Abstract
17Cr–1Si–0.5Nb–1.2Cu ferritic heat-resistant stainless steel was aged at 750 °C from 10 min to 30 h to simulate time aging and study the microstructural evolution and its effect on corrosion behavior by using optical microscopy, scanning electron microscopy, transmission electron microscopy, potentiodynamic polarization, electrochemical impedance spectroscopy, and the Mott–Schottky approach. Four types of precipitates were discovered, including ε-Cu, NbC, Fe3Nb3C, and Fe2Nb-type Laves phase. The nano-sized ε-Cu phase forms first, and its fraction follows the parabolic law change and is the largest. Compared to NbC and Fe3Nb3C particles, the coarsening of the Laves phase is the most pronounced. The aging process is divided into three parts: early-aged (0–5 h), peak-aged (5 h), and over-aged (5–30 h). However, the corrosion resistance is reduced in the early-aged stage of 0–2 h. Further extending the aging time to 30 h, the corrosion resistance is gradually improved. This change may be related to the competitive relationship between the beneficial effects of the Cu-rich phase and the harmful effects of Nb-containing particles. The dissolved Cu on the surface becomes more effective for the suppression of the anodic dissolution by the formation of ionic compounds of chlorine, thereby reducing the deterioration of corrosion resistance caused by Nb-rich precipitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nb-containing ferritic heat-resistant stainless steel (FHSS) is one of the most promising candidates for automotive exhaust pipes due to its ability to save Ni, its low cost, excellent stress corrosion resistance, and inherent advantages of low thermal expansion and high thermal conductivity [1, 2]. However, compared to the austenitic heat-resistant stainless steel, the Nb-containing FHSS has lower strength at high temperature due to the bcc structure of the matrix and Nb-rich particles precipitation (i.e., intermetallic laves phase, carbides and nitrides) [3]. The rapid coarsening of the Laves phase can dramatically reduce the high-temperature mechanical properties. Moreover, the formation of Nb-rich particles provides corrosion vulnerability to FHSS, thus limiting its application to some extent.
Alloying is a main technique to complement the comprehensive properties of FHSS. Much work has been conducted to investigate the aging precipitation and mechanical properties of FHSS modified by Mo, Ti, W, etc. [4]. These alloying elements can effectively improve the high-temperature properties of FHSS, but are expensive. In recent years, Cu as an alloying element has received great attention in the development of advanced steels [5]. The sufficient addition of Cu in steels and appropriate heat treatment can induce precipitation of the nano-sized ε-Cu phase. It is reported that the ε-Cu phase not only increases the room temperature strength of the steels, but also has a positive effect on improving the high-temperature mechanical properties of austenitic stainless steels due to its relatively high thermal stability and coherent relationship with the substrate [6]. Therefore, research on Cu-alloyed FHSS able to form a ε-Cu phase is of great significance for performance improvement. However, to the best of our knowledge, there are still few reports on this topic.
For Cu-bearing FHSSs, as the ε-Cu phase precipitates out during the aging process, the Nb-rich phases also form from the ferrite matrix, because they have the same sensitive precipitation temperature range. This may cause the complicated precipitation behavior. For example, Luo et al. [7] found co-precipitation: side-by-side Cu-rich nanoparticles and the Nb-rich phase in 15-5PH steel. In fact, these precipitates with proper size and amount will act as strengthening carriers [8]. However, considering the widespread use of FHSSs in exhaust pipes, it is essential to investigate the corrosive behavior of the Cu-bearing FHSSs, and provide a basis for understanding the relationship between the aging precipitates and corrosion properties. Despite the fact that previous studies have reported about Cu alloying on the corrosion resistivity of Fe–Cr steels [9, 10], the corrosion behavior of Cu-bearing FHSSs in chloride ion solutions still lacks a comprehensive and in-depth understanding. Oguzie et al. [9] reported the effects of Cu addition on the electrochemical corrosive behavior of austenitic, ferritic, and martensitic stainless steels in 0.1 M H2SO4 and found Cu could improve the corrosion resistance and facilitate passivation, but did not notably affect the resistance of the passive films. Seo et al. [10] indicated that Cu could be enriched on the Fe–26Cr steel surface during active dissolution in the sulfuric acid solution and had a detrimental effect on the passivity due to its dissolution in the form of copper ions.
In this work, the microstructures of 17Cr–1Si–0.5Nb–1.2Cu FHSS after different aging treatments were characterized. The influence of aging time on the corrosion behavior was studied by potentiodynamic polarization, electrochemical impedance spectroscopy, and the Mott–Schottky approach. The present study will provide a better understanding of the relationship between aging precipitation and corrosion resistance of Cu-bearing FHSS, which is important in the design of new heat-resistant materials used in automotive exhaust manifolds.
2 Experimental
2.1 Material and Heat Treatment
The material used in this work was 17Cr–1Si–0.5Nb–1.2Cu FHSS, and the chemical composition is shown in Table 1. The studied material was melted in a 50 kg vacuum induction furnace, cast into cylinder-shaped ingots with a diameter of 120 mm and a height of 420 mm, hot-forged into a thickness of 40 mm, and hot-rolled into a 10 mm plate. The as-received steel was solution-treated at 1100 °C for 30 min and immediately quenched in room temperature water. Then the as-received solution-treated samples were isothermally aged at 750 °C for different times ranging from 10 min to 30 h, together with water quenching.
2.2 Microstructural Characterization and Hardness Test
The microstructural observation was carried out by optical microscopy (OM, DM13000H). The samples were cut into 10 mm × 8 mm × 10 mm shapes from the steel plate with different heat treatments. The sample surface was ground with SiC paper of grit grade 400–1200 and then polished with 2.5 μm diamond powders. The polished samples were eroded for ~ 3 min in an etching agent composed of 5 g FeCl3, 50 ml HCl, and 100 ml H2O.
The precipitates were examined using scanning electron microscopy (SEM, Zeiss Gemini supra 40) equipped with energy-dispersive spectroscopy (EDS, Apollo XLT SDD). Moreover, the detailed information of the precipitates and the selected area diffraction pattern (SADP) were characterized by transmission electron microscopy (TEM, FEI Talos™ F200S). The thin foil samples for TEM analysis were prepared by a twin-jet polishing technique with electrolytes consisting of 15 ml perchloric acid, 45 ml n-butanol, and 90 ml methanol. The volume fraction of precipitates was measured by Leica image analysis software. For the irregular precipitates, the equivalent diameter was used to evaluate the particle size [11]. The hardnesses of the samples with different aging times were tested using a Vickers hardness tester (FM-700) that performed under the detection force of 100 gf with a dwell time of 15 s. The obtained values were the average of five measurements per sample.
2.3 Electrochemical Measurements
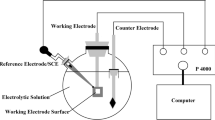
The samples for electrochemical measurements were cut into 10.1 mm × 10.1 mm × 2 mm slices, and the measured surface was ground with SiC paper of grit grade 400–2000 and sealed with an epoxy resin to a working area of 0.5 cm2. Electrochemical measurements were taken on an electrochemical workstation (IviumStat.h). A three-electrode system in 3.5 wt% NaCl solution was used, where the platinum electrode was the auxiliary electrode and the Ag/AgCl electrode was the reference electrode. Open-circuit potential (OCP), electrochemical impedance spectrum (EIS), and potentiodynamic polarization curves were aimed at investigating the electrochemical corrosion behaviors of the steels with different aging times. Before the measurement, the OCP of the steel was measured for at least 5 min until it reached a stable value. The test frequency was 100 kHz–0.01 Hz, and the amplitude of the sinusoidal excitation signal was 10 mV. ZSimpWin software was used to analyze the obtained EIS. The scanning rate of the potentiodynamic polarization curves was 4 mV/s, and the test potential interval was from − 2 to 2 V. The corrosion current density (icoor) and pitting potential (Eb) were acquired by the Tafel epitaxial method. Each experiment was repeated at least three times under the same condition to confirm reproducibility. In addition, the Mott–Schottky approach was used to evaluate the semiconductive properties of the passive film. Before measuring the Mott–Schottky curves, the cathodic polarization at a potential of − 1.5 V was conducted to remove oxides on the surface of the studied steel. The passivation potential was determined according to the anode portion of the obtained polarization curve. A stable passive film was formed at a potential of − 0.5 V for 1 h in 3.5 wt% NaCl solution.
2.4 X-Ray Photoelectron Spectroscopy Spectrum (XPS) Measurement
XPS was measured in the ultra-high-vacuum room with an attached energy electronic analyzer, where the vacuum degree of the sample analysis room was about 10–9 torr, and the anode target of the X-ray source was Al Kα. Before the XPS measurement, the samples were degreased with acetone and rinsed with alcohol. To form a stable passive film, the samples were immersed in a 3.5 wt% NaCl solution for 24 h. Then, they were transferred to the XPS analyzer chamber immediately after the surfaces were rinsed with distilled water and dried. XPS was used to analyze the passive film composition. All peaks in the pattern were analyzed by XPSPEAK 4.1 software and corrected with C1s peak (285.0 eV).
3 Results and Discussion
3.1 Hardness
The Vickers hardness results for the aged samples at 750 °C for 0–30 h are shown in Fig. 1. It can be seen that the hardness of the as-received solution-treated steel is ∼ 173 HV. With an increase in the aging time within the first 5 h, the hardness increases to a maximum value of ∼ 253 HV, an increase of 46% compared to the as-received solution-treated steel. After that, the hardness begins to decrease significantly and trends toward stabilization at 10 h. As a result, the aging process can be divided into three stages: early-aged (0–5 h), peak-aged (5 h), and over-aged (5–30 h).
3.2 Microstructures
Figure 2 shows the OM microstructures of the samples aged at 750 °C for 0 h (solution-treated status), 1 h, 5 h, 15 h, and 30 h. The as-received solution-treated sample contains equiaxed ferrite grains with an average size of ~ 89 μm (Fig. 2a). As the aging time increases to 1 h, the ferrite grains coarsen due to the recovery; the average grain size reaches ~ 100 μm (Fig. 2b). However, there is no significant change in the grain size after prolonged aging (Fig. 2c–e). This may be related to the formation of precipitates during aging. It is challenging to observe these precipitates by OM. To characterize the aged precipitates, SEM observations were performed on the samples with different aging times, and the results are shown in Fig. 3.
For the as-solution-treated sample, a few bulk precipitates can be observed both in the interior of the grain and at the grain boundary (Fig. 3a). EDS results of the typical precipitates are shown in Fig. 3b. It is confirmed that they are Nb-containing carbonitrides generally formed at elevated temperatures due to the high affinity of Nb with C and N. Generally, once these Nb-containing carbonitrides are formed, it is difficult to remove them later by heat treatment [9]. As to the sample aged for 2 h, the Cu-rich phase and more fine Nb-rich phase precipitate at both grain boundaries and grain interiors (Fig. 3c), which are evidenced by the EDS analysis at points 3–6 (Fig. 3d, e). The size of the precipitates increases significantly with the increase in aging time and some of the punctate particles become rodlike or strip-like precipitates, while the number of precipitated particles decreases (Fig. 3f, g). Precipitate-free zones are easily observed around the precipitated particles, especially on both sides of the grain boundary. In addition, some Nb-rich precipitates distributed along a straight line are visible inside the grains, as shown in Fig. 3h. A similar phenomenon has been reported by Lu et al. [12], where these precipitates are estimated to be Laves phase. Since a large amount of precipitates are formed at the grain boundaries and grain interiors with aging time, the movement of dislocations and the migration of grain boundaries can be hindered due to the pinning effects of precipitates, thereby suppressing grain growth.
Figure 4 displays the TEM images of the samples aged at different aging times. It is clear that the nanoscale and submicroscale precipitates disperse at the grain boundaries and inside the grain, which is consistent with the observations by SEM. Four types of precipitates are detected, namely ε-Cu, NbC, Fe3Nb3C, and Fe2Nb-type Laves phase, according to the calibration of SADPs of different precipitates circled in Fig. 4a, c, and g. After 10 min of aging (Fig. 4a), a large number of punctate fcc ε-Cu phases with an average size of ~ 21 nm are diffusely precipitated, while a small amount of ε-Cu phase has evolved into a rod shape. For the precipitation process of ε-Cu phase in ferrite, it generally exhibits two stages: In the initial stage, a bcc-structured Cu-rich region having a size of 2.5–3 nm is preferentially formed, since the sizes of the Fe atom and Cu atom are similar. When the size of the Cu-rich region increases to ~ 10 nm, it develops into a stable fcc-structured ε-Cu phase [13]. As the ε-Cu phase grows to ~ 30 nm, the shape of the ε-Cu begins to evolve from sphere to rod, and it follows the Kurdjumov–Sachs (K–S) orientation relationship of [11\(\overline{ 1}\)]ε-Cu // [\(\overline{ 1}\)10]α-Fe and [110]ε-Cu // [111]α-Fe with the matrix [14]. When the aging time extends to 1 h (Fig. 4c–h), the size of the rod-shaped ε-Cu phase increases significantly, and the average size is increased to ~ 32 nm. In addition, the Nb-rich precipitates can be easily found at the grain boundary and inside the grain. After the calibration, the long strip precipitate is identified as the Fe2Nb–Laves phase, and the average size is ~ 270 nm, while the blocky particle is NbC without a specific orientation relationship within the matrix, and the average size is ~ 170 nm; the corresponding SADPs are shown in Fig. 4d, e. A few diamond-shaped precipitates are also observed, and they are identified as Fe3Nb3C carbide by the analysis of SADP in Fig. 4h, and the average size is ~ 250 nm. Moreover, the tangled dislocations can be observed in the vicinity of the precipitates, indicating the pinning effect on the dislocations. When the sample is aged for 30 h (Fig. 4i), all the precipitates are coarsened. The average sizes of the ε-Cu, NbC, Fe3Nb3C, and Laves phase are ~ 130 nm, ~ 280 nm, ~ 490 nm, and ~ 780 nm, respectively. Compared to Nb-containing carbides, the coarsening rate of the intermetallic Fe2Nb-type Laves phase is significantly higher. This phenomenon agreed well with the observation in other Nb-modified FHSSs [15]. This is because the interface between the Laves phase and ferritic matrix was incoherent, and the coarsening rate of the Laves phase at 700 °C can reach 7.01 × 10−27 m3/s [16]. Figure 5 presents the amounts of precipitates, such as ε-Cu, Fe2Nb-type Laves phase, and carbides (NbC and Fe3Nb3C) in the samples aged at 750 °C for various time. The area fraction of all precipitates increases to 6% as the aging time increases from 0 to 15 h, after which it approximately remains at a stable value. Among these precipitates, the amount of the Cu-rich phase is the highest, while the fraction of carbides NbC and Fe3Nb3C is the lowest.
3.3 Corrosion Behavior
3.3.1 Potentiodynamic Polarization Curves
Figure 6 shows the polarization curves of the samples with different aging times in a 3.5 wt% NaCl solution. All samples exhibit a representative passive region, indicating that the passive film can spontaneously form on the surface of the test steel. The current density increases at a potential higher than about − 0.1 V, which may have been due to the pitting corrosion caused by local breakdown of the passive film [17]. It is well known that the cathodic reaction of metals and alloys in a near neutral solution is an oxidation reaction at room temperature [18]. From the cathode portion of the polarization curve, there is no significant difference in the cathodic polarization process of the samples with different aging times, indicating that the aging time does not affect the oxidation reaction. The detailed electrochemical parameters are listed in Table 2. The corrosion potential (Ecorr) and pitting potential (Epit) are obtained from the polarization curves directly, and the corrosion current (icorr) was calculated by Tafel extrapolation. It can be seen that the icorr value increases to 0.297 mA/cm2 with increasing aging time within 2 h, indicating that the corrosion resistance of the samples decreases. However, as the aging time is extended to 30 h, the icorr value gradually decreases, suggesting an improvement in corrosion resistance. Opposite trends are observed for Ecorr and Epit. As the sample aged at 750 °C for 2 h, the values of Ecorr and Epit are − 1.100 V and 0.004 V, respectively, showing the lowest corrosion resistance.
3.3.2 Electrochemical Impedance Spectroscopy
The Nyquist plots of the samples aged at 750 °C for different aging times in a 3.5 wt% NaCl solution are shown in Fig. 7a. Over the entire frequency range, each curve presents a single capacitive arc resistance, indicating that the corrosion rate is primarily controlled by the charge transfer process generated by the electrochemical reaction on the electrode surface [19]. As the aging time increases, the radius of the capacitive arc resistance first decreases at 2 h and then significantly increases. This shows that the stability and protection of the passive film on the surface of the samples aged for a short period of time are easily broken, while the corrosion resistance can be improved after aging for more than 2 h. The corresponding Bode plots are shown in Fig. 7b. The linear relationship between log |Z| and log f indicates that the passive film is stable and exhibits a good protective effect on the material over a wide frequency range, thereby inhibiting the penetration of corrosive medium into the substrate through the passive film [20]. The maximum value of the phase angle is less than − 80°, indicating that a stable passive film is formed on the surface of the sample in the NaCl solution [21].
To quantitatively analyze the impedance measurements, an equivalent electrical circuit model was established by using ZSimpWin software, as shown in Fig. 7c. In this model, Rs represents the solution resistance, CPE1 and CPE2 represent the capacitances of the passive film and double layer, respectively, Rc is the resistance of the film formed on the sample surface, and Ret is the charge transfer resistance. Table 3 lists the fitting parameters of the equivalent electrical circuit. It is shown that although the Ret value is reduced in the initial aging stage, it increases when the aging time is greater than 2 h. In particular, the Ret value of the sample aged for 30 h is calculated to be 15,300 Ω cm−2, which is six times that of the sample aged for 2 h. The charge transfer between the metal and electrolyte solution is related to the characteristics of the passive film of metals formed in the electrolyte solution [22, 23]. Generally, the higher the Ret value, the stronger the interface suppresses the charge transfer ability and the better the corrosion resistance of the metal material. Hence, the charge transfer between the interfaces speeds up when aged within 2 h, and the dissolution rate of the passive film gradually accelerates, and thus, the corrosion resistance of the steel deteriorates. After 2 h of aging, the trend is the opposite, as the charge transfer between the metal and electrolyte solution becomes difficult. A similar trend is also found on the film resistance (Rc value). However, the Rs value slightly increases within the aging time, indicating that the erosion ability of the solution is reduced. It is well known that the smaller the value of CPE1, the larger the corrosion resistance [12]. As is shown in Table 3, the value of CPE1 first increases to 5.335 × 10−5 Ω−1 cm−2 sn and then rapidly decreases with aging time up to 30 h. Therefore, the protective effect of the passive film can be improved by increasing the aging time.
3.3.3 Mott–Schottky Measurements
Passive films composed of metal oxides possess semiconductor properties, which is important in evaluating the corrosion resistance of metals and alloys. p type refers to a semiconductor with hole concentration much higher than the free electron concentration, while n-type semiconductors are an impure semiconductor whose free electron concentration is much higher than the hole concentration [24, 25]. For the two semiconductor types, the electrode capacitance as a function of electrode potential can be determined by:
where Csc is the space charge capacitance, ε0 is the vacuum permittivity (8.854 × 10–14 F cm−1), ε is the dielectric constant of the passive film at room temperature, where the value is usually 15.6, e is the electron charge (1.602 × 10–19 c), ND and NA are the donor concentration and acceptor density of the passive film, respectively, E is the applied voltage, EFB is the flat band potential, K is the Boltzmann constant, T is the thermodynamic temperature, and KT/e is about 25 mV at room temperature, which is usually negligible.
Figure 8 shows the Mott–Schottky curves of the passive films of the aged samples in a 3.5 wt% NaCl solution, which reflects the semiconductor properties of the passive films. As the potential increases, all curves in Fig. 8 are divided into three regions. In the R1 region (from − 0.90 to − 0.58 V), the negative slope indicates that the passive film is a p-type semiconductor. Generally, Cr2O3, NiO, MoO2, and FeCr2O4 play a major role in p-type semiconductor characteristics [26, 27]. The larger the slope, the more unstable the passivation film becomes and the more easily it is destroyed. However, the R3 region (from − 0.36 to 0 V) represents the n-type semiconductor passive film due to the positive slope. Fe2O3, MoO3, and FeOH typically exhibit n-type semiconductor properties. Conversely, the high slope in the R3 region indicates that the passive film has better corrosion resistance. Ningshen et al. [28] illustrated that the passive film of stainless steel in a NaCl solution usually has a double-layer structure of the dual polarity, and the inner layer is Cr oxide or hydroxide of p type, and the outer layer is Fe oxide or hydroxide of n type. Such passive films not only prevent the cations from diffusing from the substrate to the solid–liquid interface, but also prevent Cr and other corrosive anions entering the matrix [29], As a result, the films provide good protection for stainless steel. From this figure, the slope in the R1 region obviously decreases after aging more than 2 h, while the slope in R3 shows an increasing tendency when the semiconductor characteristics of the passive film are converted to the n type (Table 4). This indicates that the passive film becomes stable and the possibility of corrosion is small. R1 and R3 are separated by a narrow potential plateau, region R2, representing a transitional process.
In addition, the flat band potential (EFB) and defect density (N) also reflect the performance of the passive film, and the calculated results are shown in Table 4. As given in Table 4, the sample aged for 2 h shows the highest flat band potential and defect density. The flat band potential generally determines the pitting potential of the metal [30]. The high flat band potential indicates that the pitting potential will shift negatively and the corrosion resistance of the metal decreases. Meanwhile, an increase in the defect density in the passive film can promote the progress of the electrode reaction, increasing the degree of damage to the substrate [31]. On the contrary, when the defect density of the passive film is small, the electron donor concentration will be low, resulting in a small space charge capacitance. This causes difficulty in charge transfer, so it is difficult for the interface reaction on the passive film to proceed, thus improving the corrosion resistance. As given in Table 4, the sample aged for 2 h shows the highest flat band potential and defect density, indicating that the passive film formed has the worst stability. When the aging time is prolonged to 30 h, the flat band potential and defect density decrease, the stability of the passive film is remarkably enhanced, and therefore, the possibility of corrosion is reduced.
3.4 Analysis of Passive Film Composition and Corrosion Resistance
Based on the analysis of the potentiodynamic polarization curves, the electrochemical impedance spectroscopy, and the semiconductor properties of passive films in Sect. 3.3, it is obvious that the corrosion resistance of the studied steel varies with aging time. In the early-aged stage of 0–2 h, the corrosion resistance is reduced though it is improved with further aging time increase to 30 h. It is noted that the change in corrosion resistance is inconsistent with hardness. To better understand the corrosion mechanism of the aged samples, the surface composition of the passive film with typical aging time is identified by XPS [32] where Fe, Cr, Cu, Nb and O, as the possible constituent elements of the passive film, were measured.
The full XPS spectra of passive films of the aged samples formed after immersing for 24 h in a 3.5 wt% NaCl solution are shown in Fig. 9. It can be concluded that Cr–Fe compounds are the main components of the passive film in a 3.5 wt% NaCl solution. The high-resolution spectra of Cr2p3/2 in the passive film are shown in Fig. 10. Four different peaks can be identified in this figure. At the binding energy of ∼ 573.7 ± 0.1 eV, the metallic state peak is visible, and other three peaks are detected at ∼ 575.4 ± 0.2 eV, ∼ 576.2 ± 0.3 eV, and ∼ 578.1 ± 0.2 eV corresponding to Cr2O3, Cr(OH)3, and CrO3, respectively [33, 34]. Generally, CrO3 cannot be formed in the passive film of stainless steels [7]. However, previous studies have confirmed that CrO3 can coexist with Cr2O3 in the inner oxide due to similar standard free enthalpies or the short-range-order feature [35, 36]. More importantly, the glassy oxides of XCr2O3·YCrO3 can serve as barrier layers and resist chloride attack, thereby providing greater corrosion resistance and preventing the passivation film from breakdown in a NaCl solution. In this work, the intensity of CrO3 is significantly enhanced when the aging time is increased up to 30 h, indicating that the corrosion resistance of the passive film is reinforced. Figure 11 shows the peak position fitting results of the XPS spectra of Fe2p2/3. The spectrum of Fe2p2/3 can also be divided into several constituent peaks, representing the iron metallic state (706.5 ± 0.3 eV), divalent (Fe2+), and trivalent (Fe3+) substances, which mainly exist in the form of FeOOH (711.8 ± 0.2 eV), Fe2O3 (709.3 ± 0.3 eV), and FeO (708.1 ± 0.2 eV) [37]. Figure 12a shows the XPS spectra of Cu2p in the passive films obtained at different aging times, where little or no Cu can be observed. This illustrates that a large amount of Cu-rich particles formed during aging is dissolved in the NaCl solution, and no deposited Cu or oxide is formed. Generally speaking, Cu deposited on the surface can protect the passivation film, thus improving the corrosion resistance of the material [38]. However, in the neutral chloride solution, Cu can be dissolved as Cu2+, CuCl2−, or CuCl42−. When the ionic compounds of chlorine are formed, the erosion inside a pit will be suppressed. Meanwhile, it indirectly protects the passivation film against chloride attack. The XPS spectra of Nb3d of the samples with different aging times are shown in Fig. 12b. The amount of Nb in the passive film is quite low. Similarly, there were no Nb species in the passive film of Nb-containing steel [7]. This may be caused by the Nb precipitation in the matrix, since Nb is not easily oxidized compared to Fe and Cr.
It is shown that the microstructural evolution caused by aging has a significant effect on the corrosion behavior of the Cu-bearing FHSS. It is well known that solid Cu can be used to improve the resistance to general corrosion of stainless steels [39, 40]. Nb addition can increase the stability of the passive film through Nb enrichment in the surface layer [41]. However, the precipitation of Cu-rich phase and Nb-rich particles commonly affects the beneficial effects of the solid Cu and Nb on corrosion resistance. The formation of Nb-rich precipitates can cause a potential difference in the vicinity of the precipitated particles [1], which will induce deterioration of the corrosion resistance. In this work, numerous fine precipitates formed in the early-aged stage of 0–2 h result in a decrease in corrosion resistance of the studied steel. The nano-sized ε-Cu phase is easily corroded in the chloride solution by anodic dissolution (Cu → Cu2+ + 2e), and this provides a large number of vulnerable sites for pitting initiation, since these pit sites generally have high chloride ion concentration [37]. Therefore, the chloride ions in solution are more likely to break through the passive film, leading to the discontinuity of the passive film. However, with increasing the aging time, the ε-Cu particles are coarsened in the microstructure, and their amounts increase, thus resulting in the occurrence of more dissolved copper ions. In this state, a large amount of CuCl2− is formed in chloride environment by Cu and Cu2+, and the reactions are as follows:
It is suggested that when the concentration of Cl− is greater than 0.5 mol/l, the reaction (4) can be accelerated [38]. It is well known that the concentration of Cl− is 0.6 mol/l in the 3.5 wt% NaCl solution. This most likely leads to the increased consumption of chloride ions in Cu dissolution. The content of Cr oxides, especially chromium trioxide, is increased with aging time, as confirmed by XPS analysis. Therefore, the suppression of metal corrosion becomes more effective, and the passivation film is more stable. In this process, the corrosivity of the chloride solution is reduced, which is consistent with the increase in solution resistance (Rs). Although the precipitation of Nb reduces the corrosion resistance, it can be compensated by the protection of Cu dissolution; accordingly, the corrosion resistance of the studied steel is improved. It is noted that the deposited Cu is not detected in the present work, which may be connected with the concentration of Cl− and the potential inside a pit [7, 38].
4 Conclusions
The microstructural evolution and corrosion behavior of 17Cr–1Si–0.5Nb–1.2Cu FHSS after aging at 750 °C over different durations were investigated, and the following conclusions were obtained:
-
1.
The aging time has a significant effect on the microstructures of the aged samples. Four precipitates are detected during the aging: ε-Cu, NbC, Fe3Nb3C, and Fe2Nb-type Laves phase. The recovery process of ferrite grains is restrained due to the pinning effects of the precipitates. Among these precipitates, the nano-sized ε-Cu phase forms first, and its fraction is the largest and follows the parabolic law change.
-
2.
The hardness increases to a peak value after 5 h of aging and increases 46% more than the solid solution state. After that, the hardness is reduced, but eventually tends to be stabilized. The aging process with various precipitates can be divided into three parts: early-aged (0–5 h), peak-aged (5 h), and over-aged (5–30 h).
-
3.
Electrochemistry measurements show that the corrosion resistance is reduced in the early-aged stage of 0–2 h. Further increasing the aging time up to 30 h improves the corrosion resistance. Passive films formed at different aging times exhibit p–n-type semiconducting behavior, and they are mainly composed of oxides of Fe and Cr.
-
4.
Many fine ε-Cu phase and Nb-rich particles formed in the early-aged stage of 0–2 h can provide vulnerable sites for pitting initiation, which results in reduced corrosion resistance. With increasing the aging time, the ε-Cu phase coarsens and its amount also increases. More Cu dissolves as CuCl2− near the surface, consuming more chloride ions and suppressing the surface corrosion, which can compensate for the decrease in corrosion resistance caused by Nb-rich precipitation.
References
H.P. Qu, Y.P. Lang, H.T. Chen, F. Rong, X.F. Kang, C.Q. Yang, H.B. Qin, Mater. Sci. Eng. A 534, 436 (2012)
X. Li, J. Shu, L.Q. Chen, H.Y. Bi, Acta Metall. Sin. -Engl. Lett. 27, 501 (2014)
J. Han, H. Li, F. Barbaro, L. Jiang, Z. Zhu, H. Xu, L. Ma, Mater. Sci. Eng. A 63, 238 (2014)
H.H. Lu, H.K. Guo, Y. Luo, Z.G. Liu, W.Q. Li, J.C. Li, W. Liang, Mater. Des. 160, 999 (2018)
T. Xi, L. Yin, C.G. Yang, K. Yang, Acta Metall. Sin. -Engl. Lett. 32, 1537 (2019)
P. Ou, L. Li, X.F. Xie, J. Sun, Acta Metall. Sin. -Engl. Lett. 28, 1336 (2015)
H. Luo, Q. Yu, C.F. Dong, G. Sha, Z.B. Liu, J.X. Liang, L. Wang, G. Han, X.G. Li, Corros. Sci. 139, 185 (2018)
M.W. Anjum, D. Wen, Q. Wang, R. Zhang, C. Dong, P.K. Liaw, J. Nucl. Mater. 522, 19 (2019)
E.E. Oguzie, J. Li, Y. Liu, D. Chen, Y. Li, K. Yang, F. Wang, Electrochim. Acta 55, 5028 (2010)
M. Seo, G. Hultquist, C. Leygraf, N. Sato, Corros. Sci. 26, 949 (1986)
W.Q. Hu, Z. Dong, L.M. Yu, Z.Q. Ma, Y.C. Liu, J. Mater. Sci. Technol. 36, 84 (2020)
H.H. Lu, Y. Luo, H.K. Guo, W.Q. Li, J.C. Li, W. Liang, Mater. Sci. Eng. A 735, 31 (2018)
M. Murayama, Y. Katayama, K. Hono, Metall. Mater. Trans. A 30, 345 (1999)
S.H. Jeon, S.T. Kim, I.S. Lee, J.S. Kim, K.T. Kim, Y.S. Park, Corros. Sci. 66, 217 (2013)
T. Juuti, L. Rovatti, D. Porter, G. Angella, J. Kömi, Mater. Sci. Eng. A 726, 45 (2018)
G.M. Sim, J.C. Ahn, S.C. Hong, K.J. Lee, K.S. Lee, Mater. Sci. Eng. A 396, 159 (2005)
Y. Kang, W.M. Mao, Y.J. Chen, J. Jing, M. Cheng, Mater. Sci. Eng. A 677, 453 (2016)
J.K. Kim, Y.H. Kim, K.Y. Kim, Scr. Mater. 63, 449 (2010)
J.K. Kim, Y.H. Kim, S.H. Uhm, J.S. Lee, K.Y. Kim, Corros. Sci. 51, 2716 (2009)
Y.J. Kim, S.W. Kim, H.B. Kim, C.N. Park, Y.I. Choi, C.J. Park, Corros. Sci. 152, 202 (2019)
J. Jiang, D. Xu, T. Xi, M.B. Shahzad, M.S. Khan, J. Zhao, X. Fan, C. Yang, T. Gu, K. Yang, Corros. Sci. 113, 46 (2016)
B. Li, Q.L. Pan, C.P. Chen, Z.M. Yin, Trans. Nonferrous Met. Soc. China 26, 2263 (2016)
H. Wei, Y.H. Wei, L.F. Hou, N. Dang, Corros. Sci. 111, 382 (2016)
W. Lu, X. Hua, X. Zhou, J. Huang, X. Peng, J. Alloys Compd. 701, 993 (2017)
T. Xi, M. Babar Shahzad, D. Xu, J. Zhao, C. Yang, M. Qi, K. Yang, Mater. Sci. Eng. A 675, 243 (2016)
H. Tsuchiya, S. Fujimoto, O. Chihara, T. Shibata, Electrochim. Acta 47, 4357 (2002)
X.Y. San, B. Zhang, B. Wu, X.X. Wei, E.E. Oguzie, X.L. Ma, Corros. Sci. 130, 143 (2018)
S. Ningshen, U.K. Mudali, V.K. Mittal, H.S. Khatak, Corros. Sci. 49, 481 (2007)
Z. Feng, X. Cheng, C. Dong, L. Xu, X.J.C.S. Li, Corros. Sci. 52, 3646 (2010)
S. Zhang, H. Li, Z. Jiang, B. Zhang, Z. Li, J. Wu, S. Fan, H. Feng, H. Zhu, Mater. Charact. 152, 141 (2019)
H. Yan, H. Bi, X. Li, Z. Xu, Mater. Charact. 60, 204 (2009)
Z. Dong, N. Liu, W.Q. Hu, Z.Q. Ma, C. Li, C.X. Liu, Q.Y. Guo, Y.C. Liu, J. Mater. Sci. Technol. 36, 118 (2020)
M.P. Sello, W.E. Stumpf, Mater. Sci. Eng. A 527, 5194 (2010)
M.P. Sello, W.E. Stumpf, Mater. Sci. Eng. A 528, 1840 (2011)
C.R. Clayton, Y.C. Lu, J. Electrochem. Soc. 133, 2465 (1986)
C.O.A. Olsson, Corros. Sci. 37, 467 (1995)
E.E. Oguzie, J. Li, Y. Liu, D. Chen, Y. Li, K. Yang, F. Wang, J. Mater. Sci. 45, 5902 (2010)
T. Ujiro, S. Satoh, R.W. Staehle, W.H. Smyrl, Corros. Sci. 43, 2185 (2001)
Z.X. Zhang, G. Lin, Z. Xu, J. Mater. Process. Technol. 205, 419 (2008)
J.J. Guo, S. Masahiro, S. Norio, J. Chin. Soc. Corros. Prot. 10, 239 (1990)
J. Banas, A. Mazurkiewicz, Mater. Sci. Eng. A 277, 183 (2000)
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 51604034 and 51974032), the Science and Technology Project of Jilin Education Department in 13th Five-Year (No. JJKH20181008KJ), and the Science and Technology Development Program of Jilin Province (No. 20190302003GX).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195.
Rights and permissions
About this article
Cite this article
Zhang, T., Han, Y., Wang, W. et al. Influence of Aging Time on Microstructure and Corrosion Behavior of a Cu-Bearing 17Cr–1Si–0.5Nb Ferritic Heat-Resistant Stainless Steel. Acta Metall. Sin. (Engl. Lett.) 33, 1289–1301 (2020). https://doi.org/10.1007/s40195-020-01049-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-020-01049-5