Abstract
Background
Sildenafil is used to treat erectile dysfunction and pulmonary arterial hypertension and is metabolized in the liver mainly by CYP3A4, thus co-administration with drugs or herbal extracts that affect CYP3A4 activity may lead to drug-drug or drug-herb interactions, respectively. The aim of the present study was to evaluate the influence of single and multiple oral doses of methylxanthine fraction, isolated from Bancha green tea leaves on the pharmacokinetics of sildenafil in rats.
Methods
Rats were given sildenafil alone as well as simultaneously with methylxanthines or ketoconazole. The plasma concentrations of sildenafil were measured with high-performance liquid chromatography method with ultraviolet detection. The pharmacokinetic parameters of sildenafil were calculated by non-compartmental analysis.
Results
Concomitant use of sildenafil with a single oral dose of methylxanthines resulted in a decrease in Cmax (p > 0.05), AUC0-t (p < 0.05) and AUC0-inf (p < 0.05), while the administration of sildenafil after methylxanthines pretreatment resulted in an increase in Cmax (p < 0.0001), AUC0-t (p < 0.0001) and AUC0-inf (p < 0.001) compared to the sildenafil group. After co-administration of sildenafil and ketoconazole, a significant increase in Cmax, AUC0-t and AUC0-inf was observed in both of the experiments.
Conclusion
Drug-herb interactions were observed when sildenafil was co-administered with Bancha methylxanthines in rats. Further in vivo studies about the potential drug interactions between sildenafil and methylxanthines, especially caffeine, are needed to clarify mechanisms underlying the observed changes in sildenafil pharmacokinetics.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green tea is one of the most consumed beverages worldwide. It is prepared from the leaves of Camellia sinensis (L.) Kuntze, Theaceae [1]. It contains a variety of biologically active compounds, such as polyphenols, polysaccharides, alkaloids, free amino acids and saponins. The most important and well-studied tea polyphenols are catechins. They are natural antioxidants and possess various health benefits, such as cancer prevention, anti-aging, anti-inflammatory and neuroprotective properties, weight loss, reducing cholesterol levels and antibacterial effect [1, 2].
Green tea is also a source of purine alkaloids and, in particular methylxanthines. Naturally occurring methylxanthines are caffeine, theophylline and theobromine [2]. The most abundant methylxanthine in green tea is caffeine while theophylline and theobromine are present in trace amounts. Caffeine is also present in coffee and cocoa and it is a well-known stimulant of the central nervous system (CNS) as well as the cardiac and the respiratory system. It is used as an analgesic adjuvant. Theophylline is a bronchodilator and is used in the treatment of asthma and other lung diseases. Both theophylline and theobromine act as cardiac stimulants and dilators of coronary arteries. Several mechanisms contribute to the pharmacological effects of methylxanthines and, in particular, caffeine. However, the most important mechanism of action at physiologically relevant doses is considered to be the antagonism of adenosine receptors [1,2,3,4,5]. In humans, methylxanthines are metabolized in the liver mainly by CYP1A2 [6]. In addition, CYP3A4 is also involved in caffeine metabolism [7, 8].
There are different types of green tea depending on the time of harvest and subsequent manufacturing process, which determine their bioactive composition and antioxidant properties [9, 10]. Bancha green tea is a traditional Japanese tea product from the third or fourth harvest of green tea leaves [10, 11]. It is one of the main kinds of green tea consumed in Japan [12]. In this regard, it was reported that Bancha infusion contains less caffeine and L-theanine compared to other popular types of Japanese green tea, for instance, Sencha and Matcha, but there is contradictory information about its catechin content [9, 10, 13]. In addition, insufficient scientific information is available about the quantity of other bioactive compounds, health benefits, and potential herb-drug interactions of Bancha green tea leaves.
Sildenafil is a selective inhibitor of phosphodiesterase 5 (PDE5) that is used to treat erectile dysfunction and pulmonary arterial hypertension [14]. Sildenafil is administered orally and is rapidly absorbed from the gastrointestinal tract. However, it has relatively low oral bioavailability (~ 40% in humans) probably due to extensive presystemic metabolism. Sildenafil is metabolized mainly by CYP3A4 and to a lesser extent by CYP2C9 to the active metabolite N-desmethylsildenafil [15,16,17]. Due to the significant contribution of CYP3A4 in sildenafil metabolism, concomitant administration with drugs or herbal extracts that affect CYP3A4 activity may lead to drug-drug or drug-herb interactions respectively resulting in an increased toxicity or therapy failure [16]. Several studies reported drug-herb interactions when sildenafil has been administered simultaneously with certain herbs [15, 16, 18,19,20,21].
The aim of the present study was to evaluate the influence of single and multiple oral doses of methylxanthine fraction isolated from Bancha tea leaves on the pharmacokinetics of sildenafil in rats.
Methods
Chemicals and reagents
Bancha tea leaves were provided by a local herbal store and were morphologically identified by Assoc. Prof. Iliya Zhelev from the Department of Biology, Faculty of Pharmacy at the Medical University of Varna, Bulgaria. Sildenafil, ketoconazole, sodium carboxymethylcellulose, sulfuric acid, chloroform and sodium hydroxide were purchased from Sigma-Aldrich, GmbH. Methanol (≥ 99.8%, HPLC grade) was obtained from Fisher Chemicals, UK. Double-distilled water was laboratory prepared.
Extraction of methylxanthines
The extraction procedure of methylxanthines from Bancha tea leaves and their subsequent HPLC analysis were performed according to a protocol previously developed by the authors [22]. Accurately weighed amount of Bancha green tea leaves (50.0 g) was extracted under reflux with distilled water (250.0 mL) for 60 min and filtered through a Buchner funnel. The obtained aqueous extract was acidified with 25% sulfuric acid (5.0 mL) and concentrated to half of its initial volume. Then, the hot solution was filtered and extracted four times with chloroform (50.0 mL) in a separating funnel. The chloroform extract (200.0 mL) was washed twice with 5% sodium hydroxide solution (50.0 mL) and twice with distilled water (50.0 mL). After evaporation of the chloroform, the mixture of methylxanthines was obtained, and the percentage yield was calculated.
Development and validation of a HPLC-UV method for qualitative and quantitative determination of sildenafil in rat plasma
Analytical conditions
HPLC analysis was performed on a Thermo scientific AQUASIL C18 (150.0 mm х 4.6 mm, 5.0 μm) analytical column, protected by a guard-column AQUASIL C18 (10.0 mm х 4.6 mm, 5.0 μm) with a flow rate at 0.6 mL/min and UV detection at 228 nm. The volume of injection was 20.0 μL. The mobile phase was in isocratic mode and it was a mixture of methanol and double-distilled and filtered water (85:15%, v/v). The temperature of the chromatographic column and the sampler was maintained at 25°C. Systemic control, data collection and analysis were performed using the Thermo Scientific™ Chromeleon™ 7.2 Chromatography Data System software.
Standard and working solutions of sildenafil
Standard stock solution of sildenafil (0.05 mg/mL final concentration) was prepared by accurately weighing of sildenafil citrate (standard substance) and dissolving it in methanol. Subsequently, the working solutions were prepared by serial dilutions of the standard stock solution with methanol to obtain concentrations in the range of 5.0 to 100.0 ng/mL. All stock and working standard solutions were freshly prepared before the analysis.
Validation of the HPLC method
The HPLC method was validated according to the International Conference on Harmonisation (ICH) Q2(R1) Validation of Analytical Procedures: Text and Methodology [23].
Quantification of sildenafil in biological samples
Concentration of sildenafil was calculated from the calibration curve using the method of external standardization. Calibration curve was constructed via sixfold analysis of standard sildenafil solutions at ten concentration levels in the range of 5.0 to 100.0 ng/mL.
Sample preparation
A protein precipitation technique was used for the extraction of sildenafil from biological specimens. Aliquotes of 100.0 μL plasma were separately placed into 10.0 mL screw-capped glass tubes. Thereafter each of them was treated with 1.0 mL of the precipitating agent (methanol) and vortexed at 6000 x g for 30 s (ZX3 Advanced, Italy). The obtained precipitates were solidified via centrifugation at 5000 x g for 5 min (Ohaus Frontier FC5706, USA). Subsequently, each supernatant was transferred into clean 10.0 mL screw-capped glass tube and 300.0 μL of methanol were added to the precipitates. Then the latter were vortexed at 6000 x g for 30 s and centrifuged at 5000x g for 5 min. The second supernatants were combined with the first one and after that the resulted samples were evaporated to dryness under nitrogen stream at 40°C. The dry residues were dissolved in 200.0 μL of double-distilled water, vortexed for 30 s and centrifuged at 5000 x g for 5 min. Then 20.0 μL of the supernatants were injected into the HPLC-UV system.
Spiked plasma samples were used for the sample preparation and HPLC methods development. The specimens were prepared by the addition of 100.0 μL standard solutions of sildenafil (at different concentration levels) to 100.0 μL of blank rat plasma. All samples were vortexed at 6000 x g for 30 s. Thus, a series of samples with concentrations in the range of 5.0 to 100.0 ng/mL were obtained and then subjected to the aforementioned protein precipitation procedure and HPLC analysis.
Animals
Male Wistar rats (n = 144) weighing 220–250 g were used for the pharmacokinetic experiments. They were preferred to female ones due to the concern that the estrous cycle may influence the results [24]. The animals were obtained from the vivarium of Medical University, Varna, Bulgaria. They were healthy and not genetically modified. The rats were housed in stainless steel cages at 23 ± 2°C in a well-ventilated room on a 12 h light/dark cycle with free access to standard rat chow and water. The humidity of the room was 50 ± 10%. Before the beginning of the experiments, animals were acclimated for one week. For allocation of the animals, computer generated random numbers were used. To minimize the potential confounders, rodents were treated at the same time and in the same order every day. In addition, there was no change in the animals location during the experiments. Worsening of the physical condition, dyspnea and paralysis were determined as humane endpoints. Rats were monitored twice daily for signs of health problems.
This study was performed in accordance with the national requirements for protection and humane treatment of laboratory animals, complying with Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. All experimental procedures in the present study were approved by the Bulgarian Food Safety Agency (permission № 175). A protocol was not registered.
Experimental design
The doses of methylxanthines and sildenafil were chosen after conversion of human to animal dose according to the following formula [25]:
All substances were administered by oral gavage. Sildenafil and ketoconazole were suspended in 1% solution of sodium carboxymethylcellulose, while the methylxanthine fraction was dissolved in hot double-distilled water. The animals were fasted overnight, with free access to water, prior to sildenafil administration. At the end of each experiment, blood samples were taken from the rats’ sublingual vein as this technique is easy to perform and less stressful for animals [26]. The blood was collected at certain time points for determination of sildenafil plasma concentration. After the blood was obtained, the rats’ liver and kidneys were immediately removed and washed with ice-cold saline for subsequent analysis of sildenafil tissue concentration. Blood samples were centrifuged at 5000 x g for 5 min and plasma was collected. Plasma samples were frozen at −20°C, while organs were frozen at −80°C until the analysis. All rats were euthanized by cervical dislocation under diethyl ether anesthesia. The humane endpoints were not reached in any of the experiments.
Pharmacokinetics of sildenafil after a single oral dose of methylxanthine fraction
Rats were randomly divided into three groups (n = 24; 6 animals per time point to ensure statistical significance of the obtained results). All substances were administered orally as a single dose. Group 1 (sildenafil group; control group) received 1.0 mL of sildenafil (60.0 mg/kg). Rats from group 2 (positive control) received 1.0 mL of ketoconazole (10.0 mg/kg) and 1.0 mL of sildenafil (60.0 mg/kg) 30 min after ketoconazole administration. Rats from group 3 received 1.0 mL of methylxanthines solution (5.7 mg/kg) and 1.0 mL of sildenafil (60.0 mg/kg) 30 min after methylxanthines intake. Blood samples were taken at the following time points: 0.5, 1, 3 and 6 h after sildenafil dosing.
Pharmacokinetics of sildenafil after multiple oral doses of methylxanthine fraction
All experimental procedures were performed twice daily in the intervals between 8 a.m. and 10 a.m., and between 4 p.m. and 5 p.m. Rats were randomly divided into three groups (n = 24; 6 animals per time point). Group 1 (sildenafil group; control group) and group 2 (positive control) received 1.0 mL of saline twice daily for 7 consecutive days. Rats in group 1 received 1.0 mL of sildenafil (2.5 mg/kg) on the 8th day 30 min after saline administration. Rats in group 2 (positive control) received 1.0 mL of ketoconazole (10.0 mg/kg) on the 8th day and 1.0 mL of sildenafil (2.5 mg/kg) 30 min after ketoconazole administration. Rats from group 3 were pretreated with 1.0 mL of methylxanthines solution (5.7 mg/kg) twice daily for 7 consecutive days and on the 8th day received 1.0 mL of sildenafil (2.5 mg/kg) 30 min after methylxanthines intake. Blood samples were collected at the following time points: 0.5, 1, 2 and 3 h after sildenafil administration. All rats were euthanized on the 8th day of the experiment.
Pharmacokinetic and statistical analysis
The plasma concentration–time profiles of sildenafil were analyzed by non-compartmental analysis using the PKSolver version 2.0 (a menu-driven add-in program for Microsoft Excel) [27]. The following pharmacokinetic parameters were calculated: maximum plasma concentration (Cmax), time to reach maximum plasma concentration (Tmax), area under the curve from time zero to the last measurable concentration (AUC0-t), area under the curve from time zero to infinity (AUC0-inf), apparent volume of distribution (Vz/F) and apparent clearance (Cl/F). The values for Cmax and Tmax were obtained directly from the plasma concentration-time curves. The AUC0-t and AUC0–inf were determined by the linear trapezoidal method for the detected values and subsequent extrapolation to infinity for calculation of AUC0-inf.
The results are expressed as mean ± standard deviation (SD). Statistical comparisons of the pharmacokinetic parameters between the groups were performed using an analysis of variance (ANOVA) followed by Dunnett’s test. A two-tailed p value ≤0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism version 9.2.0 (332).
Results
Quantification of sildenafil in rat plasma
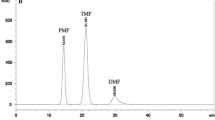
A validated HPLC-UV method was applied for quantitative analysis of sildenafil in rat plasma. The observed accuracy and precision fulfilled the criteria of ICH [23]. Linearity of the method was confirmed by the correlation coefficient (R2 > 0.998). The limits of detection and quantitation for sildenafil were 1.5 ng/mL and 5.0 ng/mL, respectively. A representative chromatogram of a test specimen is shown in Fig. 1.
The proposed protein precipitation method provided ≥93% recovery of sildenafil in all concentration levels (± 1.89% for spiked samples). In addition, no interfering endogenous or exogenous constituents were eluted in the analyte retention time, which once again proves the suitability of the sample preparation technique.
Effect of methylxanthine fraction on the pharmacokinetic of sildenafil
Both single and multiple oral doses of Bancha methylxanthines altered sildenafil pharmacokinetics in rats. However, single and multiple administration of methylxanthines affect the pharmacokinetic parameters of sildenafil in a different way.
Influence of a single oral dose of methylxanthine fraction on the pharmacokinetics of sildenafil
Concomitant use of sildenafil with a single oral dose of Bancha methylxanthines showed changes in some of the pharmacokinetic parameters of sildenafil, compared to sildenafil administration alone. When sildenafil was administered simultaneously with ketoconazole, an increase in sildenafil plasma levels was observed, compared to sildenafil group. The mean plasma concentration-time profiles of sildenafil alone and after administration of ketoconazole or methylxanthines are shown on Fig. 2. Following sildenafil intake alone (60.0 mg/kg) the mean maximum plasma concentration (Cmax) was 152.82 ± 31.41 ng/mL. The main pharmacokinetic parameters of sildenafil in each group are presented in Table 1. Oral administration of sildenafil with a single dose of methylxanthines resulted in a decrease in Cmax (29.44%), AUC0–6 (33.06%) and AUC0-inf (29.60%), compared to the sildenafil group. The reduction in Cmax was not significant (p > 0.05), unlike the reduction of AUCs that were statistically significant (p < 0.05). After co-administration of sildenafil with ketoconazole a significant increase in Cmax (p < 0.01), AUC0–6 and AUC0-inf (p < 0.05) was observed, compared to the sildenafil group. The values for Cmax, AUC0–6 and AUC0-inf were elevated by 61.97%, 49.84% and 43.45%, respectively. The Tmax values were the same in all groups (1 h). No statistically significant differences were detected in the value of Cl/F. The value of Vz/F was increased by methylxanthines and decreased by ketoconazole, compared to group 1. The differences in Cmax and AUC0-inf between the groups are shown on Figs. 3 and 4.
Influence of multiple oral doses of methylxanthine fraction on the pharmacokinetics of sildenafil
Sildenafil maximum plasma concentration (Cmax) after a single oral dose of 2.5 mg/kg in rats was 15.96 ± 2.45 ng/mL and Tmax was 0.5 h. The mean plasma concentration-time profiles of sildenafil alone, after administration of ketoconazole or after multiple doses of methylxanthines are shown on Fig. 5. Administration of sildenafil after pretreatment with Bancha methylxanthines in rats resulted in a statistically significant increase in Cmax (p < 0.0001), AUC0–3 (p < 0.0001) and AUC0-inf (p < 0.001). Furthermore, methylxanthines delayed the time to reach sildenafil peak plasma concentration (Tmax = 1 h). The main pharmacokinetic parameters of sildenafil in each group are shown in Table 2. In the ketoconazole group it was also observed an increase in sildenafil Cmax (p < 0.001), AUC0–3 (p < 0.001) and AUC0-inf (p < 0.001), as well as a Tmax delay (Tmax = 1 h). The observed values for sildenafil Cmax, AUC0–3 and AUC0-inf after pretreatment with methylxanthines were close to those observed after co-administration of ketoconazole (elevation above 100% compared to the sildenafil group). Figures 6 and 7 present a comparison of Cmax and AUC0-inf between the groups. Both methylxanthines and ketoconazole resulted in a significant decrease in Vz/F and Cl/F, compared to sildenafil administration alone.
Discussion
Nowadays, many herbs, beverages and food supplements are used daily in physiologically relevant doses for disease prevention or treatment. Some of them have been shown to inhibit or induce CYP450 activity in vitro and in vivo. Therefore, clinically significant interactions may occur if co-administered with certain drugs [28]. Green tea and coffee are among the most popular and consumed beverages worldwide and both of them may influence the pharmacokinetics of certain drugs [29, 30]. Both green tea and coffee contain a variety of bioactive compounds, including methylxanthines, mainly caffeine, that exert certain pharmacological effects [31]. Caffeine is metabolized mainly by CYP1A2 in humans and concomitant use with CYP1A2 substrates may result in an inhibition of their metabolism and increased risk of adverse reactions [29]. It is also reported that caffeine is a CYP3A4 substrate, but scarce information is available about the potential interactions when caffeine is co-administered with drugs that are predominantly metabolized by CYP3A4 [32]. In our previous study methylxanthine fraction isolated from Bancha green tea showed strong inhibition on CYP3A4 activity in vitro [22]. Another study reported that coffee inhibits CYP3A4 activity in vitro but not in vivo [33].
Тhe purpose of the present study was to assess the potential interactions between sildenafil and methylxanthines extracted from Bancha tea leaves in vivo. In the isolated methylxanthine fraction, a large amount of caffeine (87.74%) was found. The dose of methylxanthines used in the experiments was 5.7 mg/kg of dried powdered methylxanthines that is equivalent to 5.0 mg/kg caffeine. The human equivalent dose (HED) is approximately 48.0 mg of caffeine, an amount that can be found in a cup of tea [31]. In both experiments were observed changes in the pharmacokinetics of sildenafil after the co-administration of ketoconazole – a well-known inhibitor of CYP3A4 that was used as a positive control [34].
In the first experiment single doses of methylxanthines and sildenafil were orally administered with a 30-min time interval. Pharmacokinetic parameters of sildenafil after co-administration of methylxanthines were compared to those observed after sildenafil administration alone and after concomitant use with ketoconazole. A single dose of methylxanthines resulted in a non-significant reduction in sildenafil Cmax and statistically significant decrease in AUC0–6 and AUC0-inf, compared to the sildenafil group. On the contrary, a single dose of ketoconazole resulted in a statistically significant increase in sildenafil Cmax, AUC0–6 and AUC0-inf, as was expected and previously reported [35]. The observed reduction in sildenafil plasma levels may be due to decreased absorption of sildenafil by methylxanthines and, in particular, caffeine. Caffeine stimulates gastric acid secretion and decreases the gastrointestinal pH that may affect absorption of weak basic drugs, such as midazolam [29, 36]. Sildenafil, used in the form of sildenafil citrate, is a weak base and its absorption may also be decreased due to change in the gastrointestinal pH, similar to midazolam [37]. It was reported that caffeine may induce the expression of CYP1A2 and sulfotransferases in rats [38,39,40]. On the other hand, there isn’t any available data about the impact of these enzymes on sildenafil metabolism. Therefore, it cannot be considered that this is the mechanism behind the observed results. Another possible explanation for the reduced plasma levels of sildenafil is that caffeine increases heart rate and blood pressure and may also increase cardiac output resulting in an increased liver blood flow [41]. Sildenafil is an intermediate to high extraction ratio drug, thus its clearance will increase with the rise in liver blood flow [42]. Caffeine also increases glomerular blood pressure and may lead to an increased renal excretion of drugs [29]. In this study a much higher dose of sildenafil was administered to rats to obtain high plasma concentrations, since sildenafil has a lower oral bioavailability, shorter plasma half-life and higher metabolic clearance in male rats compared to humans [43]. The dose of 60.0 mg/kg was selected based on toxicological studies [44].
In the second experiment sildenafil was administered to rats after methylxanthines pretreatment and its pharmacokinetic parameters were compared to those after its administration alone, as well as after simultaneous intake of ketoconazole. The dose of sildenafil (2.5 mg/kg) was selected after conversion of human equivalent dose of 25.0 mg to animal dose. In this case, methylxanthines increased sildenafil Cmax, AUC0–3, AUC0-inf and decreased Vz/F and Cl/F, compared to group 1. The values were close to those observed after ketoconazole administration. The results may be explained with inhibition of CYP450 enzymes involved in sildenafil metabolism. Biotransformation of sildenafil in male rats is mediated by CYP3A4 and CYP2C11 [39, 45]. The CYP1A2, CYP2C11 and CYP3A4 enzymes are involved in caffeine metabolism in rats [46, 47]. Therefore, it is possible that both sildenafil and caffeine compete for the same enzyme (CYP3A4 or CYP2C11) and this may result in a decreased enzyme ability to metabolize drugs. Green tea extract and green tea catechins inhibit the activity of CYP3A4, CYP2D6, P-glycoprotein and organic anion transporting polypeptides (OATPs) but little is known about the impact of methylxanthines on this enzymes and transporters [48, 49]. One study reported that decaffeinated green tea extract is unlikely to alter the pharmacokinetics of CYP3A4 and CYP2D6 substrates which supports the assumption that caffeine may affect CYP3A4 activity [50].
This study has some limitations. One of them is the insufficient scientific information about the possible impact of methylxanthines on the activity of CYP3A4, as well as possible drug interactions with CYP3A4 substrates. However, as it was already mentioned, in our preliminary study we observed that methylxanthines show strong inhibition of CYP3A4 activity in vitro. Thus, we decided to undertake an in vivo study for confirmation of the in vitro results. Another possible limitation may be the variability in drug pharmacokinetics between humans and rats [51,52,53]. Despite the potential differences between species, it was reported that rat is an appropriate model for investigation of sildenafil pharmacokinetics. Sildenafil is metabolized mainly by CYP3A and CYP2C enzymes in humans and rats. The isoforms of these enzymes are very similar in both species and have more than 70% homology. In addition, the major metabolite is the same in humans and rats [54, 55]. Therefore, the results obtained should be taken into account when considering simultaneous administration of methylxanthines with sildenafil and subsequent in vivo studies should be performed.
Conclusion
In summary, drug-herb interactions were detected when sildenafil was co-administered with Bancha methylxanthines in rats. The observed changes in sildenafil Cmax, AUC0-t and AUC0-inf indicate that the duration of methylxanthines intake may be essential for the drug’s pharmacokinetics. However, further in vivo studies are needed to clarify the mechanism underlying the observed interactions. In conclusion, at this state of knowledge, it is recommended to avoid the concomitant use of sildenafil with beverages and dietary supplements that contain large amounts of methylxanthines, especially caffeine.
Data availability
All data are included within the article.
References
Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao T. A review of the role of green tea (Camellia sinensis) in Antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients. 2019;11(2):474. https://doi.org/10.3390/nu11020474.
Tang G-Y, Meng X, Gan R-Y, Zhao C-N, Liu Q, Feng Y-B, et al. Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci. 2019;20(24):6196. https://doi.org/10.3390/ijms20246196.
Gottwalt B, Tadi P. Methylxanthines. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK559165/: StatPearls Publishing LLC, 2021. Accessed 2021 15 Aug 2021
Monteiro JP, Alves MG, Oliveira PF, Silva BM. Structure-bioactivity relationships of Methylxanthines: trying to make sense of all the promises and the drawbacks. Molecules. 2016;21(8):974. https://doi.org/10.3390/molecules21080974.
Camandola S, Plick N, Mattson MP. Impact of coffee and cacao purine metabolites on neuroplasticity and neurodegenerative disease. Neurochem Res. 2019;44(1):214–27. https://doi.org/10.1007/s11064-018-2492-0.
Carrillo JA, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet. 2000;39(2):127–53. https://doi.org/10.2165/00003088-200039020-00004.
Briguglio M, Hrelia S, Malaguti M, Serpe L, Canaparo R, Dell'Osso B, et al. Food bioactive compounds and their interference in drug pharmacokinetic/Pharmacodynamic profiles. Pharmaceutics. 2018;10(4):277. https://doi.org/10.3390/pharmaceutics10040277.
Thorn CF, Aklillu E, McDonagh EM, Klein TE, Altman RB. PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012;22(5):389–95. https://doi.org/10.1097/FPC.0b013e3283505d5e.
Candela L, Formato M, Crescente G, Piccolella S, Pacifico S. Coumaroyl Flavonol glycosides and more in marketed green teas: an intrinsic value beyond much-lauded Catechins. Molecules. 2020;25(8):1765. https://doi.org/10.3390/molecules25081765.
Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea Catechins. Int J Mol Sci. 2020;21(5):1744. https://doi.org/10.3390/ijms21051744.
Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, et al. Green tea drinking and subsequent risk of breast cancer in a population-based cohort of Japanese women. Breast Cancer Res. 2010;12(5):R88. https://doi.org/10.1186/bcr2756.
Makiuchi T, Sobue T, Kitamura T, Ishihara J, Sawada N, Iwasaki M, et al. Association between green tea/coffee consumption and biliary tract cancer: a population-based cohort study in Japan. Cancer Sci. 2016;107(1):76–83. https://doi.org/10.1111/cas.12843.
Burana-osot J, Yanpaisan W. Catechins and caffeine contents of green tea commercialized in Thailand. J Pharm Biomed Sci. 2012;22(22):17.
Andersson KE. PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175(13):2554–65. https://doi.org/10.1111/bph.14205.
Al-Mohizea AM, Ahad A, El-Maghraby GM, Al-Jenoobi FI, AlKharfy KM, Al-Suwayeh SA. Effects of Nigella sativa, Lepidium sativum and Trigonella foenum-graecum on sildenafil disposition in beagle dogs. Eur J Drug Metab Pharmacokinet. 2015;40(2):219–24. https://doi.org/10.1007/s13318-014-0199-4.
Abdelkawy KS, Donia AM, Turner RB, Elbarbry F. Effects of lemon and Seville Orange juices on the pharmacokinetic properties of sildenafil in healthy subjects. Drugs R D. 2016;16(3):271–8. https://doi.org/10.1007/s40268-016-0140-1.
Graziano S, Montana A, Zaami S, Rotolo M, Minutillo A, Busardò F, et al. Sildenafil-associated hepatoxicity: a review of the literature. Eur Rev Med Pharmacol Sci. 2017;21:17–22.
Hsueh TY, Wu Y-T, Lin L-C, Chiu AW, Lin C-H, Tsai T-H. Herb-drug interaction of Epimedium sagittatum (Sieb. Et Zucc.) maxim extract on the pharmacokinetics of sildenafil in rats. Molecules. 2013;18(6):7323–35. https://doi.org/10.3390/molecules18067323.
Mallah E, Walid S, Rayyan W, Abu Dayyih W, Elhajji F, Mansoor K, et al. Dose-dependent synergistic effect of pomegranate juice on the bioavailability of sildenafil in rats by using HPLC method. Lat Am J Pharm. 2016;35:1277–84.
Mallah E, Saleh S, Rayyan W, Abu Dayyih W, Elhajji F, Mima M, et al. The influence of Eruca sativa (arugula) on pharmacokinetics of sildenafil in rats. Neuro Endocrinol Lett. 2017;38:295–300.
Mekjaruskul C, Sripanidkulchai B. Pharmacokinetic interaction between Kaempferia parviflora extract and sildenafil in rats. J Nat Med. 2015;69(2):224–31. https://doi.org/10.1007/s11418-014-0882-4.
Georgiev KD, Radeva-Ilieva M, Stoeva S, Zhelev I. Isolation, analysis and in vitro assessment of CYP3A4 inhibition by methylxanthines extracted from Pu-erh and Bancha tea leaves. Sci Rep. 2019;9(1):13941. https://doi.org/10.1038/s41598-019-50468-7.
ICH. Topic Q2 (R1) Validation of analytical procedures: Text and methodology (CPMP/ ICH/ 381/ 95), 1995. 2005.
Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the Design of pre-clinical Studies on sex differences in metabolism. Cell Metab. 2017;25(6):1216–30. https://doi.org/10.1016/j.cmet.2017.04.033.
Food and Drug Administration (FDA). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers [database on the Internet]July 2005. Accessed: https://www.fda.gov/media/72309/download
Heimann M, Käsermann HP, Pfister R, Roth DR, Bürki K. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab Anim. 2009;43(3):255–60. https://doi.org/10.1258/la.2008.007073.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft excel. Comput Methods Prog Biomed. 2010;99(3):306–14. https://doi.org/10.1016/j.cmpb.2010.01.007.
Mouly S, Lloret-Linares C, Sellier P-O, Sene D, Bergmann JF. Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and saint-John’s wort? Pharmacol Res. 2017;118:82–92. https://doi.org/10.1016/j.phrs.2016.09.038.
Belayneh A, Molla F. The effect of coffee on pharmacokinetic properties of drugs : a review. Biomed Res Int. 2020;2020:7909703. https://doi.org/10.1155/2020/7909703.
Teschke R, Xuan TD. How can green tea polyphenols affect drug metabolism and should we be concerned? Expert Opin Drug Metab Toxicol. 2019;15(12):989–91. https://doi.org/10.1080/17425255.2019.1697228.
Sanchez JM. Methylxanthine content in commonly consumed foods in Spain and determination of its intake during consumption. Foods (Basel, Switzerland). 2017;6(12):109. https://doi.org/10.3390/foods6120109.
Hodges RE, Minich DM. Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. J Nutr Metab. 2015;2015:760689. https://doi.org/10.1155/2015/760689.
Dresser GK, Urquhart BL, Proniuk J, Tieu A, Freeman DJ, Arnold JM, et al. Coffee inhibition of CYP3A4 in vitro was not translated to a grapefruit-like pharmacokinetic interaction clinically. Pharmacol Res Perspect. 2017;5(5):e00346. https://doi.org/10.1002/prp2.346.
Mandlekar SV, Rose AV, Cornelius G, Sleczka B, Caporuscio C, Wang J, et al. Development of an in vivo rat screen model to predict pharmacokinetic interactions of CYP3A4 substrates. Xenobiotica. 2007;37(9):923–42. https://doi.org/10.1080/00498250701570269.
Schwartz B, Kloner R. Drug interactions with Phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88–95. https://doi.org/10.1161/CIRCULATIONAHA.110.944603.
Jo JH, Kim SJ, Nam WS, Seung EJ, Lee S. Decreased absorption of midazolam in the stomach due to low pH induced by co-administration of Banha-sasim-tang. Environ Health Toxicol. 2016;31:e2016016. https://doi.org/10.5620/eht.e2016016.
Akula P, P.K L. Effect of pH on weakly acidic and basic model drugs and determination of their ex vivo transdermal permeation routes. Brazilian. J Pharm Sci. 2018;54:e00070. https://doi.org/10.1590/s2175-97902018000200070.
Vaynshteyn D, Jeong H. Caffeine induces CYP1A2 expression in rat hepatocytes but not in human hepatocytes. Drug Metab Lett. 2012;6(2):116–9.
Warrington JS, von Moltke LL, Shader RI, Greenblatt DJ. In vitro biotransformation of sildenafil (Viagra) in the male rat: the role of CYP2C11. Drug Metab Dispos. 2002;30(6):655. https://doi.org/10.1124/dmd.30.6.655.
Zhou T, Chen Y, Huang C, Chen G. Caffeine induction of sulfotransferases in rat liver and intestine. J Appl Toxicol. 2012;32(10):804–9. https://doi.org/10.1002/jat.1698.
Abdulla M, Mallah E, Abu Dayyih W, Rayyan W, Elhajji F, Bustami M, et al. Influence of energy drinks on pharmacokinetic parameters of sildenafil in rats. Biomed Pharmacol J. 2018;11:1317–28. https://doi.org/10.13005/bpj/1494.
Mehrotra N, Gupta M, Kovar A, Meibohm B. The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int J Impot Res. 2007;19(3):253–64. https://doi.org/10.1038/sj.ijir.3901522.
Walker DK. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29(3):297–310. https://doi.org/10.1080/004982599238687.
Abbott D, Comby P, Charuel C, Graepel P, Hanton G, Leblanc B, et al. Preclinical safety profile of sildenafil. Int J Impot Res. 2004;16(6):498–504. https://doi.org/10.1038/sj.ijir.3901232.
Lin Y-K, Sheu M-T, Tzen T-Z, Ho H-O. Biotransformation of sildenafil in the male rat: evaluation of drug interactions with testosterone and carbamazepine. Drug Dev Ind Pharm. 2008;34(11):1219–26. https://doi.org/10.1080/03639040802005032.
Kot M, Daniel W. Effect of cytochrome P450 (CYP) inducers on caffeine metabolism in the rat. Pharmacol Rep: PR. 2007;59:296–305.
Kot M, Daniel WA. Relative contribution of rat cytochrome P450 isoforms to the metabolism of caffeine: the pathway and concentration dependence. Biochem Pharmacol. 2008;75(7):1538–49. https://doi.org/10.1016/j.bcp.2007.12.017.
Albassam AA, Markowitz JS. An appraisal of drug-drug interactions with green tea (Camellia sinensis). Planta Med. 2017;234(06):496–508.
Knop J, Misaka S, Singer K, Hoier E, Müller F, Glaeser H, et al. Inhibitory effects of green tea and (−)-epigallocatechin Gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS One. 2015;10(10):e0139370. https://doi.org/10.1371/journal.pone.0139370.
Oga EF, Sekine S, Shitara Y, Horie T. Pharmacokinetic herb-drug interactions: insight into mechanisms and consequences. Eur J Drug Metab Pharmacokinet. 2016;41(2):93–108. https://doi.org/10.1007/s13318-015-0296-z.
Musther H, Olivares-Morales A, Hatley OJD, Liu B, Rostami HA. Animal versus human oral drug bioavailability: do they correlate? Eur J Pharm Sci. 2014;57:280–91. https://doi.org/10.1016/j.ejps.2013.08.018.
Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CECA, et al. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos. 2015;43(3):367. https://doi.org/10.1124/dmd.114.061580.
Toutain P-L, Ferran A, Bousquet-Mélou A. Species differences in pharmacokinetics and pharmacodynamics. In: Cunningham F, Elliott J, Lees P, editors. Comparative and veterinary pharmacology. Berlin: Springer Berlin Heidelberg; 2010. p. 19–48.
Bae S, Bae SK, Lee B. Effect of hepatic CYP inhibitors on the metabolism of sildenafil and formation of its metabolite, N-desmethylsildenafil, in rats in vitro and in vivo. J Pharm Pharmacol. 2009;61:1637–42. https://doi.org/10.1211/jpp/61.12.0008.
Shin HS, Bae SK, Lee MG. Pharmacokinetics of sildenafil after intravenous and oral administration in rats: hepatic and intestinal first-pass effects. Int J Pharm. 2006;320(1):64–70. https://doi.org/10.1016/j.ijpharm.2006.04.005.
Acknowledgments
The authors would like to thank the entire team of Science and Research Department at Medical University “Prof. Dr. Paraskev Stoyanov” for their support.
Funding
This study was funded by the Science Fund of Medical University “Prof. Dr. Paraskev Stoyanov” (project № 20019).
Author information
Authors and Affiliations
Contributions
MRI performed the literature review and wrote the manuscript. IZ extracted Bancha methylxanthines. MRI carried out the randomization and was aware of the group allocation. MRI, NH, SS and KG conducted the experiments. SS and MRI performed the HPLC analysis. All authors contributed to the design of the study and data analysis. All authors read and approved the final version of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial and non-financial conflicts of interest to declare.
Ethics approval
The study protocol was approved by the Bulgarian Food Safety Agency and all the requirements for the protection and humane treatment of laboratory animals according to the Directive 2010/63/EU were followed.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radeva-llieva, M., Stoeva, S., Hvarchanova, N. et al. Influence of methylxanthines isolated from Bancha green tea on the pharmacokinetics of sildenafil in rats. DARU J Pharm Sci 30, 75–84 (2022). https://doi.org/10.1007/s40199-022-00433-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-022-00433-z