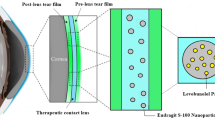
Abstract
Background
Increased intraocular pressure is a common symptom of glaucoma. In severe circumstances, it may result in loss of eyesight. Glaucoma treatment is difficult due to ocular physiological barriers that prevent medications from reaching the afflicted area. Traditional formulations (eye drops) have a short residence period and are rapidly drained away via the nasolacrimal duct, resulting in increased adverse drug responses and lower efficacy. The usage of nanoparticles such as niosomes could be one potential answer to these problems. While niosomes improve drug penetration, they have little effect on ocular retention of the medication. Contact lenses containing niosomes can assist to overcome this disadvantage.
Objective
This study aims to prepare and evaluate Brimonidine niosomes laden contact lenses for the treatment of Glaucoma.
Methods
Brimonidine niosomes were prepared using thin film hydration method and evaluated. The contact lenses were soaked in the niosomal formulation at varying intervals (3–10 days). Thereafter, the contact lenses were evaluated for %transmittance, %swelling index, drug quantification and in vitro drug release. The pharmacodynamic studies were conducted to assess the reduction in intraocular pressure (IOP) in albino rabbits. The research compared the results of the reduction in intraocular pressure caused by Brimonidine niosomes laden contact lenses with a marketed preparation of niosomes.
Results
Higher concentration of the drug was loaded in contact lenses loaded with Brimonidine niosomes compared to the marketed formulation, by soaking method. The contact lenses exhibited an optimal %transmittance of 98.02 ± 0.36 and %swelling index of 50.35 ± 0.57. Increase in the soaking time up to 7 days led to an increase in the drug concentration in the contact lenses. However, no further increase was observed after the 7th day due to saturation of the contact lenses. Brimonidine niosomes laden contact lenses provided a reduction in intraocular pressure that was similar to the marketed preparation. Further, the contact lenses provided extended release up to 20 h.
Conclusion
Brimonidine niosomes laden contact lenses exhibited superior drug loading through the soaking method, displaying optimal %transmittance and %swelling index. Soaking for 7 days increased drug concentration in contact lenses with no further increase due to saturation. These lenses reduced intraocular pressure like the marketed formulation, offering extended release for 20 h.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is an ocular disorder attributed to increased intraocular pressure. This increase in intraocular pressure may arise from a high content of aqueous humour or its reduced outflow from the trabecular meshwork and the uveoscleral pathway [1]. Over time, this increase in intraocular pressure creates intense pressure on the optic nerve and can lead to the deterioration of the retinal ganglion cells (RGCs). It might result in vision loss over a long period [2]. While glaucoma is not curable, there are various strategies to reduce its progression.
Current treatment option for glaucoma includes eye drops. The challenges with eye drops include reduced ocular retention, side effects such as conjunctival hyperemia and allergic conjunctiviti, and a higher dosing frequency [3]. The daily dose of brimonidine eye drops (Alphagan P (0.2%)) is three times daily. Eye drops have a bioavailability of about 50% [4]. Additionally, most of the drug goes into the conjunctiva and the nasolacrimal drainage leading to systemic adverse drug reactions [5].
Glaucoma can be treated with a variety of drugs. Some of these drugs decrease the secretion of aqueous humor (Alpha-2 adrenergic agonists, beta-1 adrenergic antagonists and carbonic anhydrase inhibitors) while others increase the uveoscleral outflow (Cholinergics, prostaglandins and prostamides) Brimonidine (alpha-2 adrenergic agonist) acts on alpha-2 receptors. It reduces the production and the outflow of aqueous humour [6]. Brimonidine increases the transcription of neurotrophic factors to increase the neuronal growth and differentiation of retinal ganglion cells (RGCs). It provides neuroprotection to the RGCs, reducing the possibility of vision loss [7]. Some of the common side effects of Brimonidine include CNS depression leading to fatigue and lethargy, and dry eyes. Incorporating the drug in niosomes is a potential system to overcome these side effects. Niosomes are novel nanovesicles, composed of non-ionic surfactant that show higher stability than micelles and liposomes [8]. While niosomal eye drops can provide a controlled drug release, they have lower retention on the ocular surface, and prevent optimal drug delivery.
Drug loading in contact lenses can be a potential treatment option for the treatment of glaucoma. The contact lens lies in proximity to the cornea and provides a polymeric surface to the drug. This increases the ocular retention of the drug. Further, it prevents the spilling of the drug outside the eye or drainage in the nasolacrimal fluid. Drug-laden contact lenses show sustained and controlled drug release that helps with frequent dosing and can aid in patient compliance. However, contact lenses loaded with drug solution show a high initial burst release [9]. This may lead to dose dumping. As a result, the researchers have used nanoparticles to load into the contact lenses.
Incorporating drug laden niosomes in contact lens can help to overcome lower ocular retention of the drug. Fathala et. al, demonstrated a sustained reduction in the intraocular pressure (IOP) with latanoprost niosomes [10]. Previous studies demonstrate the loading of micelles [11], microemulsions [12] and metallic nanoparticles [13] in contact lenses for treating glaucoma. Some of the past studies have reported Bimatoprost microemulsion [12], Levobunolol Eudragit nanoparticles [9] and timolol gold nanoparticles in contact lenses [13] that offered drug release up to a few days. However, they did not discuss the practical implications of overnight use of contact lenses. Moreover, the drug-laden contact lenses demonstrate a common issue of drug leaching. As a result, the storage of contact lenses after use is a challenge.
In this study, we have aimed to prepare brimonidine niosome laden contact lenses for daily application (once a day). The study explores the efficacy of brimonidine niosomes-laden contact lenses compared to the marketed formulation, by checking the effect of the formulations on the intraocular pressure of rabbits.
Materials and methods
FDC Ltd. (Mumbai, India) provided the gift sample of Brimonidine. Research labs (Mumbai, India) provided Cholesterol, Spans 20, 40, 60 and 80, and Tween 80. Thermo Fisher Scientific India Pvt. Ltd., (Mumbai, India) provided the chloroform and methanol (HPLC grade). Hioxifilcon A contact lenses (ACME, USA) were used in the study.
Preparation and characterization of niosomes
Selection of surfactant
Niosomes were prepared with different grades of Spans (20,40,60 and 80). Accurately weighed ratio of the Span and Cholesterol were added in the Round Bottom flask (RBF) followed by addition of 3:1 ratio of cholesterol and methanol. The RBF was rotated on rotary evaporator (Heidolph Base Hei-Vap ML Adv/Pre, EU) at a temperature of 50ͦ C and 60 °C (depending on the melting point of spans) and 100 rpm, for 10 min. A thin film was obtained which was hydrated with pH 7.4 Phosphate buffer. The formed dispersion was probe sonicated for 5 min at 30% amplitude [11, 14, 15]. The surfactant was selected based on the obtained vesicle size and zeta potential.
Preparation of niosomes
Preliminary lab trials
Initially, 500 mg of Span 60 and 100 mg of cholesterol were taken in a 250 ml Round Bottom Flask (RBF) and a 3:1 ratio of the organic solvents (Chloroform: Methanol) were added. The resultant solution was bath sonicated for 10 min to completely dissolve the surfactant and cholesterol in the organic solvents. The RBF was rotated on rotary evaporator (Heidolph Base Hei-Vap ML Adv/Pre, EU) at a temperature of 50 °C and 100 rpm under vacuum for 10 min. However, Span 60 flakes were observed and no thin film formation occurred. This is because of the higher phase transition temperature (Tc) of Span 60 (56–58 °C) [16]. The temperature was then increased to 60 °C, keeping other parameters constant. This led to the formation of bubbles in the RBF and no thin film was formed. The formation of bubbles was attributed to the high amount of surfactant. The amount of surfactant was reduced to 100 mg, keeping the other parameters constant. This led to the formation of the thin film. Thereafter, 10 ml of pH 7.4 Phosphate buffer (PbS) was added to the RBF for hydration. No hydration of the thin film was observed initially at this stage. This might be because of the high hydrophobicity of the surfactant. Further, an amount of 0.5 ml of tween 80 was added to the surfactant-cholesterol mixture to enhance hydrophilicity of vesicles and to improve hydration. No change was made in the other parameters and the RBF was rotated for thin film formation. However, a small amount of tween 80 remained in the liquid phase and did not contribute to the formation of thin film. This was because of higher volume of Tween present in the mixture. The volume of tween was further reduced to just 1 drop, keeping the other parameters constant. Finally, a uniform thin film was observed which was hydrated with 10 ml of the buffer. This led to incomplete hydration. As a result, the volume of the hydrating liquid was increased to a total volume of 20 ml. This led to complete hydration of the film. The resultant niosome dispersion was probe sonicated for 5 min, (50 s play, 10 s pause) at 30% amplitude [17]. The experiment was repeated with different concentration ratios of span 60 and cholesterol for an optimum vesicle size and zeta potential.
Preparation of niosomes
Millimolar ratios of span 60 and cholesterol were added to a 250-ml round-bottom flask. Thereafter, 3:1 ratio of the organic solvents was added to the RBF. The mixture was bath sonicated for 10 min. After bath sonication, the RBF was connected to the rotary evaporator (Heidolph Base Hei-Vap ML Adv/Pre, EU) and niosomes were formed by thin film hydration technique at 60 °C and 100 rpm, and vacuum for 10 min. Thereafter, 20 ml of 7.4 PbS buffer containing 10 mg of Brimonidine, was added to hydrate the thin film [18,19,20]. This dispersion was probe-sonicated (5 min, 50 s pause, 10 s play, 30% amplitude) [17]. The formed niosomes were characterized for vesicle size, zeta potential, DSC, FTIR, and encapsulation efficiency.
Characterization of niosomes
Vesicle size and zeta potential
The vesicle size, polydispersity index, and zeta potential of niosomes were determined using Malvern Nano Zetasizer [21]. The niosome formulation was diluted ten times with distilled water, poured in a cuvette, and the parameters were determined at 25 ͦC using Zetasizer software [17]. Triplicates of the sample were analyzed for each.
Differential scanning calorimetry (DSC)
DSC of Brimonidine and brimonidine-loaded niosomes was performed by DSCI, Mettler Toledo (Switzerland). Approximately 3 mg of the drug and the formulation were weighed and hermetically sealed in aluminium crucibles. The scanning range of the sample was 50–350 ͦC with constant heating at 5 °C. The sample was purged with nitrogen at a rate of 100 ml/min [22].
FTIR analysis
Spectroscopic analysis of Brimonidine, excipients and their physical mixture was carried out using ATR-FTIR instrument (Spectrum 2, Perkin. United States). The sample was placed on the sample compartment and an optimum pressure of 70 psi was applied. Baseline correction was done, and an IR spectrum was obtained in the range 4000–400 cm-1. The observed spectra were smoothened and labelled [23].
Transmission electron microscopy (TEM)
The morphology of drug loaded niosomes was examined by Transmission Electron Microscope (TEM) [TEC-12, TECNAI G2 SPIRIT BIOTWIN] using Tungsten “W” filament. 20 µl of the sample were diluted in 6 ml distilled water. Two microliter of this sample was casted on cu-carbon grids obtained from Electron microscopy Sciences, USA. The sample was dried with infrared lamp for 5 min. The niosomes were negatively stained with 5 µl phosphotungstic acid for 20 s. Excess of phosphotungstic acid was blotted on to filter paper. The sample was dried at room temperature for 2 min [24]. Thereafter, the morphology of the niosomes was observed at 100 kV [25] using Olympus soft imaging solutions VELETA CCD Camera and analysed using Tecnai imaging & Analysis software.
Encapsulation efficiency of the niosomes
The encapsulation efficiency of Brimonidine niosomes was estimated using UV–Vis spectrophotometer (Shimadzu, UV 1900i, Japan) [26, 27]. Two millilitres of the brimonidine niosomes were taken in centrifuge tube. The sample was centrifuged at 20,000 rpm and 4C using ultracentrifuge, for 1 h. 1 ml of the supernatant was then diluted with 7.4 pH buffer (PbS) up to 10 ml. The concentration of the unentrapped drug was quantified by UV analysis at 246.3 nm [26].
In -vitro drug release of niosomes
The in-vitro release study of niosomes was carried out in Franz diffusion cell (Lab Model (Assembled) DIE CELL, India). The temperature of the Franz cells was maintained at 34ͦ C by a heat circulator connected to the water jacket. Initially, 23 ml of simulated tear fluid (STF) was added to the receptor compartment. The receptor and donor chambers were isolated by a dialysis membrane (10,000 D molecular weight cut-off), pre-soaked in simulated tear fluid, overnight. One millilitre (0.428 mg) of the formulation was added to the donor chamber (inlet) and the mouth of the receptor chamber (outlet) was covered with foil to avoid evaporation of the formulation. The temperature of the cells was set at 34 °C and the rotation speed was set at 50 rpm [27]. One millilitre sample was withdrawn at the time intervals of 0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h, 10 h and 16 h from the receptor compartment. Equal amounts of STF were added to the receptor chamber after each withdrawal to maintain the sink conditions. The studies were performed in triplicate and analysed using UV Spectrophotometer (Shimadzu, UV 1900i, Japan) at 246.3 nm.
Drug loading and evaluation of contact lenses
Choice of contact lens
The selection of the contact lenses was based on the oxygen permeability of the material, swelling index, and wear time of the lenses.
Drug loading and characterization of contact lenses
The contact lenses were evaluated for %Transmittance and %swelling index before soaking them in the formulations (brimonidine niosomes and marketed preparation). During soaking of contact lenses, the drug molecules get incorporated in the internal aqueous ducts of contact lenses [28, 29]. The contact lenses were placed in 10 ml glass vials containing the niosome formulation. Initially 0.7 ml (equivalent to daily dose of 0.3 mg of Brimonidine) of niosome formulation was added. However, negligible loading of the drug was observed. Thereafter, the volume of the soaking solution was increased to 2 ml [11] Similarly, the contact lenses were soaked in 2 ml of marketed preparation and the drug content was evaluated in the contact lenses on 3rd, 5th, 7th and 10th days.
Transmittance of contact lenses
The transmittance of the blank, niosome-laden and marketed preparation (Alphagan P) laden contact lenses was determined with a UV spectrophotometer (Shimadzu, UV 1900i, Japan). Baseline was measured using distilled water. Thereafter, the lenses were scanned from 200 to 800 nm. The % transmittance was measured at 480 nm [30], for comparison.
Swelling index
Swelling Index of the contact lens is the extent to which the polymer absorbs and retains water. A high swelling index is crucial for higher oxygen permeability and hence higher comfort. To evaluate the swelling index, the contact lenses were removed from the packet using forceps and the dry weight of the blank contact lens was measured (Wd). The contact lenses were weighed again after soaking in niosome formulation and marketed preparation (wet weight). The Swelling Index of the contact lenses was determined using the following formula [31]:
- Ws:
-
Weight of soaked contact lens
- Wd:
-
Weight of dry contact lens (before soaking)
Quantification of the drug in contact lenses
The content of Brimonidine in the contact lenses was evaluated using the orbital shaker (LJE-0580, Eltek India). The soaked contact lenses were removed from the soaking solution using forceps. They were then added to a centrifuge tube containing 5 ml methanol. The setup was placed in the orbital shaker and the rotation speed was set to 100 rpm, overnight [32]. One millilitre of the sample was withdrawn and analysed using UV Spectrophotometer (Shimadzu, UV 1900i, Japan) at 246.5 nm [9].
In vitro drug release
The niosome laden contact lenses were immersed in 3 ml STF (tear fluid/day) at 34 ͦC. The vials were then agitated at 50 rpm using orbital shaker (LJE-0580, Eltek India). One millilitre of the sample was withdrawn at determined time points. Equal quantity of the STF was added to maintain sink conditions. The obtained samples were analysed at 246.3 nm using UV spectrophotometer Shimadzu, UV 1900i, Japan) [9].
Sterilization of contact lenses
Gamma sterilization of the Brimonidine niosome laden contact lenses was carried out at Agrosurg irradiators Pvt. Ltd. (Mumbai, India) as per the ISO standard 11137-2. The contact lenses were hermetically sealed in Polyethylene terephthalate (PET) sheets and exposed to Gamma radiation from a Cobalt-60 source at 25kGY for 6 h at room temperature. The temperature was maintained at 25 C during the sterilization process [27, 32].
Animal studies
Evaluation of IOP in rabbits
The in-vivo IOP lowering study was approved from the Institutional Animal Ethics Committee (IAEC) of SVKM’s NMIMS, Mumbai (CCSEA/IAEC/P-03/2023). The study design involved albino rabbits weighing 1.5–2.5 kg. One eye of the rabbit was kept as control, while marketed preparation (Alphagan P (0.2%)) and brimonidine niosomes laden contact lenses were instilled in the other eye. All these studies were done in triplicate for each timepoint. The rabbit was kept in a holder that allowed only neck movement. 2 drops of Lidocaine (0.2%) eye drops were instilled before instilling the preparations and measuring the IOP. The intraocular pressure was measured at set intervals (1 h, 2 h, 4 h, 6 h, 8 h, 10 h and 24 h) using Schiotz tonometer (Germany) [33, 34].
Results
Preparation of niosomes
Selection of the surfactant
Among all the spans, smallest vesicle size was obtained with Span 60. The results were in accordance with the literature review. As a result, Span 60 was selected as the surfactant.
Preliminary lab trials
The parameters for preparation of niosomes were finalized. Weighed quantities of surfactant and cholesterol were added in the 250 ml RBF, followed by 3:1 ratio of organic solvents. The mixture was bath sonicated for 10 min and thereafter rotated on the rotary evaporator for 10 min at 60 C, 100 rpm and vacuum. The resultant solution was hydrated with 20 ml pH 7.4 buffer and probe sonicated for 5 min at 30% amplitude (50 s play, 10 s pause) [17].
The preliminary batch trials revealed that niosomes prepared using weight ratios of span 60 and cholesterol had a higher vesicle size (> 600 nm) and low zeta potential values. This might be because of higher concentration of the surfactant and cholesterol. Thereafter, millimolar ratios of Span 60 and cholesterol were selected (40:100, 50:100 and 70:100). The optimized batch was selected based on encapsulation efficiency of the niosomes.
Preparation of optimized niosomes
The niosomes were prepared with varying millimolar ratios of span 60 and cholesterol. These niosomes were further characterized for vesicle size, zeta potential, encapsulation efficiency and in vitro drug release.
Characterization of niosomes
Vesicle size and zeta potential
After the preliminary screening of varying ratios of span 60: cholesterol (Table 1 and 2), vesicle sizes of niosomes were observed to vary between 100–300 nm. An increase in the vesicle size was observed upon addition of Brimonidine to the blank niosomal formulation. However, the vesicle size remained in the desired range.
Differential scanning calorimetry (DSC)
The thermogram of Brimonidine (pure drug) and the Brimonidine loaded niosomes is represented in Fig. 1A. The sharpness of the peaks represents the crystallinity of the drug. An endothermic peak of the drug is obtained at 213.45ͦ C, representing the melting point of the drug. The peak was observed in accordance with the reported data. An exothermic peak of the drug is obtained at 268.64ͦ C [35, 36]. The peaks for span 60, tween 80 and cholesterol were observed at 49.7ͦ C, 115ͦ C [37] and 147.87ͦ C [38], respectively. An endothermic peak of the niosome formulation is obtained at 101.72 °C. A shift from the reported values of the peaks of span 60, tween 80 and cholesterol was also observed in the niosome formulation.
FTIR analysis
The FTIR spectra of Brimonidine, Cholesterol, Span 60, Tween 80 and the physical mixture of the constituents were observed using the ATR-FTIR instrument (Fig. 1B). The observed peaks are listed in Table 3. The spectra were found to be similar to the reported values [39]. The FTIR of cholesterol revealed a broad band at 3392.71 cm-1, which represents the –O-H stretching. -C-H stretch was observed at 2929.34 cm-1. -C = C stretch was observed at 1643 cm-1, and -C-H stretch was observed at 952 cm-1 [40]. The FTIR spectra of Span 60 revealed –C-CO-O- stretch at 1172.21 cm-1, -C = O stretch at 1732.6 cm-1, asymmetric C-H stretching at 2916.26, symmetric -C-H stretching at 2849.06 cm-1 and -CH2 rocking vibrations at 721.17 cm-1 [41]. The FTIR spectra of Tween 80 revealed –O-H stretching at 3497.15 cm-1, -CH2 stretching at 2859.12 cm-1, -C = O stretching at 1735.06 cm-1 and –H-O-H bending at 1642.03 [42].
Transmission electron microscopy (TEM)
Transmission electron micrograph (TEM) of optimized brimonidine niosomes (DNS6T8F3) is shown in Fig. 1C. Sphere shaped niosomes were observed.
Encapsulation efficiency of niosomes
The amount of brimonidine niosomes encapsulated in the niosomes was estimated by UV spectrophotometer. The encapsulation efficiencies were in the range of 40%-85% (Table 2). groups of surfactants [43].
In vitro drug release from niosomes
The Franz Diffusion cell apparatus (Lab Model (Assembled) DIE CELL, India). was used to determine drug release from Brimonidine loaded niosomes. 99.32% ± 2.37 of the drug in the niosome formulation was released within 16 h. The amount of drug equivalent to the daily dose (0.3 mg) of Brimonidine was observed to release within 10 h (Fig. 2A).
The drug release data was fitted into zero order, first order, Higuchi and Korsmeyer-Peppas drug release kinetic models (Table 4). The brimonidine niosomes showed the highest linearity with Higuchi model of release kinetics, followed by Zero order release kinetics and Korsemeyer-Peppas respectively. The n value for Korsemeyer-Peppas plot was found to be 0.791 which was within the range of 0.45 – 0.89 signifying a non-fickian release behaviour [44].
Drug loading and characterization of contact lenses
Choice of contact lens
Planar Hioxifilcon A (ACME 55 Aspheric) contact lenses were selected for this study. Hioxifilcon A is formed by the copolymerization of Hydroxyethyl Methylacrylate (HEMA) and Glycerol Methacrylate (GMA) [45].
Transmittance
Transmittance is a critical parameter for contact lenses. A decrease in transmittance can lead to blurred vision. %Transmittance of brimonidine niosome and the marketed preparation laden contact lenses was analysed using UV spectrophotometer (Shimadzu, UV1900i, Japan). Higher drug loading led to decreased transmittance of contact lenses (Table 5). The observed values of % transmittance was analysed by paired t-test using GraphPad Prism 9 Software. The analysis revealed a significant (p < 0.05) decrease in the %transmittance of the BT niosomes (Fig. 3c). and the marketed preparation laden contact lenses (Fig. 3d) compared to the blank contact lenses. No change in colour of the contact lens was observed upon loading the niosome preparation. However, the contact lens turned greenish yellow because of the greenish yellow colour of the marketed preparation (Fig. 3b).
a Soaking of contact lenses in Niosomal formulation and marketed preparation, b Blank contact lens, BT niosome laden contact lens, Marketed preparation laden contact lens, c, d The evaluation of Blank contact lenses (BCL), BT niosomes laden contact lenses (BNLCL) and Marketed preparation laden contact lenses (MPLCL) for %transmittance. Data are presented as mean ± S.D. with n = 12, e, f The evaluation of Blank contact lenses (BCL), BT niosomes laden contact lenses (BNLCL) and Marketed preparation laden contact lenses (MPLCL) for % Swelling Index. Data are presented as mean ± S.D. with n = 12
Swelling index
The Swelling Index is the extent to which the contact lens polymer absorbs or retains fluids. The higher the water content, the higher is the comfort. As a result, the contact lenses with high swelling index can be worn for longer periods. Swelling index of the contact lens is seen to decrease with the loading of BT niosomes and the marketed preparation in the contact lens (Table 5). This change in Swelling Index was analysed by paired t-test using GraphPad Prism 9 Software. An insignificant (p > 0.05) change was observed in the Swelling Index of both BT niosomes laden contact lens (Fig. 3e) and the marketed preparation loaded contact lens (Fig. 3f).
Drug quantification in contact lenses
The drug uptake in contact lenses soaked in brimonidine niosomes and the marketed preparation (Alphagan P (0.2%)) were studied (Table 5). Brimonidine eye drops are delivered thrice a day as a 0.2% formulation, which equates to 300 µg per day. The concentration of brimonidine niosome formulation was 0.5 mg/ml, therefore, an initial volume of 0.7 ml (containing 300 mcg of brimonidine) was considered. However, negligible uptake of the formulation was observed. Thereafter, the volume of the niosomal preparation was increased to 2 ml (Fig. 3a) An increased drug uptake occurred within the same time period (3 and 5 days). Soaking the contact lenses for 7 days showed a drug uptake of 304 ± 1.73 mcg/lens while, the lenses soaked in marketed preparation showed a drug uptake of 97.33 ± 3.78 mcg/lens. Niosome formulation laden contact lenses showed higher concentration than lenses soaked in marketed preparation.
In-vitro drug release of contact lenses
The drug release from contact lenses was measured up to 20 h using the orbital shaker. The percentage cumulative drug release from brimonidine niosomes and marketed preparation-soaked contact lenses is shown in Fig. 2B
The contact lenses soaked in Aphagan P showed high burst release within 1 h (52.25% ± 1.17), with 98.63% ± 2.29 within 6 h. However, a sustained release was observed with the brimonidine niosomes laden contact lenses for a period of 20 h. A cumulative release of 85.46% ± 2.97 was obtained at 18 h and 93.72% ± 2.37 at 20 h. The release of drug from the Brimonidine niosome laden contact lenses was found to be two times slower than the release observed for the drug niosomes.
The drug release data was fitted into zero order, first order, Higuchi and Korsmeyer-Peppas drug release kinetic models. (Table 6) The brimonidine niosomes showed the highest linearity with Higuchi model of release kinetics. This was followed by Korsmeyer-Peppas and Zero order release kinetics. The n value for Korsmeyer-Peppas plot was found to be 0.748 which was within the range of 0.45 – 0.89.
Sterilization of contact lenses
The Brimonidine niosomes laden contact lenses were sterilized using gamma sterilization. The sterilized contact lenses were further used of animal studies.
Animal studies
Evaluation of IOP
The albino rabbits were allowed to acclimatize for two weeks as per the CPCSEA guidelines. The marketed formulation (0.2% Alphagan P) was administered in the eye twice, once at the beginning (0 h) and again after 8 h. The contact lenses were kept on the rabbits' eyes for a total of 18 h, simulating the average waking time of humans, including 6 h of sleep (Fig. 4A).
The change in intraocular pressure in the eyes of albino rabbits was determined using Schiotz tonometer (Germany). 0.2% lidocaine eye drops were instilled in the rabbit’s eye before measuring the intraocular pressure. The changes in intraocular pressure were recorded at specific time intervals: 1 h, 2 h, 4 h, 6 h, 8 h, 10 h, and 24 h after the initial eye treatment. The final measurement at 24 h was crucial to confirm whether there was a lasting reduction in IOP after removing the contact lenses overnight (Fig. 4B).
Furthermore, the change in the intraocular pressure caused by the marketed formulation (Alphagan P) and the brimonidine niosomes-laden contact lenses was compared. The latter showed reduced intraocular pressure even after removing the contact lenses. This indicates that the niosomes adhered to the corneal surface offer sustained drug release [46].
Discussion
The Hydrophilic Lipophilic Balance (HLB) value acts as a crucial parameter for selecting a surfactant. As the hydrophobic moieties in the niosomes increase, the surface free energy decreases. As a result, spans with higher HLB values form niosomes with higher particle sizes [14]. As a result, Span 20 and Span 40 are less preferred. Branching in a surfactant decreases the surface energy and impacts the entrapment efficiency of the vesicle [15, 16]. Straight-chain surfactants like Span 60 show higher entrapment efficiency than Span 65 which is a branched surfactant [17, 18]. The optimal vesicle size for drug loading in contact lenses ranges from 50–300 nm. Gulsen et. al reported that DMPC liposomes up to a few micrometres show even distribution in the contact lenses matrix [35]. In their research Elshaer et. al, prepared prednisolone nanoparticles laden contact lenses. The researchers reported an average nanoparticle particle size of 347 ± 11.9 nm. The optimized preparation had a particle size of 294 ± 1.8 nm [36]. Higher span and cholesterol ratio lead to reduced vesicle size of the niosomes. The presence of surfactant creates repulsion between the molecules and prevents agglomeration of vesicles. As a result, increase in surfactant concentration decreases the vesicles size [37]. The increase in vesicle size for drug loaded formulation indicates the encapsulation of the drug. Span 60 is composed of highly permeable alkyl groups. In the presence of cholesterol and a temperature around the phase transition temperature, the alkyl chains become disorganized. Upon hydration with drug solution, hydrocarbon chain is extended and the drug gets incorporated in the surfactant vesicles. This leads to increase in the vesicle size [38].
The sharpness of the peaks of the DSC thermogram of Brimonidine represents the crystallinity of the drug. An endothermic peak represents the melting point of the drug while the exothermic peak depicts the thermal decay of Brimonidine. The peaks of Brimonidine are not visible in the thermogram of drug niosomes, this suggests than the drug is not present in crystalline form in the formulation and has been incorporated in the niosomes. An endothermic peak of the niosome formulation is obtained at 101.72 C. This shift from the reported values of the peaks of Span 60 (49.7 C), Tween 80 (115 C) [40] and Cholesterol (147.87 C) [41] is due to interaction between the constituents to form niosomes.
The FTIR spectra of Brimonidine, Cholesterol, Span 60, Tween 80 and the physical mixture of the constituents were observed using the ATR-FTIR instrument. The spectra were found to be similar to the reported values [42]. The FTIR of cholesterol revealed a broad band at 3392.71 cm-1, which represents the –O-H stretching. -C-H stretch was observed at 2929.34 cm-1. -C = C stretch was observed at 1643 cm-1, and -C-H stretch was observed at 952 cm-1 [43]. The FTIR spectra of Span 60 revealed –C-CO-O- stretch at 1172.21 cm-1, -C = O stretch at 1732.6 cm-1, asymmetric C-H stretching at 2916.26, symmetric -C-H stretching at 2849.06 cm-1 and -CH2 rocking vibrations at 721.17 cm-1 [44]. The FTIR spectra of Tween 80 revealed –O-H stretching at 3497.15 cm-1, -CH2 stretching at 2859.12 cm-1, -C = O stretching at 1735.06 cm-1 and –H-O-H bending at 1642.03 [46]. The data was found to be in accordance with reported data. This indicates no interaction between the drug and the excipients.
A change in the encapsulation efficiency of the niosomes was observed with change in the ratios of concentrations of Span 60 and Cholesterol. Higher surfactant concentration led to higher drug encapsulation. The lack of branching and unsaturated double bonds in Span 60 decreases permeability of niosomes. Further, increase in the length of alkyl chain leads to better entrapment [47]. In their research, Mavaddati et al., reported that higher concentration of cholesterol leads to higher bilayer hydrophobicity and stability of the niosomes. As a result, higher entrapment of drugs is seen. However, increase in the amount of cholesterol after 25–50 mol % leads to disruption in the structure of vesicles. The drug and cholesterol compete for incorporation in the niosome bilayer. As a result, less amount of drug is encapsulated in the niosome [48]. In their research, Barakat et. al reported that a combination of span and tween lead to higher drug entrapment of hydrophilic vancomycin compared to individual span or tween. The polar head groups of the hydrophilic drugs form hydrogen bonds the polar head groups of surfactants. This results in a more hydrophilic bilayer membrane and higher encapsulation of hydrophilic drugs [49].
Niosomes are novel nanovesicles. These non-ionic surfactant vesicles comprise of a membrane bilayer enclosing a central cavity. This central cavity forms a depot for hydrophobic and hydrophilic drugs. The release from the niosome vesicles depends upon the surfactant and cholesterol. High molecular weight surfactants such as Span 60 are less permeable than low molecular weight spans. As a result, slow rate of drug release is observed with span 60. Furthermore, the head (hydrophilic) of Span 60 reacts with OH groups present in cholesterol. This leads to formation of a hydrogen bond. This provides mechanical rigidity to the vesicles [50]. As a result, the span 60 vesicles offer controlled drug release.
The brimonidine niosomes showed the highest linearity with Higuchi model of release kinetics, followed by Zero order release kinetics and Korsemeyer-Peppas respectively. The n value for Korsemeyer-Peppas plot was found to be 0.791 which was within the range of 0.45 – 0.89. This indicates that the brimonidine niosome formulation follows a non-Fickian drug release mechanism. In their study, Fetih et al., prepared niosomal gels for treating corneal fungal infections. Both the niosomes and the niosomal gel formulation followed Higuchi drug release kinetics. Further, the formulations showed a dominant non Fickian release kinetics [51].
Silicone hydrogel contact lenses have a higher water content than HEMA contact lenses. It makes them more prone to microbial contamination. HEMA contact lenses have optimal oxygen permeability for daily wear and are more cost-effective than their silicone hydrogel counterparts. Planar Hioxifilcon A (ACME 55 Aspheric) contact lenses were selected for this study. Hioxifilcon A is formed by the copolymerization of Hydroxyethyl Methylacrylate (HEMA) and Glycerol Methacrylate (GMA). The presence of GMA increases hydroxyl groups and forms strong bonds between these hydroxyl groups and water. As a result, these contact lenses show less dehydration than other HEMA based contact lenses [52]. Therefore, these contact lenses prevent dry eyes and show improved comfort [53]. Further, the non-ionic nature of hioxifilcon A contact lenses prevents protein adhesion due to high charge repulsion [45].
A decrease in %transmittance of contact lenses was observed with higher drug concentration. In their study, Maulvi et. al, reported a decrease in %transmittance with increased loading of Bimatoprost [5]. Mun et al. also reported similar results wherein the loading of micelles in HEMA contact lenses led to a decrease in transmittance [54]. The decrease in transmittance is the result of drug loading in contact lenses. The decrease in transmittance of marketed preparation laden contact lenses might be because of change in colour of the contact lens due to the greenish yellow colour of the formulation. However, the values of %transmittance were above 95% indicating high visual clarity of the contact lenses.
The % swelling index of contact lenses reduced upon increase in drug loading in contact lenses. In their research, Xu et. al demonstrated a decrease in Swelling Index of contact lenses upon loading of drug loaded micelles. This decrease in Swelling Index was attributed to the incorporation of the micelles in the hydrophilic channels of the contact lenses [11]. The values of Swelling Index were in accordance with the reported 33–75% of marketed contact lenses [54].
Niosome formulation laden contact lenses showed higher concentration than lenses soaked in marketed preparation. This difference in drug loading is due to formation of niosomes that leads to higher surface area of the drug particles for a higher uptake. Higher loading of the drug was achieved upon increasing the volume of the niosome formulation from 0.7 ml to 2 ml. In their study, Maulvi et. al, confirmed an increase in loading of drug with the increase in concentration [13]. Further, the time of soaking of the contact lenses is of utmost importance in drug loading by soaking method. An increase in drug loading was obtained with a soaking time of 7 days. However, no further increase was observed beyond a week. This might be because of saturation of the contact lens. The mechanism by which niosomes increase drug loading in contact lenses is attributed to their structure and composition. Niosomes can encapsulate both hydrophilic and lipophilic drugs due to their bilayer structure, composed of non-ionic surfactants like polysorbates, and cholesterol [1]. This characteristic allows niosomes to accommodate a wide range of drug molecules, thereby increasing the drug loading capacity of the contact lens.
The release of drug from the Brimonidine niosome laden contact lenses was found to be two times slower than the release observed for the drug niosomes. This reduced drug release was attributed to the hydrophilic polymer of the contact lens. It acts as a barrier and hinders the drug release. The presence of a hydrophobic non-ionic surfactant in the contact lens decreases the rate of water penetration. Further, the formation of niosomes creates a diffusion barrier around the drug molecules, hindering drug release. This prevents the burst release effect, as observed in case of the marketed preparation [12]. The brimonidine niosomes showed the highest linearity with Higuchi model of release kinetics. This was followed by Korsmeyer-Peppas and Zero order release kinetics. The n value for Korsmeyer-Peppas plot was found to be 0.748 which was within the range of 0.45 – 0.89. This indicates that the brimonidine niosome formulation follows a non Fickian drug release mechanism.
The presence of tween 80 in the niosomes, that adheres to the ocular surface and aids in sustained release of the formulation [51, 55]. The Brimonidine niosomes laden contact lenses offered comparable reduction in IOP to the marketed formulation. The former showed an extended reduction in IOP for up to 24 h even after removal of contact lens. The inclusion of Tween 80 within the niosomes is a critical element that contributes to the adhesion of these vesicles to the ocular surface and facilitates the sustained release of the drug formulation. This feature plays a significant role in improving the bioavailability [56]. Tween 80 functions as a surfactant, enhancing the stability and dispersibility of the niosomes, thereby facilitating enhanced medication penetration into the ocular tissues [57, 58]. Moreover, the biocompatibility of this substance guarantees limited ocular discomfort, rendering it a highly suitable option for ocular drug delivery systems. The utilization of water-soluble surfactants, such as Tween 80, in niosomes has been found to enhance ocular bioavailability. This is attributed to the surfactants' ability to function as penetration enhancers, facilitating the removal of the mucus layer and disruption of junctional complexes between ocular tissue cells. This phenomenon facilitates better drug absorption and distribution within the ocular region, resulting in improved therapeutic effects. In addition, the incorporation of surfactants within niosomes can contribute to the extension of drug release duration, so ensuring a persistent therapeutic impact and diminishing the need for frequent administration.
Conclusion
The study has shown that incorporating hydrophobic and hydrophilic surfactants to prepare niosomes enhances drug loading in hydrophilic contact lens polymers like HEMA. Loading drug-loaded niosomes into a Hema-based contact lens takes about seven days. The amount of drug loaded in the contact lens depends on the concentration of the drug in soaking solution. Loading of niosomes in contact lenses is more effective than loading hydrophobic drug solution. Niosome-laden contact lenses offer a sustained release effect and can reduce the dosing frequency of the drugs and increase their ocular retention. The loading of niosomes does not significantly alter the desired % transmittance and the % swelling index of the contact lens. Niosomes adhere to the corneal surface and offer drug release even after removing the contact lens. This strategy helps to overcome the issue of overnight wear of the lenses and offers a significant reduction in IOP.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Any other data required can be made available upon request to the authors.
References
Yadav KS, Rajpurohit R, Sharma S. Glaucoma: current treatment and impact of advanced drug delivery systems. Life Sci. 2019;221:362–76. https://doi.org/10.1016/j.lfs.2019.02.029.
Yadav KS, Sharma S, Londhe VY. Bio-tactics for neuroprotection of retinal ganglion cells in the treatment of glaucoma. Life Sci. 2020;243:117303. https://doi.org/10.1016/j.lfs.2020.117303.
Peral A. Contact lenses as drug delivery system for glaucoma: a review. Appl Sci. 2020;10(15):5151. https://doi.org/10.3390/app10155151.
Sartini F. In vivo efficacy of contact lens drug-delivery systems in glaucoma management. A systematic review. Appl Sci. 2021;11(2):724. https://doi.org/10.3390/app11020724.
Maulvi FA, Shetty KH, Desai DT, Shah DO, Willcox MDP. Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration. Int J Pharm. 2021;608:121105. https://doi.org/10.1016/j.ijpharm.2021.121105.
Oh DJ, Chen JL, Vajaranant TS, Dikopf MS. Brimonidine for the treatment of glaucoma. Expert Opin Pharmacother. 2019;20:115–22. https://doi.org/10.1080/14656566.2018.1544241.
Cantor LB. Brimnidine in the treatment of glaucoma and ocular hypertension. Ther Clin Risk Manag. 2006;2(4):337–46. https://doi.org/10.2147/2Ftcrm.2006.2.4.337.
Alyami H. Nonionic surfactant vesicles (niosomes) for ocular drug delivery: Development, evaluation and toxicological profiling. J Drug Deliv Sci Technol. 2020;60:102069. https://doi.org/10.1016/j.jddst.2020.102069.
Kumar N, Aggarwal R, Chauhan KM. Extended levobunolol release from Eudragit nanoparticle-laden contact lenses for glaucoma therapy. Future J Pharm Sci. 2020;6:109. https://doi.org/10.1186/s43094-020-00128-9.
Fathala D, Fouad EA, Soliman GM. Latanoprost niosomes as a sustained release ocular delivery system for the management of glaucoma. Drug Dev Ind Pharm. 2020;46:806–13. https://doi.org/10.1080/03639045.2020.1755305.
Xu J, Ge Y, Bu R. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J Control Release. 2019;305:18–28. https://doi.org/10.1016/j.jconrel.2019.05.025.
Xu W, Wanzhen J. Bimatoprost loaded microemulsion laden contact lens to treat glaucoma. J Drug Deliv Sci Technol. 2019;54:101330. https://doi.org/10.1016/j.jddst.2019.101330.
Maulvi FA, Patil RJ, Desai AR, Shukla MR, Vaidya RJ, Ranch KM, Vyas BA, Shah SA, Shah DO. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: In vitro and in vivo evaluation. Acta Biomater. 2019;86:350–62. https://doi.org/10.1016/j.actbio.2019.01.004.
Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm Sin B. 2011;1(4):208–19. https://doi.org/10.1016/j.apsb.2011.09.002.
Verma A. Emerging potential of niosomes in ocular delivery. Expert Opin Drug Deliv. 2021;18:55–71. https://doi.org/10.1080/17425247.2020.1822322.
Gugleva V, Titeva S. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int J Pharm. 2019;567:118431. https://doi.org/10.1016/j.ijpharm.2019.06.022.
Gupta P, Yadav KS. Formulation and evaluation of brinzolamide encapsulated niosomal in-situ gel for sustained reduction of IOP in rabbits. J Drug Deliv Sci Technol. 2022;67:103004. https://doi.org/10.1016/j.jddst.2021.103004.
Moiseev RV, Morrison PW. Penetration enhancers in ocular drug delivery. Pharmaceutics. 2019;11(7):321. https://doi.org/10.3390/2Fpharmaceutics11070321.
Waqas MK, Sadia H. Development and characterization of niosomal gel of fusidic acid: in-vitro and ex-vivo approaches. Des Monomers Polym. 2022;2022:165–74. https://doi.org/10.1080/2F15685551.2022.2086411.
Khan DH, Bashir S. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J Drug Deliv Sci Technol. 2019;50:27–33. https://doi.org/10.1016/j.jddst.2019.01.012.
Verma P, Yadav KS. Quality by design (QbD) enabled and Box-Behnken design assisted approach for formulation of tranexamic acid loaded stratum corneum lipid liposomes. J Drug Deliv Sci Technol. 2023;86:104571. https://doi.org/10.1016/j.jddst.2023.104571.
Silva LD, Htar TT. Characterization, optimization, and in vitro evaluation of Technetium-99m-labeled niosomes. Int J Nanomed. 2019;14:1101–17. https://doi.org/10.2147/2FIJN.S184912.
Singh KH, Shinde UA. Chitosan nanoparticles for controlled delivery of Brimonidine to the ocular membrane. Pharmazie. 2011;66:594–9. https://doi.org/10.1691/ph.2011.0349.
Bayindir ZS, Yuksel N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech. 2012;13:826–35. https://doi.org/10.1208/2Fs12249-012-9805-4.
Mohamad EA, Fahmy HM. Niosomes and liposomes as promising carriers for dermal delivery of Annona squamosa extract. Braz J Pharm Sci. 2020;56:e18096. https://doi.org/10.1590/s2175-97902019000318096.
Eldeeb AE, Salah S, Ghorab M. Proniosomal gel-derived niosomes: an approach to sustain and improve the ocular delivery of Brimonidine; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019;26:509–21. https://doi.org/10.1080/10717544.2019.1609622.
Zheng J, Clogston DJ, Patri AK. Dobrovolskaia AM, McNeil SE. Sterilization of silver nanoparticles using standard gamma irradiation procedure affects particle integrity and biocompatibility. J Nanomed Nanotechnol 2011:95–101. https://doi.org/10.4172/2F2157-7439.S5-001.
Padala S, Sripada R, Gundabattula SB, Tadi KJ, Nallamothula PR, Raveshi F, Magharla DD. A comparative study on the efficacy of brinzolamide/timolol versus brinzolamide/brimonidine fixed drug combinations in primary open-angle glaucoma. Future J Pharm Sci. 2020;6(1):1–7.
Dubey A, Prabhu P. Development and investigation of niosomes of Brimonidine and timolol maleate for the treatment of glaucoma. Int J PharmTech Res. 2014;6(3):942–50.
Maulvi FA, Soni TG, Shah DO. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016;23:3017–26. https://doi.org/10.3109/10717544.2016.1138342.
Maulvi FA, et al. Controlled bimatoprost release from graphene oxide laden contact lenses: in vitro and in vivo studies. Colloids Surf B. 2020;208:112096. https://doi.org/10.1016/j.colsurfb.2021.112096.
Jung HJ, Jaoude MA. Glaucoma therapy by extended release of timolol from nanoparticle loaded. J Control Rel. 2012;2012:82–9. https://doi.org/10.1016/j.jconrel.2012.10.010.
Nguyen DC. Pharmaceutical-loaded contact lenses as an ocular drug delivery system: A review of critical lens characterization methodologies with reference to ISO standards. Cont Lens Anterior Eye. 2021;44(6):101487. https://doi.org/10.1016/j.clae.2021.101487.
Pereira-da-Mota AF, Lopez MV. Atorvastatin-eluting contact lenses: effects of molecular imprinting and sterilization on drug loading and release. Pharmaceutics. 2021;13(5):606. https://doi.org/10.3390/pharmaceutics13050606.
Gulsen D, Li CC, Chauhan A. Dispersion of DMPC liposomes in contact lenses for opthalmic drug delivery. Curr Eye Res. 2005;30:1071–80. https://doi.org/10.1080/02713680500346633.
Jain N, Verma A, Jain N. Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: intraocular pressure measurement in white albino rabbits. Drug Deliv. 2020;27:888–99. https://doi.org/10.1080/10717544.2020.1775726.
Elshaer A, et al. Nanoparticle-laden contact lens for controlled ocular delivery of prednisolone: Formulation optimization using statistical experimental design. Pharmaceutics. 2016;8(2):14. https://doi.org/10.3390/pharmaceutics8020014.
Basiri L, Rajabzadeh G, Bostan A. α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food Chem. 2017;221:620–8. https://doi.org/10.1016/j.foodchem.2016.11.129.
Abdelkader H, Ismail S, Kamal A, Alany RG. Preparation of niosomes as an ocular delivery system for naltrexone hydrochloride: Physicochemical characterization. Pharmazie. 2010;65:811–7.
Souza JF, Maia KN. Ocular inserts based on chitosan and Brimonidine: development, characterization and biocompatibility. J Drug Deliv Sci Technol. 2016:21–30. https://doi.org/10.1016/j.jddst.2016.01.008.
Pramod K, Ali J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. Jo Anal Sci Technol. 2015. https://doi.org/10.1186/s40543-015-0073-2.
Mohamed HB, El-Shanawany SM, Hamad MA, Elsabahy M. Niosomes: a strategy toward prevention of clinically significant drug incompatibilities. Sci Rep. 2017;7(1):6340. https://doi.org/10.1038/s41598-017-06955-w.
Sharma PK, Chauhan MK. Optimization and characterization of Brimonidine Nanoparticles-loaded in situ gel for the treatment of glaucoma. Curr Eye Res. 2021:1703–1716. https://doi.org/10.1080/02713683.2021.1916037.
Gupta U. Spectroscopic studies of cholesterol: Fourier Transform Infra-red and vibrational Frequency Analysis. Materials Focus. 2014:211–217. https://doi.org/10.1166/mat.2014.1161.
Soltys-Robitaille CE. The relationship between contact lens surface charge and in-vitro protein deposition levels. Biomaterials. 2001:3257–3260. https://doi.org/10.1016/S0142-9612(01)00163-6.
Farmoudeh A, Akbari J, Saeedi M. Methylene blue-loaded niosome: preparation, physicochemical characterization, and in vivo wound healing assessment. Drug Deliv Transl Res. 2020. https://doi.org/10.1007/s13346-020-00715-6.
Hao YM, Li K. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011:245–253. https://doi.org/10.1016/j.ijpharm.2010.10.027.
Mavaddati MA, Moztarzadeh F, Baghbani F. Effect of formulation and processing variables on dexamethasone entrapment and resease of niosomes. J Clust Sci. 2015:2065–2078. https://doi.org/10.1007/s10876-015-0908-4.
Barakat HS. Vancomycin-eluting niosomes: a new approach to the inhibition of staphylococcal biofilm on abiotic surfaces. AAPS PharmSciTech. 2014:1263–1274. https://doi.org/10.1208/2Fs12249-014-0141-8.
Gharbhavi M, Amani J. Niosome: a promising nanocarrier for natural drug delivery through Blood brain barrier. Adv Pharmacol Sci. 2018:6847971. https://doi.org/10.1155/2018/6847971.
Franco P, Marco ID. Contact lenses as ophthalmic drug delivery systems: a review. Polymers. 2021;13(7):1102. https://doi.org/10.3390/2Fpolym13071102.
Moreddu R, Vigolo D, Yetisen AK. Contact lens technology: from fundamentals to applications. Adv Healthc Mater. 2019;8(15):1900368. https://doi.org/10.1002/adhm.201900368.
Riley C, Chalmers RL, Pence N. The impact of lens choice in the relief of contcat lens related symptoms and ocular surface findings. Cont Lens Anterior Eye. 2005;23:13–9. https://doi.org/10.1016/j.clae.2004.09.002.
Mun J, Jeong S, Cho S. Drug-eluting contact lens containing cyclosporineloaded cholesterol-hyaluronate micelles for dry eye syndrome. RSC Adv. 2019;9(29):16578–85. https://doi.org/10.1039/C9RA02858G.
Kesavan K, Kant S, Singh PN, Pandit JK. Mucoadhesive chitosan-coated cationic microemulsion of dexamethasone for ocular delivery: in vitro and in vivo evaluation. Curr Eye Res. 2013;38(3):342–52. https://doi.org/10.3109/02713683.2012.745879.
Aj R, Hn Y, Sb S. Natural gums as sustained release carriers: development of gastroretentive drug delivery system of ziprasidone HCl. DARU J Pharm Sci. 2012;20:58. https://doi.org/10.1186/2008-2231-20-58.
Rad MS, Mohajeri SA. Simultaneously load and extended release of betamethasone and ciprofloxacin from Vitamin E-loaded silicone-based soft contact lenses. Curr Eye Res. 2016:1–7. https://doi.org/10.3109/02713683.2015.1107591.
Ammar HO. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTEch. 2009:808. https://doi.org/10.1208/s12249-009-9268-4.
Funding
The work did not receive any fundings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The work did not involve any human study so no need of any ethical approval.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tripathi, S., Yadav, K.S. Development of brimonidine niosomes laden contact lenses for extended release and promising delivery system in glaucoma treatment. DARU J Pharm Sci 32, 161–175 (2024). https://doi.org/10.1007/s40199-023-00500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-023-00500-z