. 2019;1(15):187–208. https://doi.org/10.2147/VHRM.S209241.
Okello S, Millard A, Owori R, et al. Prevalence of lower extremity peripheral artery disease among adult diabetes patients in southwestern Uganda. BMC Cardiovasc Disord. 2014;14(1):75.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5-67.
Fowkes FGR, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156.
McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599–606. https://doi.org/10.1001/jama.286.13.1599.
Criqui MH. Peripheral arterial disease-epidemiological aspects. Vasc Med. 2001;6:3–7.
Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metabclin North Am. 2006;35(3):491–510.
Garg A. Insulin resistance in the pathogenesis of dyslipidemia. Diabetes Care. 1996;19(4):387–9.
Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiology. 1999;83(9):25–9.
Schneider DL, Sobel BE. Diabetes and thrombosis. Diabetes and cardiovascular disease. Totowa: Humana Press. 2001
Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47:457–63.
Steinberg HOBA. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45:623–34.
Garofolo L, Ferreria SRG, Junior FM. Association between peripheral arterial disease and C-reactive protein in the Japanese-Brazilian population. Rev Cir Bras Cir. 2014;41(3):168–75.
Chambers RE, Hutton CW, Dieppe PA, Whicher JT. Comparative study of C reactive protein and serum amyloid A protein in experimental inflammation. Ann Rheum Dis. 1991;50:677–9.
Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care.1999;22:1971–1977.
Jialal I, Verma S, Devaraj S. Inhibition of endothelial nitric oxide synthase by C-reactive protein: Clin. Chem. 2009;55:206–8.
Shukla V, Fatima J, Ali M, Garg A. A study of prevalenceof peripheral arterial disease in type 2 diabetes mellitus patients in a teaching hospital. J Assoc Physicians India. 2018;66(5):57–60.
Eshcol J, Jebarani S, Anjana RM, Mohan V, Pradeepa R. Prevalence, incidence and progression of peripheral arterial disease in Asian Indian type 2 diabetic patients. J Diabetes Complications. 2014;28(5):627–31.
Jude EB, Oyibo SO, Chalmers N, Boulton AJM. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24(8):1433–7.
Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. 1999–2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–7. doi: https://doi.org/10.2337/diacare.27.7.1591.
Longo CM, Montero MG, Marquez DT, Romero LB, et al. Obesity and vascular events in type 2 diabetes mellitus. Rev Esp Cardiol. 2015;68(2):151–62.
Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845.
Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–5.
Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2011;34:1642–7.
Bowlin SJ, Medalie JH, Flocke SA, Zyzanski SJ, Goldbourt U. Epidemiology of intermittent claudication in middle-aged men. Am J Epidemiol. 1994;140:418–30.
Curb JD, Masaki K, Rodriguez BL, Abbott RD, Burchfiel CM, Chen R, et al. Peripheral artery disease and cardiovascular risk factors in the elderly. The Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1996;16:1495–1500.
Tseng CH. Prevalence and risk factors of peripheral arterial obstructive disease in Taiwanese type 2 diabetic patients. Angiology. 2003;54:331–8.
Heffron SP, Dwivedi A, Rockman CB, Xia Y, Guo Y, Zhong J, et al. Body mass index and peripheral artery disease. Atherosclerosis. 2020;292:31–6.
Al-Qaisi M, Nott DM, King DH, Kaddoura S. Ankle Brachial Pressure Index (ABPI): an update for Practitioners. Vascular Health and Risk Management. 2009;5:833–41.
Ranjan P, Sarvottam K, Yadav U. Association between modified ankle brachial pressure index and indices of adiposity. Indian J Physiol Pharmacol. 2021;65(1):21–7.
Saely CH, Schindewolf M, Zanolin D, Heinzle CF, Vonbank A, Silbernagel G, et al. Data on the impact of peripheral artery disease and of type 2 diabetes mellitus on the risk of cardiovascular events. Data Brief. 2018;21:1716–20. https://doi.org/10.1016/j.dib.2018.10.153.
Bundo M, Munoz L, Perez C, Montero JJ, Montella N, Toran P, et al. Asymptomatic peripheral arterial diasease in type 2 diabetes patients. A 10 year follow–up study of the utility of ABI as a prognostic marker of cardiovascular disease. Annals of vascular surgery. 2010;8:985–993.
Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, et al. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study, 1987–2001. BMC Cardiovasc Disord. 2007;7:3. https://doi.org/10.1186/1471-2261-7-3.
Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurements and Interpretation of the ankle brachial index. Circulation. 2012;126(24):2890–909.
US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930–2.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii,1–253.
NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998. NIH publication 98–4083.
Lau DCW, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. Obesity Canada Clinical Practice Guidelines Expert Panel. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ. 2007;176(8):S1-S13.
National Collaborating Centre for Primary Care. Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute of Health and Clinical Excellence; 2006.
Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond). 2008;32:619–28.
Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond). 2007;31:1786–97.
Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev. 2006;7:183–200.
Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64:2361–8.
Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy non exercising men. JAMA. 2008;299(11):1261–3. https://doi.org/10.1001/jama.299.11.1259.
Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:1228–37.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–67.
Pinckard K, Baskin KK, Stanford KI. Effect of exercise to improve cardiovascular health. Front Cardiovasc Med. 2019;6:69.
Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–27.
Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47(8):600–11.
Zoppini G, Targher G, Zamboni C, Venturi C, Cacciatori V, Moghetti P, Muggeo M. Effects of moderate intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2006;16(8):543–9.
Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–36.
Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69.
Mika A, Macaluso F, Barone R, Di Felice V, Sledzinski T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front Physiol. 2019;10:26.
Lemieux P, Birot O. Altitude, Exercise, and Skeletal Muscle Angio-Adaptive Responses to Hypoxia: A Complex Story. Front Physiol. 2021;12:735557.
O’Leary V, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–9.
Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc. 2013;45(9):1684–93.
Emanuele NV, Swade TF, Emanuele MA. Consequences of alcohol use in diabetics. Alcohol Health Res World. 1998;22(3):211–9. PMID: 15706798; PMCID: PMC6761899.
Campagna D, Alamo A, Di Pino A, Russo C, Calogero AE, Purrello F, et al. Smoking and diabetes: dangerous liaisons and confusing relationships. Diabetol Metab Syndr. 2019; 85.
Seufert J, Lubben G, Dietrick K, Bates PC. A comparison of the effects of thiazolidinediones and metformin on metabolic control in patients with type 2 diabetes mellitus. Clin Ther. 2004;26:805–18.
Palmer SC, Maviridis D, Nicolucci A, Johanson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose- lowering drugs in patients with type 2 diabetes: a meta- analysis. JAMA. 2016;316:313–24.
Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E, et al. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes. 1994;43:920–8.
Golay A. Metformin and body weight. Int J Obes. 2008;32:61–72.
Yerevanian A, Soukas AA. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr Obes Rep. 2019;8(2):156–64. https://doi.org/10.1007/s13679-019-00335-3.
Luna B, Pharm D, BCPS and Mark N, Feinglos MN. Oral agents in the management of type 2 diabetes mellitus. Am Fam Physician. 2001;63(9):1747–1757.
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303.
Feinglos MN, Bethel MA. Treatment of Type 2 diabetes mellitus. Med Clin North Am. 1998;82:757–90.
Luna B, Hughes AT, Feinglos MN. The use of insulin secreatogues in the treatment of type 2 diabetes. Prim Care. 1999;26:895–915.
American Diabetes Association. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193–203.
Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525–36.
Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–14.
Miller S, St. Sitagliptin OEL. A dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40:1336–43.
Zhi J, Melia AT, Guerciolini R, Chung J, Kinberg J, Hauptman JB, et al. Retrospective population-based analysis of the dose response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clin Pharmacol Ther. 1994;56:82e85.
Padwal R, Kezouh A, Levine M, Etminan M. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int J Obes (Lond). 2007.
Kushner RF, Manzano H. Obesity pharmacology: past, present, and future. Curr Opin Gastroenterol. 2002;18:213e220.
Walsh KM, Leen E, Lean ME. The effect of sibutramine on resting energy expenditure and adrenaline induced thermogenesis in obese females. Int J Obes Relat Metab Disord. 1999;23:1009e1015.
Gursoy A, Erdogan MF, Cin MO, Cesur M, Baskal N. Effect of sibutramine on blood pressure in patients with obesity and well controlled hypertension or normotension. Endocr Pract. 2005;11:308e312.
Finer N, Bloom SR, Frost GS, Banks LM, Griffiths J. Sibutramine is effective for weight loss and diabetic control in obesity with type 2 diabetes: randomize, double-blind, placebo-controlled study. Diabetes Obes Metab. 2000;2:105–12.
Acknowledgements
Institute of Eminence, Banaras Hindu University
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no conflict of Interest.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, U., Kumar, N. & Sarvottam, K. Role of obesity related inflammation in pathogenesis of peripheral artery disease in patients of type 2 diabetes mellitus. J Diabetes Metab Disord 22, 175–188 (2023). https://doi.org/10.1007/s40200-023-01221-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01221-5