Abstract
Aims
Diabetic peripheral neuropathy affects up to 60% of individuals and often leads to foot ulceration and eventual amputation. When oral therapy has failed to achieve pain relief, the first line local treatment is the 5% lidocaine-medicated plaster which provides local relief. Capsaicin 8% patch is considered a promising topical treatment for diabetic peripheral neuropathy. The present study investigated the efficacy, safety and tolerability of capsaicin 8% patch vs 5% lidocaine patch treatments over 24 weeks in South Asian male diabetic patients with established peripheral diabetic neuropathy.
Methods
Analgesic effectiveness was assessed by observing any change in the Numeric Pain Rating Scale (NPRS) score, Brief Pain Inventory (BPI) for painful diabetic peripheral neuropathy (BPI-DPN question 4) and Patient Global Impression of Change (PGIC). All patients received 4% lidocaine gel/cream for 60 min prior to patch application. The trial was probably underpowered, taking into account the smaller than expected number of participants from the calculated 350 sample size required for the whole study. Two hundred ninety-one individuals were divided into three groups based on treatment regimen; Group LL (Lidocaine + Lidocaine), Group LP (Lidocaine + Placebo), Group LC (Lidocaine + Capsaicin). The treatment procedure was conducted once initially and then repeated once at 12 weeks. The patients were followed up on alternate weeks till 24 weeks after the initial treatment.
Results
Group LC experienced a more significant reduction in the average pain intensity (p < 0.05) during the last twenty-four hours. Group LC showed more significant reduction of pain compared to control (p < 0.01), a baseline score of 5.4 ± 1.2 dropped to 3.2 ± 1.5 by week 24 of treatment. The change in mean daily pain intensity was – 2.2 ± 1.5 [95% CI: −2.45, −1.5]. Group LL and LC experienced a significant overall improvement (slightly, much or very much) in the health status during the study. After the second week of the treatment, patient satisfaction scores were 2.1 ± 1.1 in Group LL which increased to 3.2 ± 1.2 by week 24 of treatment. The capsaicin 8% patch appears to be reasonably well tolerated since there were no discontinuations because of serious drug-related treatment emergent adverse event (TEAEs).
Conclusions
The aim of the present study was to assess the efficacy, safety and tolerability of the 8% capsaicin patch in patients with established painful diabetic neuropathy. There was a sustained treatment response to the initial and repeat treatment of the capsaicin 8% patch over the 24 weeks. The study population was very specific so further studies are required to investigate the generalizability of the results for patients experiencing painful diabetic neuropathy. The patch could be considered as an effective long-term treatment option in individuals with painful diabetic neuropathy, particularly those experiencing inadequate pain relief or side effects from systemic therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic peripheral neuropathy (DPN) affects up to 60% of diabetics worldwide [1] The symptoms evolve from initially the small nerve fibers that can lead to burning or tingling sensation and progress to compromise the larger fibers leading to numbness [2,3,4].
In patients experiencing inadequate response at oral therapy (pregabalin and duloxetine) or at risk of adverse effects, topical analgesics are more appropriate as these allow only a small amount of medication to enter the systemic circulation. The first line drug treatment is most often the 5% lidocaine plaster [5, 6] and capsaicin (selective vanilliod receptor subtype 1 agonist) is the second in line. Capsaicin depolarizes neurons to provide rapid and sustained pain relief [7, 8]. Using the 8% capsaicin patch provides a faster impartment of a high capsaicin concentration locally to the skin which significantly reduces the frequency of application.
Previously NGX-4010, a capsaicin (8%) dermal patch has been used for non-diabetic related peripheral neuropathic pain unrelated to diabetes. Using the 8% capsaicin patch has been approved for the control of pain in diabetic neuropathy [9].
However, capsaicin can cause degeneration of epidermal and dermal autonomic nerve fibres with use [10]. The FDA and European Medicines Agency suggest that sixty minute applications is within acceptable safety limits. [11, 12] Cutaneous nerve fibres usually regenerate after discontinuing capsaicin, however effects on patients with existing neuropathy can be more pronounced and therefore caution is advised. The patch included in our study was the 8% capsaicin cutaneous patch (8% weight for weight) and is optimized for rapid delivery of a high concentration of capsaicin directly to the skin. [13]
The efficacy and safety of lidocaine 5% versus capsaicin 8% in south Asian male patients with painful diabetic peripheral neuropathy has not yet been fully elucidated. The aim of the present study was to compare the efficacy and tolerability of using lidocaine 5% patch to 8% capsaicin patch. Assessment of analgesic effectiveness was assessed by observing any change in the Numeric Pain Rating Scale (NPRS) score, average daily pain Brief Pain Inventory (BPI) for painful diabetic peripheral neuropathy (BPI-DPN Q4) and Patient Global Impression of Change (PGIC). Assessment of capsaicin and lidocaine safety and identifying treatment adverse effects were secondary endpoints in this study.
Subjects
All patients were males of age 40–60. All participants in the study had type 2 diabetes more than ten years’ duration and experiencing symptoms of peripheral neuropathic pain, were assessed using the Diabetic Neuropathic Score (DNS). Studies suggest that estrogen receptors are present throughout the nociceptive axis and hormonal dysregulation is associated with chronic pain syndromes. Our participants were males to avoid the possibility of gender and hormonal issues being confounding variables. [14, 15]
Materials and methods
This present study was a double blinded, placebo controlled, parallel group study that was approved by the Punjab Care hospital review board (Approval no. 38920), conducted in a single clinical center. The study is in agreement with the principles laid down in the Declaration of Helsinki, Good Clinical Practice guidelines, its successive amendments and also applicable regulatory requirement. Informed consent was obtained from all individual participants included in the study. The clinical trial is registered at ClinicalTrials.gov (ID: LIDCAI38920, Clinical Trial No: NCT04238208).
According to Berlin et al. for sample size calculation we estimated a sample size of 350 patients in the whole study would provide a 95% chance of observing with an incidence of 1% for adverse events [16, 17]. Power analyses using G*Power with power (1 - β) was set at 0.80 and α = 05, two-tailed to enable statistical significance at the .05 level [18].
However, due to limitations of time and volunteer availability, we were practically able to recruit 291 male patients and due to personal reasons unrelated to the study, such as change of employment, death of a spouse, home relocation and divorce, 18 individuals dropped out before the start of the study. The dropout rate was 6.18% (n = 18) in our study. Finally, 273 individuals completed the study and from whom we obtained the data. This might have led the study to be underpowered.
The expected adverse event reported in the literature and monitored in this study included symptoms experienced by the study participants included pricking sensations, numbness, burning and aching in the feet. Symptom scores were coded as follows: score 2 (2 or more symptoms), score 1 (one symptom) or score 0 (no symptoms). Inclusion criteria required that all study participants score 1 at least. Neuropathic pain was assessed by a certified physiotherapist. All results are in mean ± standard deviation (SD). All patients scored 4–8 in the Numeric Pain Scale (0 = no pain till 10 = worst pain).
Presence of diabetic foot ulcers, deformed/contracted foot, neurological complications, neurological disease, cardiovascular or peripheral vascular disease, usage of topical analgesics or implanted medical device six weeks prior to the study were all excluded.
The study participants were randomly placed into three groups based on their treatment regimens; Group LL (Lidocaine + Lidocaine), Group LP (Lidocaine + Placebo), Group LC (Lidocaine + Capsaicin). All patients received 4% lidocaine gel/cream (Aspercreme®; 4%, Chattem Inc., Pakistan) for 60 min prior to patch application. This was according to the literature available. [10, 19] At screening and at each application visit the treatment area was demarcated and a maximum of four patches equivalent to 1120 cm2 area were applied to the feet. The most painful areas were given the highest priority. In subsequent visits the patches were placed in the same sites.
Since this was a double-blind study, neither the participants or the researchers knew which participants belong to the control group, nor the test group. The codes 11 were used for the capsaicin patch, 12 for the control patch and 33 for the lidocaine patch. These codes were retained by a hospital staff member who was not involved in the study and were revealed to the researchers after all the data was collected and analysed.
Group LL patients received the 5% lidocaine patch (Lidoderm®, Endo Pharmaceuticals Inc., Malvern, USA) 10 × 14 cm containing 700 mg of Lidocaine for 60 min. Group LP received an identical placebo patch and Group LC received the 8% Capsaicin patch [8% w/w] 640 μg/cm2 of adhesive, patch area 280 cm2 (20 cm × 14 cm), (Qutenza®; capsaicin 179 mg patch, Astella Pharma Europe Ltd. Chertsey, UK). The second patch was also applied for a total of 60 min. The patches and gel were purchased through the hospital pharmacy using the research grant. The treatment procedure was conducted once initially and then repeated once at 12 weeks. The patients were followed up on alternate weeks till 24 weeks after the initial treatment.
The comprehensive validated instrument; Brief Pain Inventory (BPI) for painful diabetic peripheral neuropathy (BPI-DPN Q4) was used during the patient visits to assess pain severity on the day of the treatment [20]. The question states ‘Please rate your pain due to diabetes by circling the one number that best describes your pain on the average’. The numerical rating scale ranges from 0 (no pain) - 10 (pain as bad as you can imagine). This single question is very similar to how physicians evaluate their patients’ pain in most clinical settings and has adequate clinical relevance.
Additional efficacy assessment was conducted using Patient Global Impression of Change which measures the patient’s impression of change on 7-point scales (very much improved – very much worse) [21]. Patient satisfaction with the treatment was also measured on a 4-point rating scare (0 = poor to 4 = excellent) in response to the question “How would you rate the patch application for your pain?”
Dermal assessment for safety assessment was scored by a certified dermatologist with scores from 0 to 7 of severity. These were recorded on the treatment application day and also after any patch application, five and ninety minutes after removing the patch during the treatment visit and during follow up visits. Safety assessment also included adverse effect coded for utilizing the Medical Dictionary for Regulatory activities version 20.0. A treatment emergent adverse event (TEAE) is an adverse effect observed after the start of the patch application, or an adverse event that increased in severity after patch application. A serious TEAE was an untoward medical occurrence that was deemed life threatening or resulted in significant disability/incapacity, death, led to in-patient hospitalization or considered a medically important event [22].
Using the Gálvez et al. protocol [23]; sensory perception, reflex testing, mapping and measuring the areas of spontaneous pain and of allodynia/hyperalgesia were assessed at baseline, prior to application and at every visit.
Package for Social Science (SPSS) Version 21 (SPSS Inc., Chicago IL, USA) was used for statistical analysis. We used Student t-test to compare the tested variable between the groups. The baseline visits and the follow ups were compared using repeated measures ANOVA with a Greenhouse-Geisser correction. The Chi-square test compared the presence and absence of pain/symptoms in the control and test groups. Calculated p value was deemed significant at <0.05. To describe the absolute values and changes from baseline of average daily pain scores, NPRS scores, PGIC and areas of pain and allodynia, we utilized descriptive statistics. Summary statistics for changes from the baseline in the average daily pain was also performed.
A detailed post-hoc analysis was performed for each sensory modality. The purpose was to identify any shift in the sensory category from the baseline.
Results
Patient demographics
Baseline characteristics such as age and other demographic details were similar between the three groups. Baseline average pain scores in each group were: Group LL: 6.4 ± 1.5, Group LP: 6.1 ± 1.6, Group LC: 6.7 ± 1.9 with an average daily pain score as 6.2. Patient demographics and baseline data is detailed in Table 1.
Analgesic effectiveness
Measured by observing changes in average daily pain (BPQ Q5), NPRS Score and Patient Global Impression of Change (PGIC).
NPRS score
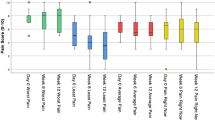
Group LL and Group LC both experienced a significant drop (p < 0.05) in the average pain intensity at the end of 24 weeks of the treatment. Group LP did not show any significant reduction in the intensity of pain (Fig. 1.).
Average daily pain
Group LL and Group LC experienced a significant (p < 0.05) reduction in average daily pain at study end compared to baseline (21.1% and 40.7%, respectively).
In Group LL the baseline BPI-DPN Q4 score for average daily pain was 5.2 ± 1.22 dropped to 4.1 ± 1.0 by Week 24. The overall change in mean daily pain intensity was 1.0 ± 1.1 [95% CI: −1.25, −0.85].
Group LC showed a more significant (p < 0.01) reduction of pain compared to control group, a baseline score of 5.4 ± 1.2 dropping to 3.2 ± 1.5 by week 24 of treatment. The overall change in mean daily pain intensity was – 2.2 ± 1.5 [95% CI: −2.45, −1.5].
In Group LP, a non-significant (p = 0.89) reduction in the scores with baseline scores of 5.0 ± 1.4 reducing to 4.6 ± 1.0 at week 24. The overall change in mean daily pain intensity was – 0.4 ± 0.3 [95% CI: −0.48, −0.35]. These results are shown in Fig. 2.
Patient global impression of change (PGIC) scores
Group LL and LC achieved a significant improvement in patients’ impression at the study end (72% ± 5.1 (n = 66) and 56% ± 6.5 (n = 51) respectively. Group LP showed no significant change.
Patient satisfaction
Patient baseline satisfaction scores 2.1 ± 1.1 in Group LL increased to 3.2 ± 1.2 by week 24 of treatment. In Group 2 LP, patient satisfaction scores increased from 2.2 ± 1.5 to 2.5 ± 1.3 by week 24. In Group LC, patient satisfaction scores were 2.0 ± 1.0 increasing significantly (p = 0.03) to 4.2 ± 1.8 by week 24 of treatment.
Adverse effects
In Groups LL, LC and LP there were 11 (12%), 8 (8.7%), and 2 (2.1%) patients respectively who reported TEAEs. The most common was application site pain (36.6%). Group 1 LL patients had mild (2%) and moderate (1%) intensity of pain. Group LP patients experience mild (1%), moderate (1%) and severe (2%) pain. Group 3 patients had mild (5%), moderate (7%) and severe (3%) pain.
Two patients (2.1%) from Group LL withdrew due to TEAEs (application site erythema, application site pain, burning sensation, renal colic) while four patients (8.7%) in Group LC withdrew due to TEAEs (application site erythema, application site pain, burning sensation, prostate adenocarcinoma). When compared, there was a non-significant difference between the two groups (p = 0.94). However, these adverse effects were not classified as serious TEAEs.
Area of allodynia/hyperalgesia
In the total population, the mean ± SD area of allodynia/hyperalgesia decreased from 141.6 ± 19.1 cm2 (interquartile range [IQR]: 62.5–123.5) before first application (n = 290) to 119.9 ± 18.7 cm2 (IQR: 36.0–182.0) at end of study (n = 282).
In Group LL, the area of allodynia/hyperalgesia decreased from 127.4 cm2 ± 168.5 (IQR: 51.5–182.5) before first treatment (n = 80) to 113.4 ± 14.4 cm2 (IQR: 43.0–199.0) at the end of the study (n = 96). This was a 14 cm2 decrease (p = 0.05).
In Group LP patients, the area decreased from 127.4 ± 18.5 cm2 (IQR: 51.5–123.5) before first treatment (n = 80) to 120.4 ± 14.4 cm2 (IQR: 43.0–166.0) at the end of the study (n = 96). This was a 7 cm2 decrease (p = 0.82).
In Group LC patients, the area decreased from 192.1 ± 19.5 cm2 (IQR: 56.0–140.5) before first application (n = 96) to 152.3 ± 12.8 cm2 (IQR: 37.0–155.0) at end of study (n = 96). This was a 40 cm2 decrease (p = 0.01).
Using ANOVA statistics, Group LC experienced the most significant reduction of area of allodynia/hyperalgesia followed by Group LL and Group LP.
Sensory perception and reflex testing
By the end of the study, in Group LL, 30.5–46.1% (depending on test), 10.1–16.7% (Group LP) and 20.1–36.7% (Group LC) of patients showed no change in any sensory category. In Group LL 24–31% (depending on test) of patients, 14–21% (Group LP) and 34–41% (Group LC) showed an improved sensory category.
In reflex testing, Group LL showed an improvement in 11.3% of patients while these values were 10.3% (Group LP) and 15.3% (Group LC).
Discussion
The present study investigated the efficacy, safety and tolerability of capsaicin 8% patch vs 5% lidocaine patch treatments over 24 weeks in diabetic patients with established peripheral diabetic neuropathy. The study was designed to mirror clinical practice with patch application dependent on the effectiveness and safety experienced by patients.
Painful diabetic neuropathy has a significant impact on the quality of life for diabetic individuals. Topical application has a lower risk of drug-drug interactions, lower systemic levels of medication and fewer side effects and overdose, when compared to systemic administration of treatment. This increase in the safety margin is of particular importance considering varying medication responses in patients that can occur due to polymorphisms and in vulnerable populations such as the elderly who are receiving multiple concomitant medication for multiple co-morbidities. The topical route skips dose titration since topical treatment involves site-specific delivery by the patients themselves to the most painful areas. [24].
At the site of local application, one of the most popularly used treatment is lidocaine that acts by nonselective blockade of the Na+ channels pore. It binds in the pore of Na+ channels on sensory afferents of small damaged or dysfunctional pain fibres. Penetration into the intact skin after transdermal diffusion does not produce a complete sensory block of Na+ channels on large myelinated Aβ sensory fibres [25]. Our results showed Group LL experienced improvement in pain reduction, this is supported by other studies that indicate lidocaine medicated plaster monotherapy is beneficial in treating peripheral neuropathic pain [6, 26,27,28].
However, a recent European label extension has allowed using 8% capsaicin, either alone or in combination with other pain medications, to be used in adults with painful diabetic neuropathy. The interactions of capsaicin with the TRPV1 receptors explains the analgesic and anti-inflammatory nature of capsaicin [32].
A potential advantage of the capsaicin 8% patch is that a single treatment can offer lasting pain relief. The present application of capsaicin 8% patch treatment is more effective in reducing painful symptoms associated with diabetic neuropathy and is well tolerated when compared to lidocaine patches. The results are in line with other studies which also reported positive results for capsaicin 8% patch treatment in patients with painful diabetic peripheral neuropathy [29].
The results also showed that capsaicin 8% patch repeat treatment over 24 weeks was well tolerated in patients with painful peripheral diabetic neuropathy. In addition to this, repeat treatment with the capsaicin 8% patch induced substantial and sustained reductions in pain over the 24 weeks of treatment. Although this goes in line with what was reported in Ostrovosky’s study, we focused on Type 2 diabetes mellitus unlike the aforementioned study that included both Type 1 and Type 2 diabetes mellitus individuals. This is because the pathophysiologic development, progression and response to treatment differs between the two types of diabetes [30, 31].
Application of the capsaicin 8% patch is associated with treatment-related discomfort. Pain relief measures are used to reduce this discomfort. Lidocaine is often the medication of choice which is why we chose to use this but there are other options including systemic tramadol to relieve the pain associated with capsaicin patch application. [19]
The major outcome of the present study concerned the analgesic effectiveness of treatment with the capsaicin 8% patch. Repeated treatment with the capsaicin 8% patch induced a sustained reduction in average daily pain intensity for the subgroup that received the treatment. The reduction in pain was consistent after the treatment, with no or minimal increase before retreatment with the capsaicin 8% patch. These results were supported by the global impression of improvement reported by about one-third of patients at the end of the study. The Haanpää et al. study compared the patch effectiveness to systemic analgesia, concluding that the patch offered more sustained pain relief and improved patient satisfaction. Our findings align with what is previously known about the effectiveness of using capsaicin [32,33,34].
In Group LC, following two treatments of the capsaicin 8% patch, there was no increase in sensory deterioration observed in the study participants. As capsaicin provides pain relief by causing defunctionalisation of hyperactive nociceptors, impaired sensory perception may have been a potential effect of the patch following repeat treatment. The standardized neurological examination enabled qualitative categorization of sensory deficits and pain. This was important to assess safety and this study did not discover any new safety concerns. These results do not support any estimation of cumulative sensory alteration at the application site. Our results are in line with the study conducted by Vinik et al. which showed no alternation in sensory perception testing of sharp, warm, cold and vibration stimuli despite the capsaicin 8% patch being used for 52 weeks. However, the Vinik et al. had participants with concomitant opioid use, a Caucasian population consisting of both male and female participants. Their findings may not be widely applicable to patients of other ethnicity. [10] The study by Webster et al. also showed that using the capsaicin 8% patch did not result in detrimental effects on the sensory function of individuals with painful diabetic neuropathy, however the study was conducted for 12 weeks which is half the time of our study. [35] The study by Simpson et al. using the capsaicin 8% patch for other types of peripheral neuropathy and followed up for 48 weeks also showed no evidence of neural impairment. [36]
The findings of the present study add to those limited data for comparing lidocaine and capsaicin 8% patch in treating chronic diabetic neuropathic pain. The capsaicin 8% patch was useful in terms of pain relief. The data indicate a clinically relevant mean reduction in pain score (NPRS) by 46% when using capsaicin 8% patches. Clinical benefit was also indicated in the global impression of change (PGIC). The results of this study support and add to the previously reported efficacy of combination of the 5% lidocaine medicated plaster with an existing partially effective systemic agent. Lidocaine can have differential effects on sensory function. Detection thresholds have been shown to be elevated for touch, pinprick pain and mechanically induced wind-up after lidocaine 5% patch application in previous studies. However, since the effect was reversed in 3 days due to the clearance of lidocaine from the skin, our sensory testing performed several days after the previous patch application, impact from using topical anaesthetics on the results is probably unlikely.
Capsaicin 8% patch treatment in patients with painful diabetic neuropathy was most commonly associated with transient application site reactions. This is consistent with studies based in patients experiencing other forms of neuropathy including diabetic neuropathy [37]. In the present study, no serious treatment related adverse effects occurred.
There was a sustained response to the initial and repeat treatment with the capsaicin 8% patch that is evident by the treatment response sustaining over the 24 weeks. Overall the capsaicin 8% patch was reasonably well tolerated and effective long-term treatment option in individuals with painful diabetic neuropathy. The 8% capsaicin patch could benefit those patients using systemic therapies and experiencing incomplete pain relief or systematic side effects.
Our study also has limitations. The trial was probably underpowered, taking into account the smaller than expected number of participants. The required sample was not attained due to limitations of time and volunteer availability. Due to this additional research may be needed to further evaluate the outcomes of this study.
Conclusion
The present study assessed, in a clinical practice, real world setting, the effectiveness of the 8% capsaicin patch in providing fast pain relief to diabetics with peripheral diabetic neuropathy. There was a sustained response to the initial and repeat treatment of the capsaicin 8% patch that is evident by the treatment response sustaining over the 24 weeks. Overall the capsaicin 8% patch was reasonably well tolerated and effective long-term treatment option in individuals with painful diabetic neuropathy. The 8% capsaicin patch could benefit those patients using systemic therapies and experiencing incomplete pain relief or systematic side effects.
Data availability
The data that support the findings of this study are available on request from the corresponding author; N.H, upon reasonable request.
References
Aslam A, Singh J, Rajbhandari S. Pathogenesis of painful diabetic neuropathy. Pain Res Treat. 2014;2014:412041.
Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Current Neurol Neurosci Reports. 2014;14(8):473–3.
Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54.
Tsuji M, et al. Painful diabetic neuropathy in Japanese diabetic patients is common but underrecognized. Pain Res Treat. 2013;2013:318352.
Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster--a review. Curr Med Res Opin. 2012;28(6):937–51.
de Leon-Casasola OA, Mayoral V. The topical 5% lidocaine medicated plaster in localized neuropathic pain: a reappraisal of the clinical evidence. J Pain Res. 2016;9:67–79.
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73.
Martini C, Yassen A, Olofsen E, Passier P, Stoker M, Dahan A. Pharmacodynamic analysis of the analgesic effect of capsaicin 8% patch (Qutenza) in diabetic neuropathic pain patients: detection of distinct response groups. J Pain Res. 2012;5:51–9.
Simpson DM, Robinson-Papp J, van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind. Placebo-Controlled Study J Pain. 2017;18(1):42–53.
Vinik AI, Perrot S, Vinik EJ, Pazdera L, Jacobs H, Stoker M, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16(1):251.
European Medicines Agency (2013) Summary of Product Characteristics: Qutenza 179 mg cutaneous patch . Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/qutenza. Accessed on 12 Jan 2020.
FDA Center for Drug Evaluation and Research. Medical review: Qutenza. MD: Silver Spring; 2009.
Hayman M, Kam PCA. Capsaicin: a review of its pharmacology and clinical applications. Current Anaesthesia Critic Care. 2008;19(5–6):338–43.
Chen X, Zhang J, Wang X. Hormones in pain modulation and their clinical implications for pain control: a critical review. Hormones. 2016;15(3):313–20.
Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends Endocrinol Metab. 2003;14(1):20–7.
Berlin JA, Glasser SC, Ellenberg SS. Adverse event detection in drug development: recommendations and obligations beyond phase 3. Am J Public Health. 2008;98(8):1366–71.
Binu VS, Mayya SS, Dhar M. Some basic aspects of statistical methods and sample size determination in health science research. Ayu. 2014;35(2):119–23.
Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28(1):1–11.
Jensen TS, et al. Tolerability of the capsaicin 8% patch following pretreatment with lidocaine or tramadol in patients with peripheral neuropathic pain: a multicentre, randomized, assessor-blinded study. European J Pain (London, England). 2014;18(9):1240–7.
Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23(2):129–38.
Perrot S, Lanteri-Minet M. Patients' global impression of change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain. 2019;23:1117–28.
Nilsson ME, Koke SC. Defining treatment-emergent adverse events with the medical dictionary for regulatory activities (MedDRA). Drug Inform J. 2001;35(4):1289–99.
Hartemann A, Attal N, Bouhassira D, Dumont I, Gin H, Jeanne S, Said G, Richard JL. Working Group on the Diabetic Foot from the French speaking Society of Diabetology. Painful diabetic neuropathy: diagnosis and management. Diabetes Metab. 2011;37(5):377–88. https://doi.org/10.1016/j.diabet.2011.06.003.
Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med. 2013;125(4 Suppl 1):25–33.
Pickering G, et al. Localized neuropathic pain: an expert consensus on local treatments. Drug Des Devel Ther. 2017;11:2709–18.
Hartemann A. et al. Painful Diabet Neuropathy: Diagnosis Manag. 2011;37(5):377–88.
Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: an update. Am Fam Physician. 2016;94(3):227–34.
Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61(6):914–8.
van Nooten F, et al. Capsaicin 8% Patch Versus Oral Neuropathic Pain Medications for the Treatment of Painful Diabetic Peripheral Neuropathy: A Systematic Literature Review and Network Meta-analysis. Clin Ther. 2017;39(4):787–803.e18.
Ostrovsky DA. Single treatment with capsaicin 8% patch may reduce pain and sleep interference up to 12 weeks in patients with painful diabetic peripheral neuropathy. Explore (NY). 2017;13(5):351–3.
Uceyler N, Sommer C. High-dose capsaicin for the treatment of neuropathic pain: what we know and what we need to know. Pain Ther. 2014;3(2):73–84.
Burness CB, McCormack PL. Capsaicin 8% patch: a review in peripheral neuropathic pain. Drugs. 2016;76(1):123–34.
Haanpaa M, et al. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur J Pain. 2016;20(2):316–28.
Hansson P, Jensen TS, Kvarstein G, Strömberg M. Pain-relieving effectiveness, quality of life and tolerability of repeated capsaicin 8% patch treatment of peripheral neuropathic pain in Scandinavian clinical practice. Eur J Pain. 2018;22(5):941–50.
Webster LR, Peppin JF, Murphy FT, Lu B, Tobias JK, Vanhove GF. Efficacy, safety, and tolerability of NGX-4010, capsaicin 8% patch, in an open-label study of patients with peripheral neuropathic pain. Diabetes Res Clin Pract. 2011;93(2):187–97.
Simpson DM, Gazda S, Brown S, Webster LR, Lu SP, Tobias JK, et al. Long-term safety of NGX-4010, a high-concentration capsaicin patch, in patients with peripheral neuropathic pain. J Pain Symptom Manag. 2010;39(6):1053–64.
Mankowski C, Poole CD, Ernault E, Thomas R, Berni E, Currie CJ, et al. Effectiveness of the capsaicin 8% patch in the management of peripheral neuropathic pain in European clinical practice: the ASCEND study. BMC Neurol. 2017;17(1):80.
Acknowledgments
We thank Dr. Fatima, Dr. Bilal and Dr. Zahra for their work and support.
Funding
The study received the hospital research grant approved by the Punjab Care Hospital Research Council [IAC-0457]. The funders did not have any role in the study design, execution, data collection or analysis and in the manuscript preparation or editing.
Author information
Authors and Affiliations
Contributions
N.H. and A.S.A.S, contributed to the design and implementation of the research. Z.K supervised the work and contributed in data collection and processing. F.J, A.H.I.A.A contributed to the analysis of the results. M.A and A.A.M contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
The authors all declare that there is conflict of interest. The research grant was not obtained from any pharmaceutical company or supplier and no author was a member of any study sponsor source.
Ethical approval
The study was approved by the Punjab Care hospital review board (Approval no. 38920), conducted in agreement with the ethical guidelines of the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable regulatory requirement.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors are in agreement to proceed for publication of this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussain, N., Said, A.S.A., Javaid, F.A. et al. The efficacy and safety profile of capsaicin 8% patch versus 5% Lidocaine patch in patients with diabetic peripheral neuropathic pain: a randomized, placebo-controlled study of south Asian male patients. J Diabetes Metab Disord 20, 271–278 (2021). https://doi.org/10.1007/s40200-021-00741-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00741-2