Abstract
Purpose
Cardiac miRNAs are the recently discovered key modulators of gene expression in the heart which have been shown to contribute to both transcriptional and post-transcriptional regulation in diabetic cardiomyopathy. The aim of this study was to evaluate the protective effects of interval and continuous aerobic training on diabetic hearts by examining the expression of myocardial miR-126, miR-222 and miR-29a genes.
Methods
Thirty male wistar rats (200 ± 20 g) were randomly divided into six groups of healthy control (HC), diabetes control (DC), continuous training (CT), interval training (IT), continuous training with diabetes (CTD), and interval training with diabetes (ITD). Nicotinamide and Streptozotocin (STZ) were injected to induce type 2 diabetes. CT was performed with a speed of 10 to 22 m/min and 20 to 30 min and IT was performed with 10 to 39 m/min and total time of 15 min, five sessions per week for 6 weeks. Muscle expression of miR-126, miR-29a and miR-222 was determined by the RT-PCR method.
Results
The results show that gene expression of miR-126 was higher in IT (p < 0.01) compare to other groups. Also expression of miR-126 was higher in the CT compare to DC (p < 0.05) group. Gene expression of miR-222 was higher in aerobic groups than other groups (p < 0.01). Also expression of miR-222 was higher in ITD compare to the DC and CTD (p < 0.01) groups. Expression of miR-29a gene was higher in the aerobic groups compare to other groups. Also miR-29a was higher in the IT compare to CT (p < 0.01) group.
Conclusion
Diabetes decreased the expression of genes associated with the development of cardiac function. It seems that IT played a more effective role in cardiac protection than CT through higher miR-126, miR-222 and miR-29a gene expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sedentary lifestyles increase all causes of mortality, double the risk of cardiovascular diseases, diabetes, and obesity [1]. Sedentary lifestyle and physical inactivity can induce diabetes. Diabetes have a destructive effect on body tissue specially on heart [2, 3]. It has been show that diabetic heart disease (DHD) is prevalence in diabetic patients [4]. DHD refers to the heart disease and develops in people with type 1 and 2 diabetes [5]. DHD is a set of chronic heart disease (CHD) or coronary artery disease (CAD), heart failure (HF), Cardiac autonomic neuropathy (CAN), or diabetic cardiomyopathy (DCM) that are characterized by structural, molecular, and functional changes. DCM has been defined as a left ventricular dysfunction in diabetics without symptoms of hypertension, congestive heart failure, or any other heart disease [6]. It has been show that diabetes, activate a network of myocardial-related internal stress signaling pathways that change the gene expression in diabetic heart disease [7].
Metabolic disorders adversely affect the cellular pathways from the earliest stages of diabetes, and are eventually manifesting as structural and functional heart changes [8]. These pathological changes are related to changes in expression of miRs as protected non-coding protein molecules that can regulate the transcription or post-translational process [9]. MicroRNAs (miRNAs) are small non-coding RNAs that play an essential role in regulating cardiac development and maintenance of cardiac function. It has been show that miR-29 overexpression in different organs, such as heart, kidney, liver, and lung, has resulted in suppression of fibrosis-related genes [10]. MiR-29 is involved in the growth, differentiation, and cardiac hypertrophy mechanisms[11]. MiR-29 regulates the expression of Types I and III collagens. Cardiac collagen expression increases in response to damage or overload that is rooted in all heart diseases, leading to mechanical heart stiffness and impaired cardiac contractility [12]. Incremental regulation of miR-29 is associated with an increase in ventricular dilatation and a significant reduction in the gene expression and types I and III collagens [11, 13]. The studies indicate that an increase in the expression of miR-29 plays an important protective role in cardiac collagen control and cardiac hypertrophy [12].
MiR-126, known as "AngiomiR-126", is expressed only in endothelial cells [14]. MiR-126 regulates the myocyte apoptosis, cardiac reconstruction after MI, and vascular inflammation [15]. Fukushima et al. [16]indicated the lower serum levels of miR-126 in patients with ischemic heart disease. In this regard, Zampetaki et al. indicated the low levels of miR-126 expression in the plasma of type 2 diabetic patients [17] and suggested it as a new diagnostic tool for the HF in patients with type 2 diabetes. It has been show that the expression of miR-126 in heart rat after aerobic exercise training increased and leading to cardiac angiogenesis through affecting the MAPK and PI3K/Akt/eNOS [18]. Also, miR-222 has been reported to play important roles in a variety of physiological and pathological processes in the heart. MiR-222 has been recently known as an important mediator of cardiac growth due to exercise training by inhibiting Cyclin-dependent kinase (CDKC) (p27), HMBOX1 and HIPK1/2 pathways. In the ischemic injury model, miR-222 protects against changes in heart structure and dysfunction of heart tissue[19].
It has been show that the high intensity interval training (HIIT) is more effective and useful in developing the aerobic training capacity and cardiovascular functions in healthy individuals and cardiovascular patients compared to moderate-intensity continuous training [20]. Carvalho et al. (2021) show that HIIT promotes cardiomyocyte hypertrophy and interstitial fibrosis, and modulates the apoptosis signaling pathway in healthy rat myocardium. Intermittent fasting reduces pro-apoptotic and increases antiapoptotic signaling, besides attenuating HIIT-induced cardiomyocyte hypertrophy and myocardial interstitial fibrosis [21]. Jeremic et al. (2020) show that cardioprotective effects of high-intensity interval training are mediated through microRNA regulation of mitochondrial and oxidative stress pathways[22]. In DCM Cassidi et al. (2017) show that high-intensity interval training impact on glucose control and cardiometabolic health [23]. Also it has been show that high-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy [24]. Another study showed no difference between moderate- and high-intensity exercises, although this later did not include the assessment of cardiac parameters [25]. More studies have concluded that the HIIT led to better performance in healthy people and diseases [26]. Limited studies investigated the comparison of this type of training with moderate-intensity continuous exercise with reference to signal pathways of miR relating to diabetic heart disease. Therefore, the hypothesis of this study was that dose the HIIT and MICT can affect on miR-126, miR-29a and miR-222 of diabetic cardiac muscle.
Methodology
Research methods and samples
Thirty wistar male rats (200 ± 20 g) were obtained from Pasteur Institute, Tehran, Iran. The rats were kept in the dark light cycle (12:12), humidity of 50%, and a temperature of approximately 22 °C. The rats had free access to water and food. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Baqiyatallah University of Medical Sciences (ethical code: IR.BMSU.REC.1396.633). Rats were randomly divided into 6 groups (n = 5 in each) of healthy control (HC), diabetes control (DC), continuous training (CT), interval training (IT), continuous training with diabetes (CTD), and interval training with diabetes (ITD). The control group did not receive any intervention.
Diabetes induction
Nicotinamide and Streptozotocin (STZ) were injected to induce type 2 diabetes. So that the nicotinamide solution at a dose of 110 mg/kg body weight of rats was first injected intraperitoneally. After 15 min, the freshly prepared solution STZ was solution in citrate buffer with pH = 4.5 and was also injected intraperitoneally at a dose of 60 mg/kg. Rats with a blood sugar above 150 mg/dl were included in the study as diabetic rats [27].
Protocol for assessing rat aerobic capacity
All Rats after prepared for exercise test before of main training protocol. This test consists of 10–20 min of running with 40% to 50% intensity of VO2max, the speed of the treadmill increased every 2 min equal to 0.03 m/s (1.8 to 2 m/min), so that other animals were unable to run. The lack of any increase in VO2max despite the increase in speed was the criterion for achieving VO2max. Speed of recorded VO2max was the speed at which VO2 reached the plateau. The plateau could be reached a lactate concentration above 6 mm/L and respiratory ratio of VCO2/VO2 equal to 1.05. Studies indicated a high correlation between treadmill speed and VO2max of rats (r = 0.94–0.98, p < 0.0005). Therefore, it was possible to calculate the VO2max of rats according to running speed of rats. Since the studies indicated that VO2max before 0.06 to 0.15 m/s was obtained from the final speed in all rats completed the aerobic capacity test, it was deducted from the final speed equal to the average value, i.e. 0.075 m/s, and the intensities were adjusted according to the speed [28].
Exercise training protocol
At first week, the training groups exercised for 3–5 days to get familiarized with running on the treadmill [29]. The training protocol in the continuous group in the first week included:
-
Warming up at a speed of 5 m per minute for 5 min
-
Main training: 20 min of continuous running at a speed of 10 m per minute
-
Cooling down at a speed of 5 m per minute for 5 min.
According to the principle of overload and development of aerobic readiness and the development of rats in the sixth week, it included warming up at a speed of 10 meters per minute for 2 minutes, 30 minutes of continuous running at a speed of 22 meters per minute, and cooling down at a speed of 10 meters per minute for 2 minutes.
The high intensity interval group in the first week included:
-
Warming up for 5 min at a speed of 8 m/min,
-
Main training: 5 sets of high intensity exercise for 2 min at a speed of 15 m/min with 5 sets of slow intensity exercise for 1 min intervals at a speed of 10 m/s
-
Cooling down lasted for 5 min at a speed of 8 m per minute.
It was performed based on the principle of overload and the development of aerobic readiness and development of rats in the sixth week, including warming for 5 minutes at a speed of 8 meters per minute, 5 fast 2 minute intervals at a speed of 39 meters per minute with 5 slow 1 minute intervals at 12 m/s, which was periodically repeated, and then cooling down for 5 min at 8 m/min.
Sampling and measurements
Twenty-four hours after the last exercise session, rats were sacrificed after a night of fasting. To collect the samples, the animals' weights were first measured, and they were then anesthetized with a combination of xylazine (10 mg/kg) and ketamine (75 mg/kg) as an intraperitoneal injection. The heart of rats was removed and washed in a physiological serum and weighed with a precision of 10–4 g at a digital scale. The left ventricle was immediately removed from the heart and weighed with a scale. To measure the level of heart hypertrophy and the left ventricle, the weights of heart muscle and the left ventricle were used as the best predictors of the hypertrophy. The left ventricular tissue was then frozen using liquid nitrogen and frozen at -80 °C for subsequent measurements.
Total RNA was extracted from tissue samples using Kiazol (Kiazist, Iran) according to the manufacturer’s instructions (Stem-loop sequence in 1). A cDNA kit was used to reversely transcribe RNA to cDNA (Parstous, Iran) according to the manufacturer’s protocol. Real-Time PCR were run for 30–40 cycles using SYBR® Premix Ex Taq ™ II (TliRNaseH Plus, RR820Q) on a Rotor-Gene Q 5plex System. GAPDH was employed as internal control. The 2-ΔΔCt method was used to normalize the expression levels of each target gene to internal control expression.
The results are represented as the Mean (± SEM) fold changes with respect to the sham control.
Statistical analysis
The necessary data were processed after collection by SPSS-19 (Chicago, USA); and all results were expressed as Mean ± SEM and then analyzed at a significant level (α ≤ 0.05). The Kolmogorov–Smirnov test was used to investigate the normal distribution of data. The one-way analysis of variance (ANOVA) and the Tukey's post hoc test were used to compare differences between groups (p < 0.05).
Results
Heart morphology
Statistical results indicated that there was a significant difference between groups in the data on weight, heart weight, and ratio of heart weight and left ventricle to body weight (p < 0.01). Tukey’s post hoc test indicated that diabetic rats' weights were significantly lower than nondiabetic rats’ (p < 0.01), but heart weights of diabetic groups were higher than other groups. The heart weight of DC was significantly higher than all other groups (p < 0.01). In ITD (p < 0.05) and CTD (p < 0.01), the heart weights were also higher than the HC. Furthermore, the CTD showed higher values of heart weight than the CT and IT (p < 0.01). Heart weight to body weight ratio in DC was significantly higher than all groups (p < 0.01) and also higher in the CTD than the HC (p < 0.05), but data of left ventricular to body weight ratio was higher in the DC than other groups (p < 0.05) (Table 2).
Expression of mir-126
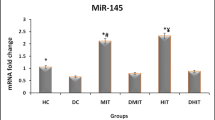
Result show that significant differences in changes of mir-126 gene expression in different groups (p < 0.01). Results of Tukey's post hoc test indicated the higher values of mir-126 gene expression in the interval aerobic training group compared to other groups (p < 0.01). Furthermore, the continuous aerobic training group indicated higher values of mir-126 gene expression than the diabetes group (p < 0.05) (Fig. 1).
Expression of miR-126 in different groups of studies. Data were show as Mean ± SEM.* indicates the difference between group IT with other groups; and # indicates difference with the CT. HC, healthy control; DC, diabetes control; CT, continuous training; IT, interval training; CTD, continuous training with diabetes; ITD, interval training with diabetes
Expression of mir-222
Result show that significant differences in changes of mir-222 gene expression in different groups (p < 0.01). Values of mir-222 gene expression in the interval and continuous aerobic training were higher than other groups (p < 0.01). Diabetes + interval group had higher amounts of mir-222 than diabetes and diabetes + continuous aerobic training (p < 0.01) (Fig. 2).
Expression of miR-222 in different groups of studies. Data were show as Mean ± SEM.* indicates the difference between group CT and IT with other groups; and # indicates difference with the DC and CTD. HC, healthy control; DC, diabetes control; CT, continuous training; IT, interval training; CTD, continuous training with diabetes; ITD, interval training with diabetes
Expression of mir-29a
Result show that significant differences in changes of mir-29a gene expression in different groups (p < 0.01). Values of mir-29a gene expression in the interval and continuous aerobic training were higher than other groups (p < 0.01). Furthermore, the gene expression of mir-29a was lower in the continuous group than the interval group (p < 0.01) (Fig. 3).
Expression of miR-29a in different groups of studies. Data were show as Mean ± SEM.* indicates the difference between group CT and IT with other groups; and # indicates difference with the CT. HC, healthy control; DC, diabetes control; CT, continuous training; IT, interval training; CTD, continuous training with diabetes; ITD, interval training with diabetes
Discussion
The available evidence indicates that miRNAs can regulate cardiac hypertrophy, myocardial fibrosis, oxidative stress and apoptosis, mitochondrial dysfunction, epigenetic modification,13 cardiac electrical remodelling and other pathophysiological changes, which are associated with diabetic cardiomyopathy [30]. The present study first measured the effects of high intensity interval training and moderate-intensity continuous training on the expression of miR-126, miR-222 and miR-29a genes in heart tissues of type 2 diabetes rats. MiR-126 with expression of 6.4 fold in the interval aerobic training group than other groups showed a significant increase. Furthermore, the expression of this gene in the continuous group was more than the diabetic group. In diabetic groups, interval and continuous training were unable to prevent a significant reduction in the expression of this gene in the cardiomyocytes. However, the expression values of this gene were higher in the diabetic interval training group than the healthy control group, but lower in the diabetic continuous group than the healthy control group. The data suggest that the high intensity interval training in the diabetic group was able to partially enhance its protective effects by increasing the expression of miR-126, but the moderate-intensity continuous training with diabetes was unable to overcome effects of diabetes. Diabetes suppresses the expression of miR-126 gene in cardiomyocytes [31]. In the present study, we had the lowest values of this gene in the diabetes group. It seems that miR-126 stimulates angiogenesis in the heart muscle by targeting the important signaling cascades AMPK PI3K/AKT/eNOS [32] that is severely reduced and suppressed by diabetes. High intensity interval training in the interval training group was able to stimulate the pathway by increasing miR-126, but the increase was not significant with interval aerobic training in the diabetic group. It seems that exercise training lead to higher expression of GLUT4 values at the cellular level [33]; and the reduction of serum glucose as well as insulin decreases the amount of inflammation, and paves the way for increasing the cellular signaling pathways leading to the higher angiogenesis. It has been reported that exercise training has a great potential for the development of cardiac angiogenesis through the expression of miR-126 and VEGF protein [34]. The positive effects were associated with increasing VEGF/Raf-1/ERK and VEGF/PI3K/AKT pathways [35]. Exercise training was also able to suppress SPRED1 and PIK3R2 proteins by increasing the miR-126 expression [18]. However, a limitation of the present study was the lack of measuring VEGF protein and capillary density. It seems that the high intensity interval training group in the present study could better increase the cardiac angiogenesis capacity that further increased the expression of miR-126.
An important intervention marker in the development of cardiac myocytes is miR-222 that increases in response and adaptation to exercise, and can be considered as a difference between physiological and pathological hypertrophy [19]. In the present study, the miR-222 gene expression of cardiomyocytes was significantly higher in the interval and continuous aerobic groups than other groups. Furthermore, values of gene expression in the diabetic interval group were significantly higher than the diabetes group and the diabetes group with continuous training. Base on this results it seems that the high intensity of aerobic training seems to play a very effective role in creating greater cardiovascular compatibility in diabetic patients as positive cardiovascular changes than low intensity of aerobic training. Liu et al. identified a key role of miR-222 in the proliferation of smooth endothelial cells of vessels [19]. When endothelial cells are challenged under conditions such as high glucose or the aging process in rats, they prevent the cell cycle from starting, and thus the endothelial cells migrate to stimulate the VEGF. It occurs at the same time as the decrease in miR-222. In fact, aerobic exercise training appears to reduce these conditions by developing the insulin sensitivity and lowering blood glucose and adjusting conditions in favor of further expression of miR-222 [19]. It has been found that the inhibition of miR-222 increases the apoptosis of cardiac cells, and thus its high expression in rats stimulates the growth and proliferation of cardiac myocytes that are associated with physiological symptoms of hypertrophy [19]. In general, aerobic training can be a powerful therapeutic strategy to replace the persistent loss of cardiomyocytes in diabetics by activation of miR-222 [19].
It has been show that cardiomyocytes MiR-29a decreases the amount of collagen protein and increases the ventricular compliance by targeting collagen I and III genes [36]. In fact, this gene is a main differentiation point between physiological and pathological hypertrophy phenomena. The heart muscle size increases by creating the training adaptations of the muscle through developing its function, but the collagen expression increases in the pathological type that is associated with ventricular stiffness and reduction of contractility [12]. In the present study, interval and continuous training groups significantly indicated the higher values of gene expression than other groups; and the increase in the high intensity interval training group was higher than the moderate-intensity continuous training. MiR-29 has been reported to be downregulated in several fibrosis-related diseases in rodents as well as in humans, and has been shown to target mRNAs that encode fibrosis-promoting proteins in different cell types/organs [37]. In apparent agreement with this, experimental elevation of miR-29 was found to repress collagen transcripts in cultured cardiac fibroblasts [38]. It seems that non-diabetic training groups had developed cardiac functions; hence, there is a need for further study due to the lack of measurement of collagen in the present study.
In the present study, the heart weight to body weight ratio in diabetic rats indicated a significant increase compared to other groups. In the diabetic group with continuous aerobic training, the increase was observed compared to the healthy control group, but the diabetes group along with interval aerobic training did not show any increase. However, the ratio of left ventricular weight to body weight increased significantly only in the diabetes group compared to all other groups. It seems that diabetes caused the cardiac hypertrophy in diabetic control rats and was to some extent controlled by training. However, the interval aerobic training was more effective in preventing the structural changes than continuous aerobic training. In the present study, an increase in heart mass of diabetic groups was higher than non-diabetic groups; however, the increase was associated with much lower levels of expression of miR-126, miR-222 and miR-29a gene in diabetic groups, indicating pathological changes caused by diabetes. Due to exercise training interventions in diabetic groups, values of the pathological changes were moderated to some extent, and the high intensity interval training had a greater effect on reducing the pathological changes than continuous aerobic training. In the present study, proteins and structural changes in cardiac muscle tissue were not measured and also the dose of exercise training was equalized in two protocols. If the limitations are overcome, roles of miRs seem to be closely investigate in training interventions in the diabetic heart.
Diabetes with down-regulation of miR-126, miR-222 and miR-29a genes can develop diabetic cardiomyopathy, while exercise, especially interval exercise, can play a preventive role in diabetic cardiomyopathy with increase of miR-126, miR-222 and miR-29a. While during diabetes, regular and interval and continuous exercise can minimize the damage of diabetes on the heart tissue. However, more studies are needed in this area, especially on the human sample.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- STZ:
-
Streptozotocin
- DHD:
-
Diabetic heart disease
- CHD:
-
Chronic heart disease
- CAD:
-
Coronary artery disease
- HF:
-
Heart failure
- miRNAs:
-
MicroRNAs
- CDKC:
-
Cyclin-dependent kinase
- HIIT:
-
High intensity interval training
References
Alkhatib A, Tuomilehto J. Lifestyle diabetes prevention. In: Encyclopedia of endocrine diseases. Elsevier; 2019. p. 148–59.
Arnold SV, et al. Type 2 diabetes and heart failure: insights from the global DISCOVER study. ESC Heart Fail. 2021;8(2):1711–6.
El Hayek MS, et al. The role of hyperglycaemia in the development of diabetic cardiomyopathy. Archives of Cardiovascular Diseases; 2021.
Raffield LM, et al. Associations of coronary artery calcified plaque density with mortality in type 2 diabetes: the Diabetes Heart Study. Cardiovasc Diabetol. 2018;17(1):1–8.
Rajbhandari J, et al. Diabetic heart disease: A clinical update. World J Diabetes. 2021;12(4):383.
Byrne NJ, et al. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radical Biol Med. 2021;169:317–42.
Diao X, et al. Differentially expressed microRNAs and their target genes in the hearts of streptozotocin-induced diabetic mice. Mol Med Rep. 2011;4(4):633–40.
Muriach M, et al. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014.
Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–42.
Boon RA, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109(10):1115–9.
Fernandes T, Soci U, Oliveira E. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz J Med Biol Res. 2011;44:836–47.
Soci UPR, et al. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics. 2011;43(11):665–73.
Liu M-N, et al. miR-29 family: a potential therapeutic target for cardiovascular disease. Pharmacol Res. 2021; 105510.
Esser JS, et al. Bone morphogenetic protein 4 regulates microRNAs miR-494 and miR-126–5p in control of endothelial cell function in angiogenesis. Thromb Haemost. 2017;117(04):734–49.
Chang J, et al. Ginkgolide B promotes cell growth in endothelial progenitor cells through miR-126 and the Akt signaling pathway. Mol Med Rep. 2017;16(4):5627–32.
Fukushima Y, et al. Assessment of plasma miRNAs in congestive heart failure. Circ J. 2011;75(2):336–40.
Zampetaki A, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–7.
Song W, et al. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats. J Cell Mol Med. 2020;24(22):12970–9.
Liu X, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21(4):584–95.
Hussain SR, Macaluso A, Pearson SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev. 2016;24(6):273–81.
Carvalho MR, et al. Influence of high-intensity interval training and intermittent fasting on myocardium apoptosis pathway and cardiac morphology of healthy rats. Life Sci. 2021;264:118697.
Jeremic N, et al. Cardioprotective effects of high-intensity interval training are mediated through microRNA regulation of mitochondrial and oxidative stress pathways. J Cell Physiol. 2020;235(6):5229–40.
Cassidy S, et al. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia. 2017;60(1):7–23.
Novoa U, et al. High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid Med Cell Longev. 2017;2017.
Taylor JD, et al. Effects of moderate-versus high-intensity exercise training on physical fitness and physical function in people with type 2 diabetes: a randomized clinical trial. Phys Ther. 2014;94(12):1720–30.
Maturana FM, et al. Effectiveness of HIIE versus MICT in improving cardiometabolic risk factors in health and disease: a meta-analysis. Med Sci Sport Exer. 2020;53:559–73.
Qasem MA, et al. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ. 2018;6:e4788.
Ghahramani M, Karbalaeifar S. A comparison of the effect of eight weeks of high intensity interval training on PGC-1α gene expression levels in the slow twitch (ST) and fast twitch (FT) muscles of rats with myocardial infarction. J Basic Res Med Sci. 2019;6(4):45–51.
Mirdar S, et al. The effects of tapering with and without ethanolic extract of Nigella sativa on Hypoxia Inducible Factor-1α and exercise-induced bronchial changes. J Mil Med. 2019;21(2):131–41.
Zhang W, et al. Non-coding RNA involvement in the pathogenesis of diabetic cardiomyopathy. J Cell Mol Med. 2019;23(9):5859–67.
Pishavar E, Behravan J. miR-126 as a therapeutic agent for diabetes mellitus. Curr Pharm Des. 2017;23(22):3309–14.
Pan Q, et al. MicroRNA-126 priming enhances functions of endothelial progenitor cells under physiological and hypoxic conditions and their therapeutic efficacy in cerebral ischemic damage. Stem Cells Int. 2018;2018.
Christ-Roberts CY, et al. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism. 2004;53(9):1233–42.
Dastah S, et al. Aerobic exercise leads to upregulation of Mir-126 and angiogenic signaling in the heart tissue of diabetic rats. Gene Reports. 2020;21:100914.
Lew JKS, et al. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol. 2017;16(1):1–20.
Xiao L, et al. Effects of miR-29a and miR-101a expression on myocardial interstitial collagen generation after aerobic exercise in myocardial-infarcted rats. Arch Med Res. 2017;48(1):27–34.
Fang Y, et al. miR-29c is downregulated in renal interstitial fibrosis in humans and rats and restored by HIF-α activation. Am J Physiol-Renal Physiol. 2013;304(10):F1274–82.
Van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci. 2008;105(35):13027–32.
Author information
Authors and Affiliations
Contributions
All author equally contributes in preparation of this manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Baqiyatallah University of Medical Sciences.
Consent to participate
Not applicable.
Consent for publication
All participants gave written informed consent for the publication of study findings.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbari, J., Shirvani, H., Shamsoddini, A. et al. Investigation of expression of myocardial miR-126, miR-29a and miR-222 as a potential marker in STZ- induced diabetic rats following interval and continuous exercise training. J Diabetes Metab Disord 21, 189–195 (2022). https://doi.org/10.1007/s40200-021-00957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00957-2