Abstract
DNA-based testing has become routine in modern health care. Today, genetic testing for BRCA1 or BRCA2 germline mutations is routinely offered to women with personal and/or family history of breast cancer (BC) and/or ovarian cancer (OC). The identification of a pathogenic mutation in an index case allows relatives to be offered predictive testing and to provide clinical advice-related risk management to women with a high risk of BC and OC. A pathogenic BRCA1 or BRCA2 mutation is identified in less than 20 % of index cases tested, while variants of unknown biological and clinical significance (VUS) are detected in at least another 10 %. Additional BC predisposition genes (PALB2, RAD51C, RAD51D, XRCC2, RINT1, etc.) have been recently identified, and as a consequence of the introduction of new sequencing technologies into clinical testing laboratories, some of these genes are already being screened as part of gene panel testing. However, the use of these new BC-predisposing genes remains limited in clinical practice because their associated cancer risks are not precisely estimated at the present time due to the small number of families that are known to carry variants in these genes. Many more BC and OC genes with an expected wide range of associated risks remain unidentified. In this review, we will focus on recent advances in the field of BC genetics and will discuss future challenges for clinical utility of new tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most commonly occurring invasive cancer among women, and approximately one million new cases are diagnosed worldwide each year. One of the strongest risk factors for the development of BC is having a close relative affected with the disease. On the basis of the increased relative risk of BC in first-degree relatives of a woman with BC and segregation studies on cases of BC in the families of affected women, it has been estimated that 5 % of these women carry a genetic risk factor transmitted according to a Mendelian dominant model. Twenty years ago, the BRCA1 and BRCA2 genes were identified by genetic linkage studies. Following the cloning of these two major BC genes, diagnostic testing for mutations in BRCA1 and BRCA2 has been routine clinical practice in many developed countries. It facilitates risk estimation and implementation of cancer prevention strategies and has the potential to influence cancer therapy [1, 2].
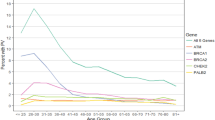
Since the discovery of the major predisposing genes BRCA1 and BRCA2 and the identification of the syndrome-related high-risk susceptibility genes PTEN, CDH1 and STK11, substantial progress has been made in identifying genetic causes of BC. Despite these advances, about 80 % of the familial relative risk remains unexplained. Current data suggest that a large proportion of the remaining familial aggregations of BC will be explained by several genes with a wide range of associated risks; moreover, sporadic cases may also be explained by a combination of susceptibility alleles [3]. Very simplistically, BC susceptibility genes have been classified into three groups, each associated with a different level of average BC risk and mutation prevalence in the population [4]: high-risk genes such as BRCA1 and BRCA2 in which rare monoallelic mutations convey high risk of BC and OC and explain about 25 % of the familial risk; common very modest-risk SNPs detected by genome-wide association studies (GWAS) explain about another 10 % of genetic susceptibility [5••, 6, 7], and rare, intermediate-risk variants have been identified more recently by a candidate gene approach and the resequencing of large numbers of familial BRCA1/BRCA2 mutation-negative cases and control groups (ATM, BRIP1, CHEK2, PALB2, RAD51C, RAD51D) or by an agnostic exomic sequencing approach [8, 9•, 10•] (Fig. 1).
This review focuses on resources and different study designs that have been developed over the last two decades to identify new BC susceptibility genes and discusses the challenges that remain to orientate appropriate clinical management for carriers of a likely deleterious variant in one of these genes.
Hereditary Breast and Ovarian Cancer and High-Penetrance Variants
Rare, large families with multiple-cases of BC affecting several generations provided clear evidence that inherited factors are important causes of BC. The two major BC-predisposing genes BRCA1 and BRCA2 were identified by genetic linkage studies in the early 90s using microsatellite markers. Microsatellites are tandem repeats of simple sequences that occur abundantly throughout the genome. These genetic polymorphisms are stable enough that they can be used in genetic analyses. Linkage analysis statistically compares the genotypes between affected and unaffected individuals within a pedigree and looks for evidence that some alleles of the typed polymorphic markers are inherited along with the disease trait. Different categories can be assigned to putative gene carriers and non-carriers in the family, depending on their age and whether or not they have developed cancer (liability classes). When a marker and the gene harbouring a mutation responsible for the disease are close together on a chromosome, there is a higher likelihood that they will be inherited together as familial recombination events during meiosis are less likely to have interfered. The likelihood that a specific allele of a microsatellite and the studied trait will be inherited together is measured as a logarithm-of-odds (LOD) score. A high LOD score (3.0 or higher) indicates a strong chance that the gene is located near the given marker (≥1000:1 in favour), whereas a low score (lower than −2.0) means that the gene is almost certainly not near that marker locus.
Following the localization of BRCA1 and BRCA2 on chromosomes 17q21 and 13q12, respectively, the two genes were subsequently cloned [11–14]. BRCA1 counts 24 exons (22 of which are coding) and BRCA2 counts 27 exons (26 of which are coding). The two genes share little structural homology but are involved in the same cell-signalling pathway. Their protein products (1863 and 3418 amino-acid long, respectively) are expressed in a wide range of tissues and are especially critical in controlling DNA damage repair, helping to prevent the accumulation of mutations in cancer-related genes [15]. Hence, BRCA1 and BRCA2 work as tumour suppressor genes, that is, a mutation in these genes causes loss or reduction in their function. Somatic loss of heterozygosity (LOH), the loss of a normal functioning allele at a heterozygous locus, is a genetic event frequently observed in the multistep process of BC tumour development and progression [16]. Carriers of a germline mutation in BRCA1 or BRCA2 are at risk of acquired loss of the wild-type allele, one of the common mechanisms of inactivation in hereditary BC [17].
Several hundreds of distinct germline mutations have been described for both BRCA1 and BRCA2, with few mutational hotspots [18]. Mutations are spread throughout the genes, and most of them are loss-of-function (LOF) mutation causing premature truncation of the protein, due to nonsense substitutions, small insertions or deletions, splice site alterations or large gene rearrangements. Pathogenic missense substitutions fall in evolutionarily conserved regions of the proteins, in some functional domains (such as RING or BRCT domains in BRCA1). Founder mutations exist in some populations (for example, in families of Jewish ancestry, in French-Canadian families from Québec or in Icelandic and Polish populations), and their presence can aid genetic testing [19].
Identification of a germline pathogenic mutation in patients with hereditary breast and ovarian cancer (HBOC) syndrome allows mutation carriers in the family to be included in cancer prevention programs, which are proven to be life saving. In addition, the absence of a BRCA1 or BRCA2 pathogenic mutation allows relatives reassurance and avoiding preventive oophorectomy. However, a clearly pathogenic sequence variant is found in only 10–20 % of subjects who undergo full-sequence BRCA1 and BRCA2 testing, and one complication resulting from genetic testing is that about the same proportion of patients screened for the BRCA1 and BRCA2 genes are found to carry of a variant of uncertain significance (VUS), i.e., a DNA sequence variant that is not easily classified as either pathogenic or neutral. Usually, VUS are either a missense substitution or a variant altering the splice junction consensus regions but outside of the canonical GT-AG dinucleotides [20]. Interpretation of VUS has become a major challenge for molecular diagnosis laboratories and genetic counsellors as these variants are problematic for the management of patients and their families who receive the ambiguous information. Over the last several years, several databases and open access tools have been created for biologists, clinical cancer geneticists and researchers to facilitate interpretation of novel genetic variations identified in at risk BC patients, such as the UMD-BRCA1/BRCA2 databases available at http://www.umd.be/BRCA1/W_BRCA1/ and http://www.umd.be/BRCA2/brca2, respectively [18], or the BRCA gene Ex-UV LOVD available at http://brca.iarc.fr/LOVD [21]. Several years ago, the Breast cancer Information Core (BIC) aimed to develop a system that could combine data from several independent types of analysis to arrive at a classification for BRCA1 and BRCA2 variants [22]. In February 2008, a Working Group on BRCA1 and BRCA2 VUS convened at the International Agency for Research on Cancer agreed on clinically applicable guidelines for VUS classification and began the diffusion of a tool for evaluation of VUS beyond the BC genetics community. The proposed Bayesian approach involves a multifactorial likelihood-ratio model based on co-segregation, personal and family history, tumour histopathology and co-occurrence of variants in trans. Integrated evaluation of a VUS includes a prior probability based on in silico analyses of the substitutions, both from the perspective of potential effects on mRNA splicing and potential effects on protein function. The classification system involves five classes of variants, each of which is associated with a given probability of being pathogenic. Testing recommendations associated with each class of variants have been proposed. The five category qualitative classifier is explicitly meant to help clinical cancer geneticists with patient counselling [23••], and over the last few years, a series of studies analysed many BRCA1 and BRCA2 variants, and now more 150 variants are classified with reasonable confidence [21, 24–27]. The majority of the VUS, however, are still assigned to class 3 (0.05–0.95 probability of being pathogenic) [23••]. As BRCA1 and BRCA2 VUS are individually rare, an international and interdisciplinary consortium named ENIGMA (for evidence-based network for interpretation of germline mutant allele) was been set up in 2009 to gather enough genetic, clinical and histopathological information from a worldwide network of laboratories and hospitals in order to define clinical relevance of the problematic variants (Table 1) [28].
Even classification of clearly pathogenic mutations themselves (“Class 5 variants” with >0.99 probability of being pathogenic) has several challenges. Family-based studies showed that mutations in BRCA1 and BRCA2 are inherited in an autosomal dominant fashion with incomplete penetrance: this means that the majority of subjects carrying a BRCA1 or BRCA2 mutation will develop cancer during their lifetime, but not all. Moreover, there is evidence that variation in penetrance estimate exists among mutation carriers [29]. Genetic epidemiology studies showed that breast and ovarian cancer risks in BRCA1 and BRCA2 mutation carrying families vary according to the location and the origin of the mutation [29–35]. Additionally, it has been shown that genetic and non-genetic factors influence cancer risk for BRCA1 and BRCA2 mutation carriers—a fact that may help explain variation in risks between families [29]. It is important that these inter-individual cancer risk differences in both age of cancer onset and site of the cancer could be taken into account when choosing risk reduction strategies. Two consortia are conducting studies that aim to achieve a more reliable estimation of individual cancer risks: the International BRCA1/2 Carrier Cohort Study (IBCCS) is exploring the extent to which non-genetic BC risk factors such as radiation exposure through chest X-rays or reproductive factors can modify cancer risk in BRCA1 and BRCA2 mutation carriers [36–39], while the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) is investigating the role of low-penetrance common breast and ovarian cancer genetic risk factors, discussed hereafter, in BRCA1/2 carriers [40–45] (Table 1).
Polygenic Inheritance and Low-Penetrance Variants
Overall, mutations in high-risk BC genes explain about 25 % of the familial risk, and the frequency of BRCA1 and BRCA2 mutations in BC families is highly correlated with the degree of family history. They are present at very low frequencies in the general population (minor allele frequency (MAF) <0.1 %), and explain less than 5 % of total BC predisposition [46]. Taken together, these observations have led to the hypothesis that the so-called “sporadic cases” (i.e., cases with no known affected close relatives) could be attributable in part to allelic variants present in more than 1–5 % of the population and that will confer lower risks [47, 48]. To identify those, association studies are being conducted on thousands of cases who are unselected for family history and thousands of unrelated controls. The first studies of common genetic variation and BC risk looked at candidate genes involved in specific pathways that are important in BC biology, including DNA repair, cell-cycle regulation, carcinogenesis, apoptosis, carcinogen metabolism and steroid hormone metabolism. Most of these studies were based on putative functional variants or on tag SNPs, i.e., SNPs correlated with, and therefore could serve as a proxy for, much of known remaining common variation in a region. Although numerous associations have been proposed from candidate gene analyses, few definitive susceptibility alleles were unequivocally identified through such approaches. A notable success though was the demonstration that the common missense substitution p.Asp302His in the CASP8 gene, which encodes a regulator of apoptosis, was associated with a moderate reduction in BC risk, and the association was convincingly replicated through the large multicentre Breast Cancer Association Consortium (BCAC) (p value: 1.1 × 10−7; odds ratio [OR] 0.88; 95 % CI 0.84–0.92) [49, 50]. Soon after this first success, large-scale case–control studies were greatly enhanced by the development of commercial ‘SNP chips’ or arrays that capture most, although not all, common variation in the genome. These high-throughput genotyping technologies have allowed conducting empirical GWAS with very low error rates and at very low per SNP genotyping costs. However, because associations between SNPs and BC show low ORs and given the very large number of tests that are required to obtain reliable signal, huge sample sets are needed. Different strategies have therefore been proposed to reduce genotyping costs such as procedures for improving power by inferring genotypes, by combining data across studies or by applying multiple stage designs where “best hits” from one study would be followed up in independent case–controls series [51]. As for many other complex diseases, these strategies have proven successful approaches for the identification of common low-risk loci without prior knowledge of location or function, and they have provided an opportunity to improve our understanding of the aetiology of BC. The two first GWASs of BC were conducted concurrently by the BCAC [5••] and by the Cancer Genetic Markers of Susceptibility study [6], mainly in populations of European origin. Subsequently, additional GWAS was undertaken in other populations and a recent meta-analysis of nine GWAS that included 10,052 BC cases and 12,575 controls of European, followed by a replication study on a subset of SNPs genotyped in 45,290 cases and 41,880 controls identified further genome hits leading to the identification of over 70 loci involved BC susceptibility [52]. Combined analyses now suggest that more than 1000 further loci are yet to be discovered and that part of the remaining risk result from a combination of multiple common variants, each conferring a small effect on BC risk, with ORs usually between 1.2 and 1.5 [5••, 53]. Assuming a polygenic model, many low-penetrance SNPs may have a cumulative effect on both the overall risk of disease [54] and on disease onset [55].
These case–control studies have highlighted an enrichment of genes involved in tumorigenesis in model systems, cell death and differentiation. Intriguingly, the majority of BC risk-associated SNPs fall in non-coding or intergenic regions, and the underlying biological/functional impact of these variants is yet to be elucidated. GWASs have also revealed that pattern of association is different by population (e.g., Europeans vs. Asians) [56]. In this regard, follow-up studies in different populations with different linkage disequilibrium patterns will be helpful for fine-mapping studies. Another finding is that some SNPs are associated with risk of a specific BC subtype. For instance, the association at the 6q25 locus near the oestrogen receptor gene ESR1 is found in ER-negative BC [56], and not unexpectedly, the same SNPs showed an association with BC risk in BRCA1 carriers [41]. Another lesson from GWASs has been highlighted by the large-scale collaborative genotyping experiment involving >200,000 individuals from four consortia (COGS) and the design of a custom genotyping array with about 211,000 SNPs (iCOGS chip) is the detection of pleiotropic loci associated with risk of three hormone-related cancers, namely breast, ovarian and prostate cancers. In addition, some environmental factors specifically alcohol consumption and parity seem to modify the association between some SNPs and cancer risk [57]. To pursue the dissection of the genetic architecture of BC and other cancers, a second dedicated genotyping array, the OncoArray (>600,000 SNPs), has been recently designed, and genetic analysis is on the way to further characterize the genetic and environmental bases of breast, ovarian, prostate, colorectal and lung cancers [58].
Rare Moderate-Risk Variants
Recent genetic epidemiology studies of BC have tended to focus on the third class of BC susceptibility genes. Classical linkage analyses were no longer successful as pathogenic variants in the as yet unidentified genes are either not penetrant enough or frequent enough to produce LOD score signals [59, 60], nor could these genes be identified by GWASs due to the very low frequency of these variants in the general population. Typically, the pathogenic alleles of the so-called “moderate-risk” or “intermediate-risk” genes exemplified by ATM, CHEK2, PALB2, genes of the MRN complex (MRE11, RAD50 and NBN) and RAD51 paralogs (RAD51C, RAD51D and XRCC2) [8, 9•, 46, 62–64, 64••, 65–70] confer ORs of 2.0–5.0, and for some of these genes, the summed frequency of the pathogenic alleles is close to 1 %. Approximately, 5 % of the familial risk is likely to be explained by mutations in such genes. Evidence for this class of BC-predisposing variants actually dates back to the late 80s when Swift demonstrated that carrier status for recessively inherited ataxia-telangiectasia (A-T) was associated with a threefold-elevated risk of female BC [71–75]. Recent case–control, case-only and control-only mutation screening studies of the A-T predisposing gene ATM confirmed that protein-truncating (LOF) variants confer increased risk of BC [61, 62]. Moreover, in a pooled analysis including all published ATM case–control mutation screening studies as well as new data, Tavtigian et al. conducted a separate analysis of LOF variants and of rare missense substitutions. In addition to confirming the contribution of LOF variants, this study highlighted the importance of the most severe grades of rare ATM missense substitutions (as predicted by the align-GVGD algorithm [76]) in BC susceptibility and showed that almost all of the evidence for some of the rare missense substitutions being pathogenic could be attributed to those falling in the key FAT, protein kinase and FATC domains [61]. Likewise, the spectrum of pathogenic variants in RAD50, MRE11A and NBN includes a relatively high proportion of rare (with MAF <0.1 %) missense substitutions located in the conserved functional domains of the gene products forming the MRN complex [46]. The case of CHEK2 is particular since two founder mutations are predominant in some populations: the carrier frequency of the truncating mutation c.1100delC is 0.5–1.0 % in several northern European countries and the carrier frequency of the missense mutation p.Ile157Thr is above 1.0 % among individuals of Slavic ancestry [77, 78]. However, in more mixed populations, half or more of the susceptibility alleles consist of rarer missense substitutions [63, 79], and overall, it is estimated that CHEK2 pathogenic variants account for 2.5–3.0 % of early onset or familial BC cases [80]. Finally, the characterization of PALB2 as a BC susceptibility gene through familial and population-based studies showed that LOF mutations in PALB2 confer a higher risk of BC than do LOF mutations in ATM and CHEK2 [8, 65, 81] and may overlap with that for BRCA2 mutation carriers. The mean absolute BC risk for PALB2 female carriers by 70 years of age was 35 % (95 % CI 26–46), which is comparable to the mean absolute BC risk for BRCA2 carriers estimated by Antoniou et al.: 45 % (95 % CI 31–56) [82]. This would justify routinely offering genetic testing for PALB2 along with BRCA1 and BRCA2. Hence, PALB2 is the first example among the “new” BC susceptibility genes for which identified mutation carriers could be informed of optimal clinical screening and treatment [64••, 66].
Until very recently, rare intermediate-risk variants were identified by screening carefully selected candidate genes involved in the homologous recombination repair detection and signalling pathways involving BRCA1 and BRCA2 in large numbers of familial BC cases or early onset BC series, as well as population control groups using traditional mutation detection techniques such as Sanger sequencing, dHPLC or high-resolution melt curve analysis. Their discovery is now accelerating thanks to the introduction of massive parallel sequencing (MPS) in both research and diagnosis laboratories and studies aiming to explore entire DNA repair pathways or the exome in large study samples.
Until exome sequencing of thousands of cases and matched controls becomes available, a successful approach has been to combine a two-stage study design where whole-exome sequencing of women affected with BC from highly selected multiple-case BC families is followed by case–control mutation screening of candidate gene(s) plus additional genotyping in BC pedigrees. So far, gene prioritization for follow-up studies has been based on knowledge of biological function of the genes and for more than one highly selected family to carry a variation in the gene. Hence, among the dozen of possible candidates, the RAD51 paralog XRCC2 was picked as a plausible candidate because (i) exome sequencing of multiple affected relatives from 13 high-risk BC families identified a LOF mutation partially segregating with BC in one family and a very rare likely deleterious missense variant in a second family, and (ii) Xrcc2-knockout mice present with genetic instability due to homologous recombination deficiency [83]. The confirmation of the involvement of XRCC2 in BC susceptibility came from the large-scale validation step where the entire coding sequence of the gene was screened in 1308 cases and 1320 frequency-matched controls recruited through population-based sampling by the BC Family Registry [9•, 84]. However, the association was not replicated in an independent study involving 3548 non-BRCA1/2 familial BC cases and 1435 healthy controls, although a relative risk smaller than two could not be excluded [85]. Following the same study design, the RINT1 gene was further investigated because exome sequencing identified rare mutations in 3 out of 49 multiple-case BC families, and because this tumour suppressor gene encoding the RAD50-interacting protein 1 is essential for maintaining several aspects of the Golgi apparatus dynamics as well as the integrity of the centrosome, which coordinates mitosis [10•]. Interestingly, this study showed that RINT1 carriers in BC high-risk families were also at increased risk of developing Lynch syndrome–spectrum cancers.
Beyond the laboratory challenge imposed by large-scale of resequencing projects, such studies have also illustrated the complexity of variant annotation using various bioinformatics tools, which, although imperfect, is a necessary step to classified the variants and to pool those with a a priori similar severity grade in order to increase the power of the statistical tests. Thus, a second challenge for MPS projects is to be able to conduct a statistically powerful enough analysis of the multitude of rare sequence variants identified. Moreover, combining exome sequencing data from different studies will require effort to harmonize analytical and bioinformatics pipelines for the analysis of NGS data. To this end, the COMPLEXO consortium was formed to facilitate international collaborations, an essential step to advance the identification of additional moderate-risk BC susceptibility genes by increasing the likelihood of identifying causal variants in the same gene in multiple families [86].
Despite the unclear clinical utility of the majority of the already identified moderate-risk genes, gene panel testing has already become a reality. However, for many genes the evidence for association is weak, risk estimates for carriers are imprecise and biased (if conducted at all), and very often variants in the “new” genes do not achieve clinically actionable classification, the vast majority of them being considered as VUS. Therefore, neither individuals carrying a deleterious variant nor a VUS can benefit from knowing their mutation status because risk, penetrance and appropriate clinical management strategies have not yet been established. Risks associated with tumour subtypes or other tumour localizations are even more imprecise. Hence, as for BRCA1 and BRCA2 evidences based on co-segregation, frequency and functional studies need to be gathered for variants in each gene, and improvement of in silico evaluation is required to interpret test results. Curated data repositories for clinically relevant variants reviewed by expert panels are also needed.
Eventually, identifying causal genetic variants in novel genes associated with susceptibility to BC will make it possible to improve the medical management of women at risk and to adapt the surveillance of these women according to risk assessments based on new tests. If the risk is considered high, early and regular magnetic resonance imaging (MRI)-based screening could be offered, and prophylactic surgery could be discussed.
Conclusion
Identifying specific genes and gene variants that predispose to the development of cancer is important for a greater understanding of the biological pathways that are involved in tumourigenesis, elucidating how environmental factors may exert their effects in combination with genes, and also identifying individuals whose risk is high enough to benefit from existing risk reduction strategies. Indeed, the identification of inherited mutations in a number of cancer-predisposing genes has been a major step for genetic counselling. However, currently approximately 80–90 % of families who are tested in a clinical setting for BRCA1 and BRCA2 mutations receive a negative report. The identification of the majority of BC susceptibility genes will be a great advance for these women who could be offered additional testing. At the moment, they are counselled solely on the basis of their family history; identification of additional susceptibility genes, which would allow more precise estimation of their risk, would be of great value. With the implementation of MPS technologies in genetic testing laboratories, the selection criteria for tests for these mutations are becoming increasingly wide-ranging as clinicians come to recognize the importance of such information for improving patient management.
Hence, investigating genetic factors in phenotypically well-described high-risk populations and the possibility to conduct large-scale validation in national and international research resources will be extremely useful to further decipher the genetic architecture of BC. It will provide evidence based on which a larger number of susceptibility genes for BC will be routinely screened in the future. In turn, this may open new therapeutic avenues, as it has been the case with the use of PARP inhibitors in carriers of BRCA1 and BRCA2 mutations, and significantly progress personalised medicine in areas such as BC prevention, risk estimation, early detection, screening recommendations, treatment selection (genotype specific/targeted therapies) and improved prognosis, leading to the improved clinical management of women at genetic risk of breast cancer.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Domchek SM, Weber BL. Clinical management of BRCA1 and BRCA2 mutation carriers. Oncogene. 2006;25(43):5825–31.
Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21.
Lu Y, Ek WE, Whiteman D, et al. Most common ‘sporadic’ cancers have a significant germline genetic component. Hum Mol Genet. 2014;23:6112–8.
Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nat Genet. 2008;40(1):17–22.
•• Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087–93. Conducted the first genome scan on breast cancer using a three-stage design and combining 22 case-control studies (>44,000 subjects).
Hunter DJ, Kraft P, Jacobs KB, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39(7):870–4.
Stacey SN, Manolescu A, Sulem P, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39(7):865–9.
Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39(2):165–7.
• Park DJ, Lesueur F, Nguyen-Dumont T, et al. Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet. 2012;90(4):734–9. Demonstrate the power of massively parallel sequencing in the discovery of additional breast cancer susceptibility genes when used with an appropriate study design involving both a multiple-case family study and population-based case-control mutation screening.
• Park DJ, Tao K, Le Calvez-Kelm F, et al. Rare mutations in RINT1 predispose carriers to breast and Lynch syndrome-spectrum cancers. Cancer Discov. 2014;4(7):804–15. Identified a link between rare mutations in RINT1 and hereditary breast cancer and support the possibility that these mutations also predispose to a spectrum of cancers with mismatch repair defects.
Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71.
Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–92.
Tavtigian SV, Simard J, Rommens J, et al. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996;12(3):333–7.
Smith TM, Lee MK, Szabo CI, et al. Complete genomic sequence and analysis of 117 kb of human DNA containing the gene BRCA1. Genome Res. 1996;6(11):1029–49.
Venkitaraman AR. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science. 2014;343(6178):1470–5.
Miller BJ, Wang D, Krahe R, Wright FA. Pooled analysis of loss of heterozygosity in breast cancer: a genome scan provides comparative evidence for multiple tumor suppressors and identifies novel candidate regions. Am J Hum Genet. 2003;73(4):748–67.
Osorio A, de la Hoya M, Rodriguez-Lopez R, et al. Loss of heterozygosity analysis at the BRCA loci in tumor samples from patients with familial breast cancer. Int J Cancer. 2002;99(2):305–9.
Caputo S, Benboudjema L, Sinilnikova O, Rouleau E, Beroud C, Lidereau R. Description and analysis of genetic variants in French hereditary breast and ovarian cancer families recorded in the UMD-BRCA1/BRCA2 databases. Nucleic Acids Res. 2012;40(Database issue):D992–1002.
Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev. 2004;4(9):665–76.
Gomez-Garcia EB, Ambergen T, Blok MJ, van den Wijngaard A. Patients with an unclassified genetic variant in the BRCA1 or BRCA2 genes show different clinical features from those with a mutation. J Clin Oncol. 2005;23(10):2185–90.
Vallee MP, Francy TC, Judkins MK, et al. Classification of missense substitutions in the BRCA genes: a database dedicated to Ex-UVs. Hum Mutat. 2012;33(1):22–8.
Goldgar DE, Easton DF, Deffenbaugh AM, et al. Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am J Hum Genet. 2004;75(4):535–44.
•• Plon SE, Eccles DM, Easton D, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29(11):1282–91. Developed rationale and guidelines for the integrated evaluation of BRCA1 and BRCA2 VUS to improve the interpretation of genetic test results.
Easton DF, Deffenbaugh AM, Pruss D, et al. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet. 2007;81(5):873–83.
Spurdle AB, Lakhani SR, Da Silva LM, Balleine RL, kConFab I, DE Goldgar. Bayes analysis provides evidence of pathogenicity for the BRCA1 c.135-1G > T (IVS3-1) and BRCA2 c.7977-1G > C (IVS17-1) variants displaying in vitro splicing results of equivocal clinical significance. Hum Mutat. 2010;31(2):E1141–5.
Chenevix-Trench G, Healey S, Lakhani S, et al. Genetic and histopathologic evaluation of BRCA1 and BRCA2 DNA sequence variants of unknown clinical significance. Cancer Res. 2006;66(4):2019–27.
Tavtigian SV, Samollow PB, de Silva D, Thomas A. An analysis of unclassified missense substitutions in human BRCA1. Fam Cancer. 2006;5(1):77–88.
Spurdle AB, Healey S, Devereau A, et al. ENIGMA—evidence-based network for the interpretation of germline mutant alleles: an international initiative to evaluate risk and clinical significance associated with sequence variation in BRCA1 and BRCA2 genes. Hum Mutat. 2012;33(1):2–7.
Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30.
Vos JR, Teixeira N, van der Kolk DM, et al. Variation in mutation spectrum partly explains regional differences in the breast cancer risk of female BRCA mutation carriers in the Netherlands. Cancer Epidemiol Biomarkers Prev. 2014;23:2482–91.
Tryggvadottir L, Sigvaldason H, Olafsdottir GH, et al. Population-based study of changing breast cancer risk in Icelandic BRCA2 mutation carriers, 1920–2000. J Natl Cancer Inst. 2006;98(2):116–22.
Thompson D, Easton D, Breast Cancer Linkage C. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68(2):410–9.
Thompson D, Easton D, Breast Cancer Linkage C. Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiol Biomarkers Prev. 2002;11(4):329–36.
Lecarpentier J, Nogues C, Mouret-Fourme E, et al. Variation in breast cancer risk associated with factors related to pregnancies according to truncating mutation location, in the French National BRCA1 and BRCA2 mutations carrier cohort (GENEPSO). Breast Cancer Res. 2012;14(4):R99.
Lecarpentier J, Nogues C, Mouret-Fourme E, et al. Variation in breast cancer risk with mutation position, smoking, alcohol, and chest X-ray history, in the French National BRCA1/2 carrier cohort (GENEPSO). Breast Cancer Res Treat. 2011;130(3):927–38.
Andrieu N, Easton DF, Chang-Claude J, et al. Effect of chest X-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group. J Clin Oncol. 2006;24(21):3361–6.
Andrieu N, Goldgar DE, Easton DF, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS). J Natl Cancer Inst. 2006;98(8):535–44.
Brohet RM, Goldgar DE, Easton DF, et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;25(25):3831–6.
Chang-Claude J, Andrieu N, Rookus M, et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16(4):740–6.
Antoniou AC, Beesley J, McGuffog L, et al. Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: implications for risk prediction. Cancer Res. 2010;70(23):9742–54.
Antoniou AC, Kartsonaki C, Sinilnikova OM, et al. Common alleles at 6q25.1 and 1p11.2 are associated with breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet. 2011;20(16):3304–21.
Mulligan AM, Couch FJ, Barrowdale D, et al. Common breast cancer susceptibility alleles are associated with tumour subtypes in BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res. 2011;13(6):R110.
Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–47.
Antoniou AC, Kuchenbaecker KB, Soucy P, et al. Common variants at 12p11, 12q24, 9p21, 9q31.2 and in ZNF365 are associated with breast cancer risk for BRCA1 and/or BRCA2 mutation carriers. Breast Cancer Res. 2012;14(1):R33.
Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet. 2013;9(3):e1003212.
Damiola F, Pertesi M, Oliver J, et al. Rare key functional domain missense substitutions in MRE11A, RAD50, and NBN contribute to breast cancer susceptibility: results from a Breast Cancer Family Registry case-control mutation-screening study. Breast Cancer Res. 2014;16(3):R58.
Fletcher O, Johnson N, Dos Santos Silva I, et al. Family history, genetic testing, and clinical risk prediction: pooled analysis of CHEK2 1100delC in 1,828 bilateral breast cancers and 7,030 controls. Cancer Epidemiol Biomarkers Prev. 2009;18(1):230–4.
So HC, Gui AH, Cherny SS, Sham PC. Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet Epidemiol. 2011;35(5):310–7.
Cox A, Dunning AM, Garcia-Closas M, et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet. 2007;39(3):352–8.
Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the Breast Cancer Association Consortium. J Natl Cancer Inst. 2006;98(19):1382–96.
Ghoussaini M, Pharoah PD. Polygenic susceptibility to breast cancer: current state-of-the-art. Future oncology. 2009;5(5):689–701.
Michailidou K, Hall P, Gonzalez-Neira A, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–61 61e1-2.
Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006;25(43):5898–905.
Pharoah PD, Antoniou A, Bobrow M, Zimmern RL, Easton DF, Ponder BA. Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet. 2002;31(1):33–6.
Harlid S, Ivarsson MI, Butt S, et al. Combined effect of low-penetrant SNPs on breast cancer risk. Br J Cancer. 2012;106(2):389–96.
Hein R, Maranian M, Hopper JL, et al. Comparison of 6q25 breast cancer hits from Asian and European Genome Wide Association Studies in the Breast Cancer Association Consortium (BCAC). PLoS One. 2012;7(8):e42380.
Nickels S, Truong T, Hein R, et al. Evidence of gene-environment interactions between common breast cancer susceptibility loci and established environmental risk factors. PLoS Genet. 2013;9(3):e1003284.
Sakoda LC, Jorgenson E, Witte JS. Turning of COGS moves forward findings for hormonally mediated cancers. Nat Genet. 2013;45(4):345–8.
Smith P, McGuffog L, Easton DF, et al. A genome wide linkage search for breast cancer susceptibility genes. Genes Chromosomes Cancer. 2006;45(7):646–55.
Huusko P, Juo SH, Gillanders E, et al. Genome-wide scanning for linkage in Finnish breast cancer families. Eur J Hum Genet. 2004;12(2):98–104.
Tavtigian SV, Oefner PJ, Babikyan D, et al. Rare, evolutionarily unlikely missense substitutions in ATM confer increased risk of breast cancer. Am J Hum Genet. 2009;85(4):427–46.
Renwick A, Thompson D, Seal S, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38(8):873–5.
Le Calvez-Kelm F, Lesueur F, Damiola F, et al. Rare, evolutionarily unlikely missense substitutions in CHEK2 contribute to breast cancer susceptibility: results from a breast cancer family registry (CFR) case-control mutation screening study. Breast Cancer Res. 2011;13(1):R6.
•• Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. New Engl J Med. 2014;371(6):497–506. First study estimating lifetime risk of breast cancer in families with germline loss-of-function mutations in PALB2 and suggesting the breast cancer risk for PALB2 mutation carriers may overlap with that of BRCA2 mutation carriers.
Southey MC, Teo ZL, Dowty JG, et al. A PALB2 mutation associated with high risk of breast cancer. Breast Cancer Res. 2010;12(6):R109.
Southey MC, Teo ZL, Winship I. PALB2 and breast cancer: ready for clinical translation! Appl Clin Genet. 2013;6:43–52.
Blanco A, Gutierrez-Enriquez S, Santamarina M, et al. RAD51C germline mutations found in Spanish site-specific breast cancer and breast-ovarian cancer families. Breast Cancer Res Treat. 2014;147(1):133–43.
Kushnir A, Laitman Y, Shimon SP, Berger R, Friedman E. Germline mutations in RAD51C in Jewish high cancer risk families. Breast Cancer Res Treat. 2012;136(3):869–74.
Gutierrez-Enriquez S, Bonache S, de Garibay GR, et al. About 1 % of the breast and ovarian Spanish families testing negative for BRCA1 and BRCA2 are carriers of RAD51D pathogenic variants. Int J Cancer. 2014;134(9):2088–97.
Thompson ER, Rowley SM, Sawyer S, et al. Analysis of RAD51D in ovarian cancer patients and families with a history of ovarian or breast cancer. PLoS One. 2013;8(1):e54772.
Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. New Engl J Med. 1987;316(21):1289–94.
Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia-telangiectasia. New Engl J Med. 1991;325(26):1831–6.
Thompson D, Duedal S, Kirner J, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;97(11):813–22.
Olsen JH, Hahnemann JM, Borresen-Dale AL, et al. Cancer in patients with ataxia-telangiectasia and in their relatives in the nordic countries. J Natl Cancer Inst. 2001;93(2):121–7.
d’Almeida AK, Cavaciuti E, Dondon MG, et al. Increased risk of breast cancer among female relatives of patients with ataxia-telangiectasia: a causal relationship? Br J Cancer. 2005;93(6):730–2 author reply 2.
Tavtigian SV, Byrnes GB, Goldgar DE, Thomas A. Classification of rare missense substitutions, using risk surfaces, with genetic- and molecular-epidemiology applications. Hum Mutat. 2008;29(11):1342–54.
Consortium CBCC-C. CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74(6):1175–82.
Kilpivaara O, Vahteristo P, Falck J, et al. CHEK2 variant I157T may be associated with increased breast cancer risk. Int J Cancer. 2004;111(4):543–7.
Desrichard A, Bidet Y, Uhrhammer N, Bignon YJ. CHEK2 contribution to hereditary breast cancer in non-BRCA families. Breast Cancer Res. 2011;13(6):R119.
Tavtigian SV, Chenevix-Trench G. Growing recognition of the role for rare missense substitutions in breast cancer susceptibility. Biomark Med. 2014;8(4):589–603.
Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res. 2011;71(6):2222–9.
Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30.
Deans B, Griffin CS, O’Regan P, Jasin M, Thacker J. Homologous recombination deficiency leads to profound genetic instability in cells derived from Xrcc2-knockout mice. Cancer Res. 2003;63(23):8181–7.
John EM, Hopper JL, Beck JC, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–89.
Hilbers FS, Wijnen JT, Hoogerbrugge N, et al. Rare variants in XRCC2 as breast cancer susceptibility alleles. J Med Genet. 2012;49(10):618–20.
COMPLEXO, Southey MC, Park DJ, et al. COMPLEXO: identifying the missing heritability of breast cancer via next generation collaboration. Breast Cancer Res. 2013;15(3):402.
Acknowledgements
The author is grateful to Dominique Stoppa-Lyonnet, Nadine Andrieu and Melissa Southey for reviewing and commenting on this manuscript.
Disclosure
F. Lesueur declares no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by F. Lesueur involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Cancer Genetics.
Rights and permissions
About this article
Cite this article
Lesueur, F. Breast Cancer Risk Gene Discovery: Opportunities and Challenges. Curr Genet Med Rep 3, 82–91 (2015). https://doi.org/10.1007/s40142-015-0066-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40142-015-0066-x