Abstract
Familial Hypercholesterolemia is an autosomal, dominant genetic disorder associated with premature cardiovascular disease (CVD). Mutations in the LDLR, APOB, and PCSK9 genes cause the FH phenotype, but in 20% of FH patients, mutations in other genes cause FH. In this regard, we investigated the genetic basis of an Autosomal Dominant Hypercholesterolemia (ADH) phenotype in an Iranian family via next-generation exome sequencing with a panel of hyperlipidemia. We report the first case of FH in an Iranian family due to a mutation in the APOE gene. A 10-year-old female was referred to our genetic clinic with a family history of hypercholesterolemia and high cholesterol level at the age of 3. Evaluation of the lipid profile showed the off total cholesterol of 338 mg/dl, low-density lipoprotein cholesterol (LDL-C of 247 mg/dl(. We identified a mutation in the APOE gene, c.500_502del /p. Leu167del confirmed co-segregation in three individuals of the family from three generations. This in-frame mutation identified here, the first report in Iran, confirms previous reports that ADH can be caused by mutations within the APOE gene and strongly introduces it as the 4th gene that must be checked in the genetic investigating of FH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial hypercholesterolemia is an autosomal dominant disease characterized by elevated plasma levels of LDL cholesterol [1]. Two Kind of FH is heterozygous(HeFH) and homozygous (HoFH) forms. Homozygous FH (HoFH) patients manifest a more severe clinical phenotype compared to heterozygous FH (HeFH). Homozygous FH is rare, with a reported prevalence of 1/1000 000 with plasma LDL-C of 444-1089 mg/dl (11.5-28.2 mmol/l) [2,3,4].
HeFH is the most common monogenic disorder. The prevalence is reported to be 1:200–500, but more recent reports showed a higher prevalence of 1:100–250.
FH is usually caused by a mutation in one of three genes: the low-density lipoprotein cholesterol receptor (LDLR), Apolipoprotein B gene (APOB), or a gain-of-function mutation in the gene for proprotein convertase subtilisin/ Kexin type-9 (PCSK9), but it is mainly caused by mutations in the low-density lipoprotein receptor (LDLR) gene that results in reduced uptake and clearance of LDL-C. More than 1200 different types of mutations were described in the LDLR gene [5,6,7].
Less common causes of FH are mutations in the genes encoding Apolipoprotein B-100 (APO B-100) and proprotein convertase subtilisin/Kexin 9 (PCSK9) [8].
Apolipoprotein E (APOE) is a cholesterol transport protein and a cholesterol-carrying ligand for the lipoprotein receptors of the LDL receptor gene family. It is a multi-functional glycosylated protein secreted from a variety of tissues, including the liver, brain, kidney, adrenals, adipocytes, macrophages, and immune cells. Several studies showed that it is a major risk factor for neurodegenerative diseases and it may be genetically associated with late-onset Alzheimer’s disease [9]. On the other hand, APOE mutants are associated with type III hyperlipoproteinemia characterized by high cholesterol and triglycerides levels(6is).
FH is characterized by markedly elevated LDL-C, skin manifestations such as planar xanthomas, tendinous xanthomas, and premature arcus cornpredisposesdisposing affected people to premature atherosclerotic cardiovascular disease and death. Early identification and treatment of FH patients can improve prognosis and reduce the burden of cardiovascular mortality [10, 11].
We report a 10-year-old girl with HeFH, a mutation in APOE c.500_502del /p. Leu167del coed with co-segregation in three individuals of the family from three generations.
Case presentation
Our patient is 10 years old girl of Iranian origin diagnosed with Familial hypercholesterolemia since the age of 3. She was born of a healthy non-consanguineous parent but the same region referred to the endocrine clinic with high cholesterol levels, because her mother had high cholesterol levels in childhood. At the time of referral, on clinical examination, she had no extensive tendinous xanthomas of the Achilles tendons, Arcus Cornelis, Xanthelasmataof the eyes, Xanthomas of the elbows, knee, metacarpophalangeal joints, Tuberous, palmar, or planar.
The weight of born and around her head was 3100 g and 49 cm. She was a healthy child without any diseases, metabolic disorders, or anomalies. Her baseline untreated LDL-C level was 191 mg/dl at the age of 3. Her endocrinologist advised when she will be 10 years old, she would test her lipid profile. Now she is 10 years old and imputed LDL-C level 247 mg/dl, HDL level 52 mg/dl, Total Cholesterol 338 mg/dl, and Triglicerid is 66 mg/dl. She was started on Atorvastatin 20 mg per day. Three months later she tests again (Table 1). Her mother (302) had Familial hypercholesterolemia since aged 20 and, she was started on Atorvastatin 20 mg at that time. Her aunt (303) had FH recently, and she used lipid-lower drugs, too.
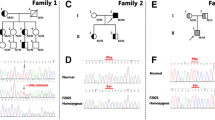
Three-member of the mother’s family were also hypercholesterolemia. The family history is shown in Table 2 and the family pedigree is shown in Fig. 1. She has a significant family history of FH and Atherosclerotic cardiovascular diseases (ASCVD). Her father and two of her uncles had Polycystic Liver and Kidney Disease, her aunts had suffered from Hypertension and Hypothyroidism.
Genomic DNA was obtained from blood samples from the proband and 6 members of her family. Informed consent was obtained from her family and this study complied with the Declaration of Helsinki Principles. Clinical information and paraclinical results were obtained for his family members with particular attention to the Lipid profile. Permission to undertake the study was obtained from the Ethics Committee of Alborz University of Medical Sciences Karaj(Ethics Code: 139585), Iran.
Mutation detection
According to the pedigree (Fig. 1), we invited the family to the Genetic clinic. 5 ml of venous blood was obtained from the proband (401) and six of her family members (203, 204, 207, 301, 302, and 303).
Targeted Exome Sequencing with 11 hyperlipidemic genes panel (including ABCG5, ABCG8, APOB, APOE, LDLR, LDLRAP1, LPL, PCSK9, SLCO1B1 CYP2C19) was performed in the proband. We removed all SNPs identified in non-coding regions or in cases where PolyPhen-2 and SIFT were not expected to cause functional defects. PCR and Sanger sequencing was then performed to confirm the variant found in the proband. After analyzing the data and confirming the role of change observed in the proband, other affected individuals, parents, and healthy individuals of the main family were also examined with Sanger sequencing [12].
Discussion
The FH is an autosomal dominant disorder that results in high levels of LDL-C. In our report, the proband [13] her mother (302), her aunt (303), and her mother’s father (203) are HeFH for a mutation in APOE c.500_502del /p. Leu167del. Her father (301) and her grandmother (204) had no mutation in this gene. Due to the pattern of the family, we expected the inheritance pattern (Autosomal Dominant) of this mutation. We report the first case of FH in an Iranian family due to a mutation in the APOE gene.
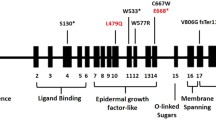
This mutation has also been previously reported by Nguyen, et al. in a patient with sea-blue histiocytosis(with splenomegaly and thrombocytopenia in two unrelated probands of French Canadian ancestry with mild hypertriglyceridemia) and was known as APOE Leu149del, due to different numbering based on the exclusion of the pro-peptide sequence [14].
The other report was two members of a French family, the proband and his brother were diagnosed with splenomegaly, thrombocytopenia, and hypertriglyceridemia. An APOE p.Leu149del mutation was found in both subjects and their mothers [15].
Rahalka et al. reported this mutation with the unusual Japanese and British ancestry subject with hyperlipidemia and (Hypertriglyceridemia) Splenomegaly [16]. Okorodudu et al. reviewed 3405 patients from a lipid clinic database and they identified four patients with dyslipidemia and splenomegaly, one of whom had the APOE p.Leu167del mutation [7].
Awan reported this mutation in a 43-year-old man of Italian origin who presented with acute myocardial infarction. He had tendinous xanthomas, xanthelasmas, and elevated levels of total and LDL cholesterol, at 11.2 and 9.69 mmol/L, respectively, with normal levels of HDL cholesterol and triglycerides at 1.62 and 1.13 mmol/L, respectively [5].
Exome sequencing did not identify any mutation in the LDLR, PCSK9, LDLRAP1, and APOB gene, but it showed that the strongest evidence of association was found for the previously identified apolipoprotein E mutation (APOE, chromosome 19:45412053-55) known as APOE Leu167del, an in-frame three base-pair deletion.
Most studies showed that there were 34 million FH cases worldwide. If it is undiagnosed and untreated, there is a significant CHD (coronary heart disease) onset presented before age 55 (men) and 60 (women). They may be presented tuberous xanthomas and xanthelasmata in the body. Half of all untreated HeFH men and 15% of women will die of CHD-induced myocardial infarction (MI) before these ages. 79% of Mutations in the LDLR gene are caused by FH cases and mutations for 5% of FH cases [6, 8, 17].
National Institute for Health and Clinical Excellence (NICE) guidelines and the National Lipid.
Association (NLA) has significant approaches to FH. Changing the lifestyle, Regular physical activity every week, a healthy diet, and normal BMI, and statins as the first line of treatment for HeFH children. The European and American guidelines recommended high doses of high potency statins in suspected genetic hypercholesterolemia with LDL cholesterol values >190 mg/dL.
Lipid-lowering drugs, regularly statins or combination treatment, is required to obtain the recommended (LDL) cholesterol goals in most affected patients. This drugs like Atorvastatin has been approved for use from age 6 and it can be started at an early and have been shown to be safe and, it has been very well demonstrated in heterozygous subjects with mutations in LDLR, APOB, and PCSK9 [18].
In FH, the treatment goal should be for LDL < 132 mg/dl (3.43 mmol/L) [3, 19, 20]. Our proband was started on an Atorvastatin 20 mg per day and after 3-month reduction of LDL level was significant.
Versmissen J, in 2008 showed that statin therapy had a 76% overall risk reduction in CHD and no increased risk of adverse effects such as increased plasma liver enzyme activity, myalgia and, less commonly, rhabdomyolysis and myopathy. Several authors suggested that the best screening method is Cascade screening (CS), it is more cost-effective than other methods [21].
Conclusions
The FH is an autosomal dominant disorder that results in high levels of LDL-C. The diagnosis was based on elevated plasma LDL-C, a family history of hypercholesterolemia and Coronary Artery Diseases. Early diagnosis and treatment of FH patients, is more cost-beneficial both economical and social with implications for mortality and morbidity, and familial screening via genetic tests seems to be the best approach to prevent Cardio Vascular Disease.
References
Wonderling D, Umans-Eckenhausen MA, Marks D, Defesche JC, Kastelein JJ, Thorogood M, editors. Cost-effectiveness analysis of the genetic screening program for familial hypercholesterolemia in The Netherlands. Seminars in vascular medicine; 2004: Copyright© 2004 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA.
Austin MA, Hutter CM, Zimmern RL, Humphries SE. Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol. 2004;160(5):421–9.
van Aalst-Cohen ES, Jansen AC, Tanck MW, Defesche JC, Trip MD, Lansberg PJ, et al. Diagnosing familial hypercholesterolaemia: the relevance of genetic testing. Eur Heart J. 2006;27(18):2240–6.
Movahedian A, Ghannadi A, Vashirnia M. Hypocholesterolemic effects of purslane extract on serum lipids in rabbits fed with high cholesterol levels. Int J Pharmacol. 2007;3(3):285–9.
Awan Z, Choi HY, Stitziel N, Ruel I, Bamimore MA, Husa R, et al. APOE p.Leu167del mutation in familial hypercholesterolemia. Atherosclerosis. 2013;231(2):218–22.
Marduel M, Ouguerram K, Serre V, Bonnefont-Rousselot D, Marques-Pinheiro A, Erik Berge K, et al. Description of a large family with autosomal dominant hypercholesterolemia associated with the APOE p.Leu167del mutation. Hum Mutat. 2013;34(1):83–7.
Okorodudu DE, Crowley MJ, Sebastian S, Rowell JV, Guyton JR. Inherited lipemic splenomegaly and the spectrum of apolipoprotein E p.Leu167del mutation phenotypic variation. J Clin Lipidol. 2013;7(6):566–72.
Subramani KS, Kolhari VB, Manjunath CN, Bhairappa S. Familial hypercholesterolaemia presenting with coronary artery disease in a young patient. BMJ Case Rep 2013;2013:bcr2013008718.
Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7(11):850–9.
Cenarro A, Etxebarria A, de Castro-Oros I, Stef M, Bea AM, Palacios L, et al. The p.Leu167del mutation in APOE gene causes autosomal dominant hypercholesterolemia by Down-regulation of LDL receptor expression in hepatocytes. J Clin Endocrinol Metab. 2016;101(5):2113–21.
Henderson R, O'Kane M, McGilligan V, Watterson S. The genetics and screening of familial hypercholesterolaemia. J Biomed Sci. 2016;23:39.
https://genetics.bwh.harvard.edu/pph2. Wsh. PolyPhen-2 prediction of functional effects of human nsSNPs accessed January 2013.
Nguyen TT, Kruckeberg KE, O'Brien JF, Ji ZS, Karnes PS, Crotty TB, et al. Familial splenomegaly: macrophage hypercatabolism of lipoproteins associated with apolipoprotein E mutation [apolipoprotein E (delta149 Leu)]. J Clin Endocrinol Metab. 2000;85(11):4354–8.
Faivre L, Saugier-Veber P, Pais de Barros JP, Verges B, Couret B, Lorcerie B, et al. Variable expressivity of the clinical and biochemical phenotype associated with the apolipoprotein E p.Leu149del mutation. Eur J Hum Genet: EJHG. 2005;13(11):1186–91.
Rahalkar AR, Wang J, Sirrs S, Dimmick J, Holmes D, Urquhart N, et al. An unusual case of severe hypertriglyceridemia and splenomegaly. Clin Chem 2008;54(3):606-10; discussion 10-1.
Mohd Nor NS, Al-Khateeb AM, Chua Y-A, Mohd Kasim NA, Mohd NH. Heterozygous familial hypercholesterolaemia in a pair of identical twins: a case report and updated review. BMC Pediatr. 2019;19(1):106.
Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425–37.
Moorjani S, Roy M, Gagne C, Davignon J, Brun D, Toussaint M, et al. Homozygous familial hypercholesterolemia among French Canadians in Quebec Province. Arteriosclerosis (Dallas, Tex). 1989;9(2):211–6.
Wintjens R, Bozon D, Belabbas K, MBou F, Girardet JP, Tounian P, et al. Global molecular analysis and APOE mutations in a cohort of autosomal dominant hypercholesterolemia patients in France. J Lipid Res. 2016;57(3):482–91.
Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ (Clinical research ed). 2008;337:a2423.
Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HA. Cost effectiveness analysis of different approaches of screening for familial h. BMJ (Clinical research ed). 2002;324(7349):1303.
Acknowledgments
The authors thank the proband family and the nurses and staff of the Genetic clinic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicting interests
The authors declared no potential conflicts of interest with respect to this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noorian, S., Razmandeh, R. & Jazayeri, R. Familial hypercholesterolemia in an Iranian family due to a mutation in the APOE gene (first case report). J Diabetes Metab Disord 21, 1201–1205 (2022). https://doi.org/10.1007/s40200-022-01007-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01007-1