Abstract
Carrier gas hot extraction (CGHE) is a commonly applied technique for determination of hydrogen in weld joints using a thermal conductivity detector (TCD) for hydrogen measurement. The CGHE is based on the accelerated hydrogen effusion due to thermal activation at elevated temperatures. The ISO 3690 standard suggests different specimen geometries as well as necessary minimum extraction time vs. temperature. They have the biggest influence on precise hydrogen determination. The present study summarizes the results and experience of numerous test runs with different specimen temperatures, geometries (ISO 3690 type B and small cylindrical samples), and factors that additionally influence hydrogen determination. They are namely specimen surface (polished/as-welded), limited TCD sensitivity vs. specimen volume, temperature measurement vs. effects of PI-furnace controller, as well as errors due to insufficient data assessment. Summarized, the temperature is the driving force of the CGHE. Two different methods are suggested to increase the heating rate up to the desired extraction temperature without changing the experimental equipment. Suggestions are made to improve the reliability of hydrogen determination depended on the hydrogen signal stability during extraction accompanied by evaluation of the recorded data. Generally, independent temperature measurement with dummy specimens is useful for further data analysis, especially if this data is used for calculation of trapping kinetics by thermal desorption analysis (TDA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen can cause serious defects in welds, typically in terms of cracking, which have serious impact on structural integrity of safety relevant components. Hydrogen-related cracking phenomena are commonly referred as hydrogen assisted cracking (HAC) [1], hydrogen induced cracking (HIC) [2], or even more general: hydrogen embrittlement (HE) [1, 3]. But many more denotations are available. During weld fabrication, those are commonly referred as cold cracking or delayed cracking. The delay is due to the decreasing diffusivity of the diffusible hydrogen in the weld joint after cooling. As a result, the necessary hydrogen desorption time increases [3, 4]. This delay time can be up to days for thick plates [5] and must be considered adequately before non-destructive testing (NDT) of welds in accordance with common standards [6, 7]. Hydrogen can also be introduced into weld joints by subsequent electroplating or in a corrosive environment. Thus, hydrogen promotes and accelerates so-called hydrogen assisted stress corrosion cracking (HASCC) [8].
Generally, HAC, HIC, HE, HASCC (and so on), is a combination of three local factors: a hydrogen concentration, within a susceptible microstructure under sufficient mechanical load [9]. Detrimental effects of hydrogen are mainly the degradation of the ductility/toughness [3], especially in welded steels due to the changed microstructure. Susceptibility for HAC generally increases with strength of the material. Hydrogen sources during weld fabrication are manifold: weld consumables and weld fluxes (SAW, SMAW) [10, 11], humidity, and contaminants (organic greases or lubricants) [4, 12, 13]. The amount and distribution of dissolved hydrogen mainly depends on the weld parameters (voltage, current, travel speed, contact tube distance) [13, 14] or the weld joint geometry (layer sequence, opening angle, or plate thickness) [15,16,17]. Big efforts are made to investigate and to limit the hydrogen concentration in welds, for example, given in [4, 13, 15, 18, 19]. For that reason, reliable hydrogen determination in welds is necessary.
Latest discussions [20,21,22] on standardization of hydrogen determination in welds by ISO 3690 [23] showed that discussion of experimental effects is necessary for the used carrier gas hot extraction (CGHE) method in terms of hydrogen collection and extraction apparatus. Particularly, the specimen temperature, the extraction time, and their interdependencies are important factors that influence the results of hydrogen extraction and collection. It is worth to mention that further boundary conditions can have impact like specimen size and surface.
The mentioned boundary conditions can have effect on the hydrogen concentration measured by CGHE with thermal conductivity detector (TCD). Our present study demonstrates how specific measurement errors or possible misinterpretation of measured values can influence the desired hydrogen concentration results. Based on the aforementioned discussions on ISO 3690 [20,21,22] and our own lessons learned, we give a review on CGHE parameters, which influence the hydrogen effusion and vice versa the measured concentration.
For that reason, the effect of the time-dependent specimen temperature vs. hydrogen effusion/desorption out of a specimen is the focus of this study. For that purpose, different specimen geometries were investigated (ISO 3690 type B specimen and small cylindrical specimens) as well as the influence of abovementioned boundary conditions. However, the next sections give a deeper understanding of hydrogen determination in welds vs. experiment design focused on hydrogen determination apparatus.
2 Hydrogen determination in welds
2.1 Preparation of test welds
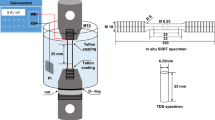
To quantify influence of welding parameter or effect of chemical compositions vs. possible hydrogen concentrations, ISO 3690 [23] standard or ANSI/AWS A4.3-93 [24]are well established as well as JSA/JIS Z 3118 [25]. In our study, we focus on ISO 3690 due to its wide application. Different sets of specimens are described in ISO 3690 that can be used for the experiment. The ISO 3690 sample contains three parts: run-on, center, and run-off piece (Fig. 1a). The run-on (labeled “a” in Fig. 1a) and run-off part (“b”) are removed after welding and the center piece (“c”) is investigated. The specimen is clamped in a suitable water-cooled welding fixture unit, as shown in Fig. 1b. The copper clamps are water-cooled to remove very fast the residual weld heat. The clamped specimen before welding is shown in Fig. 1c. Subsequently, the welded specimen is removed from the fixture and quenched in ice-water to remove residual heat. Hereafter, the specimen is stored in liquid nitrogen at − 196 °C until hydrogen determination is started to prevent further hydrogen effusion. Further details can be found in the ISO 3690 standard [23].
a ISO 3690 specimen dimensions in accordance with [23], b welding fixture for ISO samples at BAM, c clamped specimen before welding
2.2 Methods for hydrogen collection and determination
In accordance with ISO 3690 [23], different methods can be used for determination and measurement for hydrogen: (1) the mercury method and two carrier gas based methods: (2) gas chromatography (GC) and (3) hot extraction (HE). In case of (2) and (3), a thermal conductivity detector (TCD) is used for hydrogen quantification (further details in Section 2.3)
-
(1)
The mercury method is generally and critically discussed due to the use of mercury in terms of health risks as well as environmental protection. Hence, it is more and more replaced by techniques like GC or HE [26]. Further description of this technique is not given here. Details can be found in [23, 27]. Nonetheless, an additional disadvantage is the necessary long time for hydrogen collection of 15–21 days.
-
(2)
Using the GC method, hydrogen is collected from the weld specimen in a closed chamber for certain holding time at elevated temperatures. For that reason, the collection time could be reduced to some hours [28]. After, the chamber is purged with a carrier gas and the gas mixture is transferred to the GC-unit. Typically, a gas chromatograph consists of a heated column (coiled narrow tube with length up to meters) for separation of the individual gases. The separation is achieved by the different retention time of the carrier gas and hydrogen due to the interaction with the column wall. This also includes different time to reach the subsequent TCD for quantification [26]. This technique is commonly abbreviated with CG-TCD.
-
(3)
The HE method (regardless of the use of vacuum or carrier gas) is based on thermal activation of hydrogen atoms in the solid-state specimen and subsequent thermal desorption. The carrier gas hot extraction (CGHE, defined in ISO 3690) is a special kind of HE. Due to specimen heating (in our case: via infrared radiation), hydrogen is activated and effuses from the specimen. The hydrogen desorbs from the specimen surface and recombines to molecular gas. CGHE is characterized by a half-open hydrogen collection chamber that is continuously purged with an inert carrier gas (such as nitrogen). This gas flow carries the desorbing hydrogen. In accordance with ISO 3690 [23], the gas mixture is transferred to a TCD, so-called CGHE+TCD. The main advantage of the CGHE is a relatively short time (< 0.5 h) at respective temperatures for hydrogen collection [23, 29] (higher temperature effect described in Section 4.1) and easier specimen handling due to the half-open chamber for gas collection.
There are pros and cons for all introduced methods. It is also a question of available budget as well as the number of specimens that have to be analyzed (and time per specimen) which kind of equipment is used for the hydrogen determination in welds. In the following, we focus on the CGHE+TCD method.
2.3 Hydrogen determination by TCD
The principle of the thermal conductivity detector (TCD) is differential thermal conductivity of the examined gas compared to the reference gas. The TCD consists of reference cells and specimen cells mounted in a thermostat-controlled aluminum block [30]. These cells are connected via Wheatstone’s bridge with a constant electrical current. Each cell contains a thermal resistor, which changes its electrical resistance with temperature. The reference cells are rinsed with the carrier gas. This gas has a specific thermal conductivity. The specimen cells are rinsed with the gas mixture (carrier gas and desorbed hydrogen). The thermal conductivity of the gas mixture changes due to the “contamination” with hydrogen. This changed conductivity leads to a temperature change in the thermistor, and thus instantaneously to a change of the electrical resistance, hence a change of the measured voltage in the sample cells. This voltage drop can be related to the hydrogen amount desorbing within certain time increment. Therefore, calibration using specified amounts of hydrogen is needed beforehand. The summation of the incremental steps results in an integral corresponding to a hydrogen amount in the specimen. By relation of this amount to the weld specimen weight, hydrogen concentration is calculated. Assuming hydrogen is only inserted to the weld seam by the weld consumable, the weight of the deposited weld metal must be considered for calculation of the corresponding hydrogen concentration. This is experimentally ensured by dehydrogenation heat-treated ISO 3690 specimens before welding.
2.4 Specifics of using infrared radiation heat source
For CGHE, different heating methods are possible, e.g., infrared (IR) radiation or inductive heating for solid-state extraction of hydrogen as well as Joule-heating of crucibles for melt extraction (e.g., Bruker or LECO). IR radiation offers the advantage that the specimen can be heated contactless as well as a wide range of adjustable extraction temperatures and times (like in case of Bruker Corporation hydrogen analyzers G4 or G8 equipped with furnace type IR07). The main advantage of solid-state extraction is that hydrogen transport data (diffusion coefficients, thermal desorption analysis) can be additionally determined as well as temperature depended trapped amounts of hydrogen, for example, see our results in [19, 30,31,32,33]. A typical setup is shown in Fig. 2.
The IR radiation heat source is mounted beneath the specimen chamber (in our case a quartz glass tube, which is rinsed/flushed with the carrier gas). This chamber is surrounded by parabolic mirrors. Those concentrate the IR radiation on the specimen surface and are responsible for the heating by radiation absorption [21].
2.5 Temperature control of furnace
The CGHE has a thermocouple that is placed beneath the glass tube in which the weld sample is inserted. This thermocouple is used for furnace control (as shown in Fig. 2). Temperature control is mostly realized by a proportional-integral-derivative (PID) controller. This controller is a loop feedback mechanism and uses the mentioned thermocouple as measured process variable. This process variable is permanently corrected in comparison to the desired setpoint [34]. In other words, the actual temperature should be as close as possible to the desired temperature without delay or overshooting. To achieve this:
-
The proportional (P) part is set in relation to the existing discrepancy of programmed and real temperature, i.e., fast response to temperature changes. Negative effect is that fast change typically involves overshooting of the temperature, i.e,. temperature error.
-
The integral (I) term is used to calculate the magnitude and duration of this error, i.e., the integrated error sum that should have been corrected previously. The integral term accelerates the movement of the process.
-
The derivative (D) part is calculated by determination of the slope of the current error over the time.
-
It is used to flatten the error trajectory of the temperature into a horizontal line, i.e. constant extraction temperature. This is important especially for long extraction time.
In case of the most analyzers with temperature control, the PID controller is mostly of PI-type with limited effect of D-part.
3 Materials and methods
3.1 Examined materials and specimen dimensions
In this study, we present the summarized results of different CGHE experiments performed during the last years. The test materials were low-alloyed steel S690Q and creep-resistant steel T24 (7CrMoVTiB10-10). Table 1 shows the chemical composition of the investigated steel grades (determined by optical emission spectroscopy). The effect of CGHE parameters on hydrogen determination was investigated by two different specimen types.
-
The first was ISO 3690 type B specimen (only S690Q) with the dimensions lc = 30 mm, e = 15 mm, and t = 10 mm. The corresponding abbreviations are explained in Fig. 1a. All samples had the necessary surface condition (ground) and dehydrogenation heat-treated before welding. Three representative conditions were investigated to determine the influence of surface condition on temperature evolution during extraction of the ISO type B specimens: (1) polished/ground surface, (2) oxidized surface without weld seam, and (3) welded. For welding, MAG-process was used with M21 (18% CO2 and 82% Ar) shielding gas and matching filler material. After charging, the specimens were stored in liquid nitrogen in accordance with ISO 3690 [23].
-
The second was a cylindrical specimen (only T24 base material) with Ø = 3 mm and 20 mm length with ground surface (500 grit SiC paper). Electrochemical hydrogen charging offers the option to charge reproducible hydrogen amounts into a specimen, which gives some advantages for the further necessary hydrogen determination. Hydrogen electrochemical charging was performed for a couple of T24 base material specimens using 0.05 M H2SO4 acidic solution with addition of 12 mg/l NaAsO2 (recombination poison to prevent molecular H2-form). For that purpose, galvanostatic charging mode was used to obtain different hydrogen concentrations by different charging current densities, as shown in [32, 33, 35]. The specimen was the cathode and a Schott Pt1800 electrode was anode. Before charging, each specimen was cleaned in acetone using an ultrasonic bath and rinsed in inert nitrogen gas flow. After charging, the specimens were stored in liquid nitrogen in accordance with ISO 3690 [23] before hydrogen determination was done by CGHE.
3.2 Carrier gas hot extraction and temperature measurement
For hydrogen determination, the H-analyzer JUWE H-mat 221 was used. This analyzer corresponds to the commercially available analyzer Bruker G4 PHOENIX. It is a CGHE-based analyzer equipped with TCD. For some experiments, the Bruker G8 GALILEO was used with coupled mass spectrometer. Despite of two different detection techniques, both analyzers are equipped with the same IR-furnace type for solid-state extraction (IR07). This furnace contains the glass tube in which the specimen is place. For that purpose, Fig. 3a shows the furnace and quartz glass tube of the IR07. The red square indicates the approximate position, where the specimens should be placed in the glass tube. One reason is that here the IR-furnace thermocouple is installed (temperature control).
For more precise specimen temperature determination, calibration specimens were used. These had similar surface conditions and identical geometry (cylindrical specimen is shown in Fig. 3b and ISO 3690 type B specimen in Fig. 3c). An additional hole was drilled in the center of the specimens and a type K mantle-thermocouple was attached in this hole. By this external thermocouple, an independent temperature measurement was ensured that could be compared to the adjusted furnace temperature. The external thermocouple was applied in the center region of the ISO type B specimen to ensure that the specimen core temperature was measured and not the surface temperature. The reason was to exclude possible underestimation of temperature evolution due to thermal gradient between surface and specimen bulk material. In addition, this thermal gradient in thickness direction is the driving force for the thermal conductivity, i.e., the increase of the specimen temperature.
4 Results and discussion
4.1 Facts of necessary time and temperature vs. detectable TCD-signal
For hydrogen determination in accordance with [23], the necessary hydrogen collection time can be reduced at elevated temperatures, as shown in Fig. 4. For example, an (specimen) extraction temperature of 400 °C calls for a minimum dwell time of 0.35 h (21 min) compared to 72 h at 40 °C. The reason for the shorter necessary hydrogen collection time is the increasing hydrogen diffusivity at elevated temperature. Nonetheless, hydrogen diffusion is affected by the chemical composition. Typically, alloy elements decrease diffusivity compared to pure iron [36]. Even more “complex” materials like low-alloyed steels and their weld joints show this general trend [37].
Extraction temperature vs. recommended minimum dwell time, in accordance with [23]
At higher extraction temperatures, the diffusion coefficients generally increase. They have an Arrhenius-type relationship as shown in Eq. 1.
“D” is the temperature depended hydrogen diffusion coefficient, “D0” is material specific constant, “EA” is the activation energy in kJ/mol, “R” is the universal gas constant with 8.31 J/(mol × K), and “T” is the absolute temperature in K. Because of the temperature dependent diffusion, higher extraction temperature results in faster hydrogen desorption, which is directly used for the recommended values in ISO 3690 [23]. Hence, a shorter dwell time is necessary for hydrogen collection, which is one of the most important economic advantages of CGHE compared to the conventional mercury method, independently of the environmental and health risks of using mercury.
The use of a TCD has some inherent specific topics. The restrictive condition is the “limited” resolution of 0.01 μg H /1 g specimen weight (~ 0.01 ppm) and the minimum detectable amount of 0.05 μg/g. This is already a quite good resolution for hydrogen determination but limited compared to detection limit of highly sensitive methods like mass spectrometry (in ppb-range) [38]. Hence, the reliability of measured hydrogen contents by TCD strongly depends on the available hydrogen amount in the specimen and how “fast” this hydrogen is extracted. Assuming a constant hydrogen concentration in the specimen, a higher extraction temperature (high diffusivity and reduced desorption time) leads to increased signal stability in terms of improved signal-to-noise ratio. In other words, a high hydrogen peak with small width (high extraction temperature) can be better detected than a small but wide peak (low temperature) as shown in Fig. 5.
The reason for the limited resolution is the small difference of the thermal conductivity of carrier gas (nitrogen) and the analyze gas (nitrogen and hydrogen mixture) that must be measured by the TCD.
A short thought experiment clarifies the limits: The hydrogen content “HD” of a welded type B ISO 3690 specimen shall be given with maximum 5 ml/100 g; “H5”-classification, e.g., for flux cored wires [39,40,41]. Assuming the preferred distribution in the weld seam and neglecting the fused base material, the type B demands a weight of ≥ 3 g of deposited weld metal in accordance with ISO 3690 [23]. In this case, the total hydrogen amount of the deposited weld metal in the H5-specimen is approx. 0.15 ml or 150 μl (assuming 3 g as minimum weight of the deposited weld metal). This small amount is detected by the TCD. But this hydrogen volume corresponds to the integrated effusion curve (schematically represented by the green curve in Fig. 5). This curve is the sum of the detected current H-effusion rate, which is typically very low for H5-classification.
Practical examples for temperature dependent hydrogen effusion rates (in ml/100 g per s) are shown in Fig. 9 for the T24 base material. The specimen was electrochemically charged with ~5 ml/100 g Fe. This corresponds to 0.05 ml/g Fe. The real specimen weight was 1.1 g. Hence, the absolute hydrogen amount corresponds to 0.055 ml or 55 μl (dissolved in 1.1 g metal). The specimen was isothermally held at different temperatures (adjusted temperature in the software). Although, it was a small cylindrical specimen, it represents a good correlation to the ISO 3690 type B specimens considering a minimum weight of deposited weld metal of 3 g. The hydrogen effusion rate increases with increasing temperature and the total effusion time decreases. This is indicated by the time “t0.5”, which represents a value after 50% of the charged hydrogen was effused. At 400 °C it is approximately one quarter of the necessary time at 100 °C (24 vs. 91 s). It is obvious that at 100 °C a very low effusion rate was present (max. 0.02 ml/100 g per s) (Fig. 6).
Influence of extraction temperature on effusion rate and t0.5-time in accordance with [35]
If the temperature is too low, inaccurate results can occur in terms of insufficient signal-to-noise ratio, i.e., limited effusion rate. The result would be that TCD detects “no” hydrogen although small rate of hydrogen effuses. Finally, this can be a reason for underestimation of hydrogen content in the specimens, namely weld fluxes for SAW [42]. The temperature evolution, i.e., heating, to the desired extraction temperature is the most effective CGHE parameter and is consistently linked with a sufficient extraction time.
The thermodynamic process of hydrogen desorption/extraction is influenced by the specimen geometry and size. The heating of the specimen is the result of the heat conduction in the bulk by the adsorbed radiation energy. In the following, some results are shown which increase the understanding of unavoidable differences of specimen and furnace temperature as well as countermeasures.
4.2 Influence of specimen size and geometry
As mentioned in [20], different ISO specimen types resulted in different heating behavior, i.e., different extraction behavior of hydrogen. For example, the A-type needed approx. 60 min to reach constant (specimen) extraction temperature and the B-type approx. 30 min. This is consistent with our own experience. For specimen type B, an extraction temperature of approx. 350 °C (specimen) was reached after 10 min, as shown in Fig. 7. In addition, the figure shows the temperature evolution for different isothermal hot extractions from 200 to 500 °C representing different time-temperature combinations covering the information given in ISO 3690 [23]. The 100 °C extraction temperature is not shown due to the minor relevance for practical CGHE (long necessary dwell time). The 500 °C extraction temperature is shown for comparison. Although this temperature is not fixed in the ISO 3690, it emphasizes that a faster specimen heating is possible by higher temperatures. In that case, 350 °C are already achieved after 300 s. This effect can be used for further studies concerning trapping and diffusion behavior (see Section 4.4). It is obvious that a certain time is necessary to reach the desired extraction specimen temperature.
In accordance with ISO 3690, the minimum dwell time at 400 °C is 0.35 h or 21 min (Fig 4). Considering the furnace temperature and the real specimen temperature (see Fig. 8), a temperature difference of up to 25 K can occur during quasi-isothermal holding. This temperature difference was evident for all investigated temperature levels. This can be a detrimental problem for hydrogen determination if welded materials are investigated that have complex trapping behavior including retained austenite [43] or considerable amount like in case of duplex steels [44]. In accordance with the intended use of ISO 3690, 400 °C furnace temperature is high enough. A crack “critical” hydrogen amount should already be released at this temperature. Hence, from our point of view, the discussion about the final temperature and how fast it is reached is somewhat unnecessary in case of ISO 3690. The ISO standard only represents a technical recommendation with a minimum holding time. Each user must ensure reliable measurements (sufficient dwell time) by him- or herself.
In addition to the temperature depended necessary extraction time, the specimen geometry has an influence in terms of surface and volume. The reason is that the IR radiation must be absorbed at the surface and is subsequently transferred to the bulk material. How fast a temperature change occurs is influenced by the thermal conduction coefficient of the material and the specimen thickness (i.e., cross-section area). This behavior is shown for the ISO 3690 type B and the cylinder specimen in Fig. 9.
The IR-furnace temperature is controlled by a thermocouple beneath the glass tube and is only indirectly influenced by the specimen temperature (Fig. 3a). The two cases shown in Fig. 9 compare the different heating behavior for a constant adjusted (and recorded) furnace temperature using the same PI controller values (P = 1800, I = 22.5, D = 0; it must be considered that those values mostly depend on the installed firmware version of the control software and CGHE analyzer type). Independently of the desired extraction temperature (200 or 400 °C), the cylinder specimens are much faster. For example, the cylinders already had 350 °C after 200 s compared to 600 s for ISO 3690 type B.
It is assumed that the effectively heated surface is the main influence in relation to the specimen volume. To give a ranking on that, the so-called surface-area-to-volume ratio “SA : V” can be used. For the ISO 3690 type B specimens “SA : VISO(B)” is calculated by Eq. 2. Nonetheless, this is valid for all three ISO geometries. The reason is the rectangular shape of the specimens. The surface-area-to-volume ratio “SA : VCYL” for the cylinder specimens is calculated by Eq. 3.
where “lc, e” and “t” are the dimensions in accordance with ISO 3690 [23] (see Fig. 1a). The length of a cylinder (with radius “r”) is here also considered as “lc”. Considering that the specimen is placed parallel to the IR- radiation sources (see Fig. 3a, b), the front and rear surface can be neglected for both specimen types. For that reason, the ISO 3690 type B specimen surface area is reduced by 2 × e × t and for the cylinder by 2 × π × r2. The reason is that the front and rear specimen surface (ISO 3690 type B: e × t and cylinder: π × r2) are not parallel to the IR radiation sources and parallel to the mirrors that concentrate the radiation onto the specimen surface. Only parallel surfaces can effectively absorb the radiation energy (if diffraction is neglected). Hence, both equations can be simplified to the following:
and further by eliminating “lc” (both equations) and: “π × r2” (Eq. 5) reduced to:
From Eqs. 6 and 7, it is obvious that neither the length of the ISO type B specimen nor the length of the cylinder has a dominant effect. In case of the ISO specimens, especially the thickness “t” has a big impact on the surface-area-to-volume ratio “SA : V” and thus on the heating behavior. In case of the cylinder specimen, the radius “r” is the determining factor. The corresponding ratios of the ISO 3690 specimens are: type A = 0.25, B = 0.33, and C = 0.26. The cylinder has 1.33, which is much higher. From these values, two conclusions can be made: (1) the length of the specimen is negligible in the IR-furnace and (2) an increased “SA : V” results in faster heating. This is supported by the results in [20] (type B faster than A) In other words: relatively thin specimens with a suitable width warm-up faster compared to thick specimens with concurrent small width. Thin specimens are favorable for further investigations like thermal desorption analysis (TDA) or investigation of high-temperature trapping and diffusion kinetics.
This effect also can be seen in Fig. 12 (heating to 300 °C) and Fig. 15 (200 °C). In case of small specimens, the temperature is already reached within 2 min and shorter, which is way below the ISO 3690 type B specimens. The used cylinder specimens are quite unrealistic in case of welding. Nonetheless, they are a suitable specimen size for further thermal desorption analysis (TDA) to clarify hydrogen trapping and diffusion kinetics, as we successfully done in the past [31,32,33, 35]. Even in case of using such small specimens, a deviation of adjusted (in control software) and real specimen temperature up to some 10 K can occur [32, 33].
The ISO 3690 type B specimen represents a suitable specimen size, e.g., for shielded metal arc welding (GMAW) or gas metal arc welding (GMA). In [20], an additional ISO 3690 specimen type (referred as “Type D”) was suggested by other research groups due to the favorable possible faster heating. This effect is only based on the smaller sample size. Based on our experience, it is also favorable to increase the necessary extraction/dwell time at the desired extraction temperature (see Fig. 6). The advantage is that the delayed heating to the desired extraction temperature is compensated by the prolonged extraction time for hydrogen collection. A second advantage is that the established ISO 3690 specimen geometries can be used regardless of possible further geometries.
4.3 Specimen surface condition
Two possible specimen surface conditions before and after extraction were investigated for the ISO 3690 type B specimens: oxidized after welding or hot extraction and initial ground surface condition. These two different conditions must be anticipated in case of using an IR radiation source.
Figure 10 shows the difference between ground (polished) and oxidized (by furnace treatment) ISO 3690 type B specimen. Figure 11 shows comparison to an as-welded ISO 3690 type B specimen. The adjusted extraction temperature of the specimen was 400°. Both figures show that the polished condition delays the heating of the ISO 3690 type B specimen compared to the oxidized or as-welded condition. For example, the ISO 3690 type B specimen with weld seam had already 350 °C after 600 s compared to 1200 s (i.e., 20 min!) of the polished surface. Commonly, the so-called emissivity of polished steel is approximately 0.1 compared to 0.7 to 0.9 in oxidized condition [45]. This means a polished surface reflects 90% of the radiation energy compared to 30 to 10% of an oxidized surface. Hence, in case of constant radiation power, the oxidized specimen is heated much faster. Before welding, the ISO 3690 type B specimen set is in ground (less oxide scales) surface condition. This means an optional temperature assessment of welded specimens must always be done with the identical surface condition, i.e., an as-welded specimen. Finally, it can be concluded that independent temperature measurement should always be conducted (if available) when using CGHE for hydrogen determination.
In case of using IR radiation as energy source for solid-state extraction of hydrogen, some aspects must be considered during CGHE:
-
Infrared (or thermal radiation) is absorbed at the specimen surface. This process strongly depends on the surface condition of the specimen, i.e., polished/ground or oxidized, and is expressed by the absorption or emissivity coefficient. Considering a constant radiation source power, a higher amount of reflected radiation results in a delayed heating to the desired extraction temperature. In other words, the necessary dwell time for hydrogen release is directly coupled with the specimen heating behavior to the desired temperature.
-
The material is heated by the heat transfer from the surface to the bulk material that is characterized by the specific thermal conductivity of the examined material. In addition, this means a temperature deviation between the adjusted furnace temperature and the specimen surface temperature is existent as well as the temperature gradient in the specimen. Materials with higher thermal conductivity (like copper) could be heated faster compared to iron or steel. In case of high austenite amount in the weld metal, it should be considered that fcc-phase (austenite) has approximately one third lower thermal conductivity compared to bcc (ferrite) materials. This can result in a delayed heating of the specimen despite the ISO 3690 standard only refers to ferritic, bainitic, or martensitic weld microstructures [23].
The effect of temperature delay is less important for ISO 3690 samples independently of type A, B, or C (only the determination of an absolute hydrogen amount is of interest) but is very important for correct calculation of activation energy of hydrogen traps from thermal desorption analysis (TDA). During TDA, the peak temperature and a heating rate are used for calculation of the activation energy of a hydrogen trap in accordance with [46]. This calculation method is an alternative to Eq. 1. If the specimen temperature is assigned incorrect, the calculated activation energy can be somewhat misleading in terms of “diffusible” and “trapped” hydrogen. Even in low-alloyed T24 steel, we determined trapped hydrogen above 100 °C [32]. Additionally, only correct temperature measurement directly on the specimen (i.e., not the idealized linear heating rate) allowed to calculate activation energy that corresponds to residually trapped hydrogen in the microstructure. This demonstrates that it is necessary to deduce the correct extraction temperature in the specimen during CGHE experiments. This can be easily done by calibration specimen without hydrogen that undergo the same extraction time-temperature-cycle.
4.4 Countermeasures to influence the specimen temperature during heating
4.4.1 PI-parameter setting
By adjusting the PI-parameter of the IR-furnace, the heating process of the specimen can be simply somewhat “accelerated.” Major impact has typically the P-part [34]. We determined a similar behavior as shown in Fig. 12. In this figure, the temperature of the center region of the cylindrical specimen is shown for a desired extraction level of approx. 300 °C.
The P value of the PI controller is responsible for a fast temperature response of the furnace, i.e., fast heating to the desired extraction temperature. But this behavior is somewhat limited and strongly depends on the sample size. Only for the small cylindrical specimens this effect was found to be significant. The orange line (cond. 1) in Fig. 12 represents the best-case scenario in terms of fastest possible heating of the small samples. The identified parameters were P = 1800 and I = 22.5 (D = 0). But during our investigations, we recognized that a possible preheating (PH) of the glass tube in advance to the specimen insertion is quite useful. The control software allows such procedure by simple adjustments (definition of higher preheating temperature than analysis temperature). For that reason, we investigated again a set of different parameters as shown in Fig. 12 but with additional PH temperature of approximately 780 °C for 120 s prior to the specimen insertion. It is worth to see that a change of any combination of P- and I-parameter did not result in significant differences. This can be seen by comparing all conditions including certain preheating (cond. 2–6). But there was a major difference if the PH-conditions are compared to condition 1 (without PH). Hence, we decided to investigate this effect more in detail (as shown in the next paragraph.
The effect of the PID controller is limited by the specimen dimensions. An ISO 3690 type B specimen is somewhat “thick” compared to the smaller “cylindrical” specimens. Hence, it is difficult to recommend best-case PI(D)-parameters for certain extraction temperature. As best-practice recommendation, from our experience and for any desired extraction temperature, the adjusted temperature in the Bruker control software should be higher than the intended extraction temperature. Our ISO 3690 type B specimens had a difference of up to 20 K below the desired extraction temperature. But it is at least questionable if 380 or 400 °C is “better or worse.” This is up to the customer. From the point of thermodynamics and weld metal microstructure, all hydrogen should be already diffusible at this temperature despite ferrite, martensite, bainite, or pearlite (in other words: the field of application of ISO 3690). Thus, it is just a question of necessary extraction time at relatively high temperatures. Nonetheless, it is recommended to investigate the specific heating behavior of “dry” hydrogen-free specimens in advance to real extraction experiments, e.g., by suitable calibration specimens [32, 33, 35].
4.4.2 “Preheating” of extraction chamber
As shown in Fig. 12, we also investigated a certain preheating effect of the quartz glass tube on the appearing temperature profiles. We partly reported on that in [33, 35]. This effect can accelerate the heating process of the specimen significantly. The general procedure is schematically shown in Fig. 13.
Preheating with different temperatures (A to C) of extraction chamber (glass tube) for (cylindrical specimen with 3 mm Ø, 20 mm length) [33]
The “secret” is to choose a higher temperature for preheating the glass tube/furnace system compared to the desired extraction temperature. For that purpose, we investigated three conditions temperature conditions A, B, and C. The preheat time was about 120 s for conditions B and C. Condition A was the reference in terms of adjusting only the desired extraction temperature (same PID-parameters as in condition 1, shown in Fig. 12. Those features can also be easily adjusted in the IR07 furnace control software. After 120 s, the specimen is inserted and the furnace “cools” down to the desired extraction temperature.
The temperature effect of the three different preheating conditions (schematically described in Fig. 13) on the real specimen temperature is shown in Fig. 14. Two cases are shown: a desired extraction temperature of 100 and 200 °C. All temperatures represent the real specimen temperature.
Preheating of glass tube for accelerated specimen heating (cylindrical specimen with 3 mm Ø, 20 mm length), desired extraction at 200 and 100 °C [35]
It is obvious that preheat condition C results in the fastest heating of the specimen. Preheating at 325 °C (condition C) results in the highest temperature gradient (approx. 175 °C already after 50 s) during warm-up of the specimen. The reason for the accelerated heating is the higher energy, which is then already present in the furnace system. In addition, the emissivity of the IR radiation source increases with increasing preheating furnace temperature. During “cooling” of the furnace to the desired extraction temperature, the increased emissivity at 325 °C (compared to 200 °C) results in a faster heating of the specimen after insertion. The effect on the corresponding hydrogen effusion/desorption is shown in Fig. 15.
In condition C, the hydrogen effusion is accelerated at the beginning due to the increased temperature of the specimen. The peak value is already reached after 25 s compared to approx. 90 s for condition A. This also influences further data that are calculated from those experiments.
It is quite useful if hydrogen diffusion coefficients are calculated from those “quasi-isothermal” experiments. The reason is the reduced temperature deviation from the idealized isothermal condition of the specimen during hydrogen effusion/desorption. Otherwise, different methods must consider a kind of limited mean value of the temperature during hydrogen desorption [47]. That results in a certain deviation of experimental data and analytical solution, especially at the beginning of the experiment, i.e., delayed heating of the specimen. A second advantage can be found for desorption analysis experiments using a constant heating rate to calculate certain trapping characteristics from desorption spectra. We already reported on this effect, which influences the activation energy for hydrogen diffusion and trapping [31,32,33], independently from the microstructure of the welded component.
It is necessary to mention, that like in case of the PI parameters, this effect of preheating is only significant for smaller specimens (cylinder specimens compared to the ISO 3690 type B specimens).
Nonetheless and independently of the specimen size, the specimen temperature always will be below the programmed extraction temperature in the control software of the furnace! In general terms, the furnace or glass tube must be always hotter than the specimen. Hence, it is at least questionable if further specimen types should be added to the standards [20, 21] despite the specimen size itself has an impact (in terms of delayed heating of thick specimens).
4.5 Possible errors during automated calculation of hydrogen concentration
It is worth to say that a considerable factor for reliable measurements is the human factor in terms of “blind trust” on automatically generated values. This is independently of the hydrogen analyzer type (i.e. manufacturer). Setting of total hydrogen extraction time, extraction temperature, or result assessment is typically done by humans, but the total hydrogen concentration is mostly automatically calculated by the software. Two problems can typically occur during calculation of hydrogen concentration: insufficient extraction time (Fig. 16) or wrong assignment of results.
As indicated in Fig. 16, a possible reason for misinterpreting results is if “automated” measurements are intended. For example, if a hydrogen measurement is started with a defined maximum dwell time in accordance with the table “extraction temperature and dwell time” given in ISO 3690 [23]. In case of 400 °C, an extraction time of 0.35 h is recommended. If this time is set as maximum extraction time, hydrogen collection is virtually aborted with a corresponding desorbed and detected hydrogen amount of (“HDD”) before all hydrogen has left the specimen. Hence, an error occurs due to the missing and not detected part (“HDND”). As a result, the total hydrogen content (“HDGes”) would be too low. For that reason, it is strongly recommended to set a sufficient holding time for hydrogen extraction and collection, for example at least 50% prolonged compared to the recommendations given in ISO 3690 [23]. In addition, sufficient holding time at the desired temperature ensures that all diffusible hydrogen (“HDGes”) has left the specimen. This effect is strongly related to the previously manually set hydrogen collection time. It is not a problem by the control software, it is human made. Hence, it is strictly recommended to perform “surveilled” measurements from time to time by qualified staff. This is independently of the equipment manufacturer!
The second fact of automated determination is a possible wrong assignment of the TCD baseline (shown in Fig. 17). If the baseline is far away from the horizontal regular shape, the calculated total “HDGes” (i.e., the total of the integrated TCD-signal) can be over- or underestimated, as for example reported in [22]. This is especially the case for long desorption/extraction time and small signal-to-noise ratio (like shown in Fig. 5). The wrong baseline assignment by the software can influence the final hydrogen amount. The upper part of Fig. 17 shows a real hydrogen signal that was afterwards manually manipulated to emphasize the possible difference to the correct signal. The gray-shaded area in Fig. 17 (lower part) corresponds to the “not detected” hydrogen amount “HDND”. The baseline is typically set by the software automatically, based on so-called lower signal switch-off limit (set in the control software as mV-threshold). This switch-off is the most important boundary for the integration limit of the signal, i.e., the hydrogen amount in the specimen. This integration limit can be very easily manually adjusted after measurement.Footnote 1 Especially for small amounts of hydrogen dissolved in small specimens, the correct integration limits can have a significant effect. Unfortunately, it is hard to quantify in which case this fact becomes detrimental for “reliable” hydrogen determination.
Nonetheless, an empirical and methodological recommendation is to increase the hydrogen extraction temperature (more stable signal) at sufficient extraction time. For that reason, it is suggested to use at least temperatures above 200 °C as extraction temperature. Our own experience showed that even in low-alloyed bainitic or ferritic weld joints trapped hydrogen concentrations of > 1.1 ml/100 g Fe can occur below this temperature [32, 33, 35]. And this trapped hydrogen concentration must be considered for the classification of the hydrogen level of a weld consumable.
5 Summary and conclusions
Hydrogen assisted cracking is a considerable failure during and after weld fabrication. Big efforts are made by industry to limit the hydrogen content in welds (e.g., in weld consumables). For that reason, precise hydrogen measurement is reasonable. The aim of the present study is to give a review on CGHE parameters and their influence on hydrogen effusion, and vice versa, the measured hydrogen concentration. The following conclusions can be drawn:
-
In case of carrier gas hot extraction with infrared furnace (solid extraction), the extraction temperature and dwell time are the most important parameters for hydrogen determination. Sufficient time and temperature must be chosen to collect all hydrogen in the specimen affecting hydrogen assisted cracking.
-
Specimen size and geometry have significant influence on hydrogen determination for a given desired extraction temperature. In case of using IR radiation, the specimen is heated via radiation absorption and the bulk material by further heat conduction. Hence, a delay time to reach the desired temperature occurs, which directly depends on the specimen dimensions. Smaller specimens are generally heated faster. This can be useful if the CGHE analyzer is used further for determination of diffusion and trapping kinetics in microstructures, not only for ISO 3690 procedure.
-
The surface condition (polished/ground vs. oxidized/welded) has effect on the specimen temperature. The temperature in ISO 3690 type B specimens was approximately 50 K higher in the as-welded condition after 10 min holding time. Nonetheless, all investigated conditions showed a final discrepancy of 25 K during isothermal holding. Hence, ex-ante temperature assessment like the suggested dummy specimens can be helpful to estimate and asses the preliminary specimen temperature for a given furnace parameter set. For that purpose, these calibration specimens must have the same surface condition (ex-post temperature measurement with an already welded sample).
-
The heating process of the specimens can be accelerated by using adapted PI parameters and in particular by a higher preheating temperature of the glass tube, before hydrogen collection (i.e., specimen insertion) is started. In that connection, these effects are more significant for small specimens due to the instantaneous reaction of the specimen. The reason is the increased surface-area-to-volume ratio (“SA : V”). This ratio is independent of the specimen length. Specimen thickness and width (or radius in case of cylinders) are the determining factors.
-
Calculation of hydrogen concentration depends on reliable measurement of the extracted hydrogen. This depends on sufficient effusion rates, i.e.. sufficient extraction temperature and time. From our point of view, the ISO 3690 extraction time table should only be used as a general recommendation. Independent measurements for sufficient extraction time can be helpful and should be done if possible. At least, they improve the understanding if the determined results are reliable.
-
The “human” factor should be considered in terms of qualified staff. This ensures that suitable extraction time and temperature are selected for software-calculated hydrogen concentration. In addition, this ensures that the integration limits are set correctly and the manual cross-checking of selected results in case of high number of CGHE-tests. This is explicitly independent of the hydrogen analyzer equipment manufacturer.
Notes
by pressing “Ctrl -Y” in the Bruker G4/G8 control software and setting the limits manually by using the arrow keys. This is also written in the apparatus’ manual.
References
Beachem CD (1972) A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall Mater Trans B Process Metall Mater Process Sci 3(2):441–455. https://doi.org/10.1007/BF02642048
ANSI/NACE MR0175/ISO 15156 (2015) Petroleum and natural gas industries - materials for use in H2S-containing environments in oil and gas production
Robertson IM, Sofronis P, Nagao A, Martin ML, Wang S, Gross DW, Nygren KE (2015) Hydrogen embrittlement understood. Metall Mater Trans B Process Metall Mater Process Sci 46(3):1085–1103. https://doi.org/10.1007/s11663-015-0325-y
Du Plessis J, Du Toit M (2008) Reducing diffusible hydrogen contents in shielded metal arc welds through addition of flux oxidizing ingredients. J Mater Eng Perforn 17(4):50–56. https://doi.org/10.1007/s11665-007-9133-0
Pargeter R (2003) Evaluation of necessary delay before inspection of hydrogen cracks. Weld J 82(11):321s–329s
DIN EN 1090-2 (2017) Execution of steel structures and aluminium structures - Part 2: technical requirements for steel structures
EN 1011-2 (2001) Welding - recommendation for welding of metallic materials - Part 2: arc welding of ferritic steels
Hirth JP (1980) Effect of hydrogen in the properties of iron steel. Metall Mater Trans A 11(6):861–890. https://doi.org/10.1007/BF02654700
ISO 17462-1 (2004) Destructive tests on welds in metallic materials - cold cracking tests for weldments - arc welding processes - part 1: general
Kasuya T, Hashiba Y, Ohkita S, Fuji M (2013) Hydrgen distribution in multipass submerged arc weld metals. Sci Technol Weld Join 6(4):261–266. https://doi.org/10.1179/136217101101538767
Patchett BM, Yarmuch MAR (2010) Hydrocarbon contamination and diffusible hydrogen levels in shielded metal arc weld deposits. Weld J 89:262s–265s
Bailey N, Coe FR, Gooch TG, Hart PHM, Jenkins N, Pargeter RJ (2004) Welding steels without hydrogen cracking, 2nd edn. Woodhead Publishing, Oxford
Pitrun M, Nolan D, Dunne D (2004) Diffusible hydrogen content in rutile flux-cored arc welds as a function of the welding parameters. Weld World 48(1/2):2–13. https://doi.org/10.1007/BF03266408
Kannengiesser T, Lausch T (2012) Diffusible hydrogen content depending on welding and cooling parameters. Weld World 56(11/12):26–33. https://doi.org/10.1007/BF03321392
Schaupp T, Kannengiesser T, Burger T et al (2018) Einfluss der Wärmeführung auf die Wasserstoffkonzentration in geschweißten höherfesten Feinkornbaustählen beim Einsatz modifizierter Sprühlichtbogenprozesse. Schweißen und Schneiden 70(5):290–297
Schaupp T, Rhode M, Yahyaoui H, Kannengiesser T (2018) Hydrogen distribution in multi-layer welds of steel S960QL. In: Proceedings of the Third International Conference on Metals & Hydrogen, Ghent
Mente T, Boellinghaus T, Schmitz-Niederau M (2012) Heat treatment effects on the reduction of hydrogen in multi-layer high-strength weld joints. Weld World 56(7/8):26–36. https://doi.org/10.1007/BF03321362
Kannengiesser T, Boellinghaus T (2013) Cold cracking tests-an overview of present technologies and applications. Weld World 57:3–37. https://doi.org/10.1007/s40194-012-0001-7
Schaupp T, Rhode M, Kannengiesser T (2008) Influence of welding parameters on diffusible hydrogen content in high-strength steel welds using modified spray arc process. Weld World 62(1):9–18. https://doi.org/10.1007/s40194-017-0535-9
ISO/DIS 3690 - Result and comment. IIW-Doc. II-E-760-18. Presented at: Intermediate Meeting of IIW Commission II-E, Genoa, Italy
N.N. (2017) Comments on ISO/DIS 3690 by Japan Welding Engineering Society. IIW-Doc. II-E-750-17. Presented at: Intermediate Meeting of IIW Commission II-E, Genoa, Italy
The Japan Welding Engineering Society, Technical Committee - Welding Consumables Division (2016) Determination of hydrogen content in arc weld metal per ISO 3690:20 - study on the measurement conditions in hot-extraction method. IIW-Doc. II-E-721r-16
ISO 3690 (2012) Welding and allied processes - determination of hydrogen content in arc weld metal
ANSI/AWS A4.3-93 (2006) Standard methods for determination of the diffusible hydrogen content of martensitic, bainitic, and ferritic steel weld metal produced by arc welding
JSA/JIS Z 3118 (2014) Method for measurement of amount of hydrogen evolved from steel welds
Padhy GK, Komizo YI (2013) Diffusible hydrogen in steel weldments - a status review. Trans JWRI 42(1):39–62
Fydrych D, Labanowski (2011) Determining diffusible hydrogen amounts using the mercury method. Weld J 26(9):697–702. https://doi.org/10.1080/09507116.2011.592682
Jenkins N, Hart PHM, Parker DH (1997) An evaluation of rapid methods for diffusible weld hydrogen. Weld J 76(1):1s–10s
Kannengiesser T, Tiersch N (2010) Comparative study between hot extraction methods and mercury method - a national round robin test. Weld World 54(5/6):R108–R114. https://doi.org/10.1007/BF0326349
Salmi S, Rhode M, Juettner S, Zinke M (2014) Hydrogen determination in 22MnB5 steel grade by use of carrier gas hot extraction technique. Weld World 59:137–144. https://doi.org/10.1007/s40194-014-0186-z
Steppan E, Mantzke P, Steffens BR, Rhode M, Kannengiesser T (2017) Thermal desorption analysis for hydrogen trapping in microalloyed high-strength steels. Weld World 61(4):637–648. https://doi.org/10.1007/s40194-017-0451-z
Rhode M, Mente T, Steppan E, Steger J, Kannengiesser T (2018) Hydrogen trapping in T24 Cr-Mo-V steel weld joints -microstructure effect vs. experimental influence on activation energy for diffusion. Weld World 62(2):277–287. https://doi.org/10.1007/s40194-017-0546-6
Rhode M, Muenster C, et al. (2017) Influence of experimental conditions and calculation method on hydrogen diffusion coefficient evaluation at elevated temperatures. In: Somerday BP, Sofronis P (eds) International Hydrogen Conference (IHC 2016): Materials Performance in Hydrogen Environments. ASME Press, pp 495–503. https://doi.org/10.1115/1.861387_ch56
O’Dwyer A (2009) Handbook of PI and PID controller rules. Imperial College Press, London
Rhode M (2016) Hyrogen diffusion and effect on degradation in welded Microstructures of creep-resistant low-alloyed steels. BAM-Dissertationsreihe 148, Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin, Germany. ISBN: 978-3-9817853-3-3
Grabke HJ, Riecke E (2000) Absorption and diffusion of hydrogen in steels. Mater Tehnol 34(6):331–342
Boellinghaus T, Hoffmeister H, Dangeleit A (1995) A scatterband for hydrogen diffusion coefficients in micro-alloyed and low carbon structural steels. Weld World 35(2):83–96
System specifications of Bruker G4 Phoenix DH. https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/csonh-analysis/g4-phoenix-dh/technical-details.html. Accessed on 2018-06-08
EN ISO 17632 (2016) Welding consumables - tubular cored electrodes for gas shielded and non-gas shielded metal arc welding of non-alloy and fine grain steels - classification
EN ISO 17633 (2010) Welding consumables - tubular cored electrodes and rods for gas shielded and non-gas shielded metal arc welding of stainless and heat-resisting steels - classification
EN ISO 17634 (2015) Welding consumables - tubular cored electrodes for gas shielded metal arc welding of creep-resisting steels - classification
Lausch T (2015) Zum Einfluss der Wärmeführung auf die Rissbildung beim Spannungsarmglühen dickwandiger Bauteile aus 13CrMoV9–10. BAM-Dissertationsreihe 134, BAM, Berlin, Germany. ISBN: 978-3-9817149-5-1, p 222
Solheim KG, Solberg JK, Walmsley J, Rosenqvist F, Bjorna TH (2013) The role of retained austenite in hydrogen embrittlement of supermartensitic stainless steel. Eng Fail Anal 34:140–149. https://doi.org/10.1016/j.engfailanal.2013.07.025
Elhoud AM, Renton NC, Deans WF (2010) Hydrogen embrittlement of super duplex stainless steel in acid solution. Int J Hydrog Energy 35:6455–6464. https://doi.org/10.1016/j.ijhydene.2010.03.056
http://www.scigiene.com/pdfs/428_InfraredThermometerEmissivitytablesrev.pdf. Accessed on 2018-06-08
ChooWY LJY (1982) Thermal analysis of trapped hydrogen in pure iron. Metall Trans A 13(1):135–140. https://doi.org/10.1007/BF02642424
Kuhlmann M, Schwedler O, Holtschke N, Juettner S (2015) Consideration of hydrogen transport in press-hardened 22MnB5. Mater Test 57(11–12):977–984. https://doi.org/10.3139/120.110808
Acknowledgements
The authors want to thank Mr. Michael Richter for the machining of the manifold specimens. Mrs. Stefanie Groth and Mr. Jörg Steger are thanked for assistance in performing the electrochemical charging experiments. Mr. Enrico Steppan is thanked for the fruitful discussions and keeping the lab running.
Author information
Authors and Affiliations
Corresponding author
Additional information
Recommended for publication by Commission II - Arc Welding and Filler Metals
Rights and permissions
About this article
Cite this article
Rhode, M., Schaupp, T., Muenster, C. et al. Hydrogen determination in welded specimens by carrier gas hot extraction—a review on the main parameters and their effects on hydrogen measurement. Weld World 63, 511–526 (2019). https://doi.org/10.1007/s40194-018-0664-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-018-0664-9