Abstract
The microstructure, phase constitution, melting character, and 6061 aluminum alloy brazing properties of Al-Si-Cu-Zn-Re filler metals with different copper contents were investigated. The Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals had the same solidus, at about 502 °C. Increasing the copper content lowered the liquidus temperature of the filler metals. In general, increasing the copper content and brazing time decreased the average joint shear strength of joints bonded with the Al-Si-Cu-Zn-Re filler metals. The joint strength of the 6061 aluminum alloy brazed with Al-9.6Si-10Cu-10Zn-0.1Re filler metal at 530 °C for 10 min without flux was about 72.9 MPa. All of the shear fractures occurred in the filler metals near the interface between the brazing alloy and the base metal. Due to the formation of Al2Cu intermetallic compounds and Si particles, the hardness values of the filler metals were higher than that of the 6061 aluminum substrate. Increasing the copper content in the filler metals also increased the width of the brazed joint regions with higher hardness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to their combination of outstanding properties, such as high specific strength, good workability, and excellent corrosion resistance, aluminum alloys have been widely used in the automotive, aircraft, ship building, and chemical industries. Aluminum alloys also exhibit high electrical and thermal conductivities, so they are also widely used in heat exchangers and cooking utensils. One effective method for manufacturing aluminum heat exchangers, bicycle frames, and automobiles is brazing. However, brazing of aluminum and aluminum alloys has been considered to be problematic because direct wetting of the filler metals is hindered by a stable oxide layer on the surface of aluminum alloys [1, 2]. Traditional Al-12Si eutectic filler metal, which has a melting point of about 577 °C, has been adopted as a reliable braze for the brazing of some aluminum alloys [3]. Unfortunately, the working temperature of Al-12Si filler must be above 590 °C. This brazing temperature is too high relative to the melting point of most commercial high-strength aluminum alloys, which degrade or melt at high temperatures. Previous studies have reported the development of a series of low-melting-point aluminum filler metals for brazing aluminum alloys by adding copper, germanium, zinc, magnesium, or tin into Al-Si alloys [2, 4]. The addition of copper or germanium into the traditional Al-12Si alloy can significantly reduce the melting point of Al-Si filler [5, 6]. However, various factors limit the use of such alloying elements. For example, germanium is expensive, limiting its use in filler metals, and the high vapor pressure of zinc makes high concentrations of zinc unsuitable for filler metals used in the vacuum brazing process [7]. Recently, some studies have indicated that trace amounts of rare earth elements in aluminum brazes promote the wetting of the filler metals on aluminum alloys and improve the joining strengths of aluminum alloys [8–11]. This study employed three low-melting-point filler metals, Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re, all of which contained trace amounts of rare earth elements, to join 6061 aluminum alloy. The effects of the copper content on the microstructures and thermal properties of the filler metals were investigated. The effects of brazing duration on joint reliability were also analyzed by measuring the shear strengths of the brazing joints and characterizing the interfacial reaction products, microstructures, fractography, and microhardness.
2 Experimental
The filler metals used in this study were Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re. They were prepared from eutectic Al-12Si alloy (supplied by Degussa AG, Germany), high purity (99.99 %) copper and zinc, and mixed rare earth elements in a vacuum arc furnace under a high-purity inert argon atmosphere. The chemical composition of the mixture of rare earth elements (Re) used in the study was 77.82 wt% La, 16.84 wt% Pr, 3.24 wt% Ce, and 2.10 wt% Nd. The major chemical compositions of the filler metals in the study are shown in Table 1. To ensure a homogeneous composition within the filler metals, all alloys were mechanically stirred every 3 min during melting and remelted at least three times. The melted filler metals were then poured in a water-cooled copper mold with a diameter of approximately 2 cm. After solidifying, the cast ingots were rolled into 0.2-mm-thick foils. The microstructures and chemical compositions of the filler metals in the study were determined using a field emission scanning electron microscope and an electron probe microanalyzer (EPMA, JEOL TXA-8600SX). The existing phases were identified using a Siemens D5000 X-ray diffractometer with Mo Kα radiation (λ = 0.71073 Å) at a scanning rate of 0.02° per second in the 2θ range of 5–95°. The solidus and liquidus temperatures of the three Al-Si-Cu-Zn-Re filler metals were measured through differential thermal analysis (DTA, TA Instruments SDT-2960) at a heating rate of 10 °C/min from room temperature to 600 °C under an inert argon atmosphere. The material joined in this study was the 6061 aluminum alloy. The chemical composition, solidus, and liquidus of the 6061 aluminum alloy are listed in Table 2.
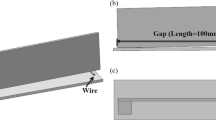
The materials for brazing were cut and machined into rectangular plates with a size of 40 × 6.5 × 3 mm. The geometry and dimensions of the brazing specimens subjected to shear testing are shown in Fig. 1. Before assembly, the bonding surfaces of the 6061 aluminum alloy and the surfaces of filler metals were ground with SiC paper down to grade 1200. Then, the 6061 aluminum alloy and filler metals were ultrasonically cleaned in acetone for 15 min and dried in air. A stainless steel fixture was designed to fix the brazing specimens into a sandwich structure, as schematically shown in Fig. 2. The filler metal foils with a thickness of 200 μm were cut into dimensions of 6.5 mm × 3 mm and then placed between the 6061 aluminum plate specimens. The overlapping length of the brazed joints in the joining experiment was 3 mm.
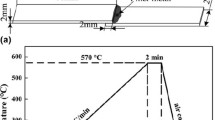
Brazing was performed at an isothermal temperature of 530 °C in a vacuum furnace under a high-purity argon atmosphere. The samples were heated to the brazing temperature at a rate of 20 °C/min. After isothermal holding for 10, 30, and 60 min, the joined specimens were finally cooled to room temperature in the furnace. To estimate the reliability of the joints, the bonding strengths of the joints were measured by shear testing. Shear tests were carried out using a tensile testing machine (Hung Ta Instrument Co., HT-2402) at a constant crosshead speed of 3 mm/min. To ascertain reproducibility, at least two measurements were performed, and the reported values are averages of the tests. Fractured surfaces from shear testing were characterized with a Philips XL40 field emission scanning electron microscope (FE-SEM) equipped with an energy-dispersive spectrometer (EDS). A set of the brazed specimens was cross sectioned and polished for microstructural observation using an electron probe microanalyzer (EPMA, JEOL TXA-8600SX) equipped with a wavelength-dispersive spectrometer (WDS). The microhardness distribution of the filler metals and the interfaces of the brazing joints were conducted using a Shimadzu microsclerometer (Shimadzu HMV-2) with Vickers diamond pyramid indenters under a 50-g load for 10 s. Five measurements were taken per sample, and the average microhardness values were determined.
3 Results and discussion
Figures 3, 4, and 5 show the microstructures of the Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals, respectively. In the figures, it can be observed the Al-rich solid solution phase (gray), Al2Cu intermetallic compounds (white), silicon particles in the dark region, and a few fine needle-like La3Al11 intermetallic compounds. Higher amounts of copper in the Al-Si-Cu-Zn alloy caused larger amounts of Al2Cu intermetallic compounds to form. Moreover, increasing the copper content increased the particle size of the silicon. Large silicon particles of 20 to 50 μm were found in the Al-8.4Si-20Cu-10Zn-0.1Re alloy, as shown in Fig. 5.
Figure 6 shows the XRD analysis of the Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals. The XRD patterns confirmed the phases observed in the microstructures of the filler metals: Al-rich solid solution, Si particles, Zn-rich phase, and Al2Cu intermetallic compounds.
Figure 7 shows the differential thermal analysis (DTA) curves of the Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals. Two endothermic peaks were found in the DTA curves of the Al-9.6Si-10Cu-10Zn-0.1Re and Al-9Si-15Cu-10Zn-0.1Re alloys. The solidus of the Al-8.4Si-20Cu-10Zn-0.1Re alloy was almost the same as those of the Al-9.6Si-10Cu-10Zn-0.1Re and Al-9Si-15Cu-10Zn-0.1Re alloys, at about 502 °C. Only one endothermic peak was found in the DTA curve of the Al-8.4Si-20Cu-10Zn-0.1Re filler metal. The melting temperature range of the Al-8.4Si-20Cu-10Zn-0.1Re filler was from 502 to 518 °C. Previous research [6] indicated that the solidus and liquidus temperatures of the traditional Al-12Si filler metal were 586.1 and 591.7 °C. The addition of 20 wt% copper, 10 wt% zinc, and 0.1 wt% rare earth elements into the Al-12Si to form Al-8.4Si-20Cu-10Zn-0.1Re filler metal lowered the solidus and liquidus temperatures by about 84 and 73.7 °C, respectively. However, melting temperatures have not been affected by adding trace amount of rare earth element (0.1 wt%) in this study. The melting points of the Al-Si-Cu-Zn-Re alloys decreased with increase of copper concentration.
Figure 8 shows the shear strengths of the 6061 aluminum alloy brazed with Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals for various periods. The shear strengths of the joints brazed with the rare earth elements containing Al-Si-Cu-Zn filler metals ranged from 40 to 80 MPa. The joints brazed with Al-9.6Si-10Cu-10Zn-0.1Re filler had a higher average joining strength than the joints brazed with Al-9Si-15Cu-10Zn-0.1Re and Al-8.4Si-20Cu-10Zn-0.1Re filler metals. The joint strengths of the 6061 aluminum alloy brazed with the Al-9.6Si-10Cu-10Zn-0.1Re filler metal at 530 °C decreased slightly as brazing time increased. The average shear strengths of the joints were about 72.9 MPa after 10 min, about 68.9 MPa after 30 min, and about 65.1 MPa after 60 min of brazing with Al-9.6Si-10Cu-10Zn-0.1Re filler metal at 530 °C, respectively. The highest joint strength, 80 MPa, was found in the 6061 aluminum alloy brazed with Al-9.6Si-10Cu-10Zn-0.1Re filler metal at 530 °C for 10 min without flux. Besides the exception at the brazing time of 30 min, which can be explained by experimental bias, in the general, the average joint shear strength decreased as copper content increased.
Figure 9a–c shows micrographs of cross sections of the interface joints of the 6061 aluminum alloy brazed with Al-9.6Si-10Cu-10Zn-0.1Re filler metal at 530 °C for 10, 30, and 60 min, respectively. Al2Cu intermetallic compounds and some Si particles were found in the zone of the filler metal. Increasing the brazing periods did not significantly alter the microstructures. Figure 10a–c shows micrographs of cross sections of interface joints for the 6061 aluminum alloy brazed with Al-9Si-15Cu-10Zn-0.1Re filler metal at 530 °C for 10, 30, and 60 min, respectively. A large number of coarse clusters of Al2Cu were embedded in the filler metal, and the Al2Cu tended to segregate at the grain boundaries of the filler metal after brazing. The microstructures of the interfaces of the 6061 aluminum alloy joints brazed with Al-8.4Si-20Cu-10Zn-0.1Re filler metal at 530 °C for 10, 30, and 60 min are shown in Fig. 11a–c, respectively. Large numbers of Al2Cu were found in the zone of the filler metal. Reliable tight joints were obtained using the Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals in this study.
Figures 12, 13, and 14 show the microhardness values of joints brazed with Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals at 530 °C for various brazing periods. The Al2Cu intermetallic compounds and Si particles formed within the filler metals had hardness values above that of the 6061 aluminum substrate. The microhardness values of the filler metals after brazing under various conditions were about 200–250Hv, much higher than that of 6061 aluminum alloy, about 54Hv. The width of the butt joint regions with higher hardness values, where copper penetrated into the 6061 aluminum, increased with higher copper content in the filler metals. Figures 15, 16, and 17 show fractographs of 6061 aluminum alloy joints brazed with the various filler metals after shear tests. The fractographs showed brittle fractures in the filler metals. In all cases, the fractured surfaces of the brazed 6061 aluminum alloy specimens were covered with filler metals. Failure of the joints occurred in the zone of the filler metals very close to the interface of the filler metal/6061 aluminum alloy. Due to the brittleness of the filler metals, a larger dispersion of results of shear strengths was observed for all brazing conditions. As the copper content in the filler metal increased, the amount of Al2Cu particles on the fracture surface increased and lowered the joint strength.
4 Conclusion
Three filler metals containing rare earth elements with a lower solidus temperature of around 502 °C, Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re, were developed for brazing 6061 aluminum alloy. Increasing the copper content lowered the liquidus temperatures. The main microstructures of all the Al-9.6Si-10Cu-10Zn-0.1Re, Al-9Si-15Cu-10Zn-0.1Re, and Al-8.4Si-20Cu-10Zn-0.1Re filler metals consisted of an Al-rich phase, Al2Cu and La3Al11 intermetallic compounds, and some Si particles. A high copper content led to the formation of a large amount of Al2Cu intermetallic compounds. The joints brazed with Al-9.6Si-10Cu-10Zn-0.1Re filler without flux had high average joining shear strengths of 65 to 73 MPa. Increasing the copper content in the filler metals decreased the average joint shear strength. The hardness values of the Al2Cu intermetallic compounds and Si particles that formed within the filler metals were higher than that of the 6061 aluminum substrate. Increasing the copper content in the filler metals also increased the width of the butt joint regions with higher hardness values. Shear tests revealed that all of the joints formed with the three filler metals failed in the zone of the filler metals.
References
Schultze W, Schoer H (1973) Fluxless brazing of aluminum. Welding Journal 10:644–651
Tsao LC, Weng WP, Cheng MD, Tsao CW, Chuang TH (2002) Brazeability of a 3003 aluminum alloy with Al-Si-Cu-based filler metals. Journal of Materials Engineering and Performance 11(4):360–364
He P, Liu YZ, Liu D (2006) Interfacial microstructure and forming mechanism of brazing Cf/Al composite with Al-Si filler. Materials Science & Engineering A 422:333–338
Dai W, Xue S, Lou J, Wang S (2012) Microstructure and properties of 6061 aluminum alloy brazing joint with Al-Si-Zn filler metal. Materials Transactions 53(9):1638–1643
Chuang TH, Yeh MS, Tsao LC, Tsai TC, Wu CS (2000) Development of a low-melting-point filler metal for brazing aluminum alloys. Metallurgical and Materials Transactions A 31A(9):2239–2245
Chang SY, Tsao LC, Li TY, Chuang TH (2009) Joining 6061 aluminum alloy with Al-Si-Cu filler metals. Journal of Alloys and Compounds 408(1):174–180
L. C. Tsao, M. J. Chiang, W. H. Lin. M. D. Cheng, T. H. Chuang, Effect of zinc additions on the microstructure and melting temperature of Al-Si-Cu filler metals, Materials Characterization, 48 (2002), pp. 341–346.
Dai W, Xue S, Lou J, Wang S (2012) Development of Al-Si-Zn-Sr filler metals for brazing 6061 aluminum alloy. Materials and Design 42:395–402
Wang S, Zhou H, Kang Y (2003) The Influence of rare earth elements on microstructures and properties of 6061 aluminum alloy vacuum-brazed joints. Journal of Alloys and Compounds 352:79–83
Chang SY, Tsao LC, Lei YH, Mao SM, Huang CH (2012) Brazing of 6061 aluminum alloy/ Ti-6Al-4V using Al-Si-Cu-Ge filler metals. Journal of Materials Processing Technology 212:8–14
Zhang G, Bao Y, Jiang Y, Zhu H (2011) Microstructure and mechanical properties of 6063 aluminum alloy brazed joints with Al-Si-Cu-Ni-RE filler metal. Journal of Materials Engineering and Performance 20(8):1451–1456
Acknowledgments
Special thanks go to the National Science Council, Taiwan, for sponsoring this research under Grant No. NSC 102-2221-E-224-013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Recommended for publication by Commission XVII - Brazing, Soldering, and Diffusion Bonding.
Rights and permissions
About this article
Cite this article
Chang, S.Y., Lei, Y.H., Tsao, L.C. et al. Effects of copper content on the microstructure and brazing properties of Al-Si-Cu-Zn-Re filler metals. Weld World 60, 109–116 (2016). https://doi.org/10.1007/s40194-015-0279-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-015-0279-3