Abstract
Purpose of review
This review aimed to evaluate the evidence of using non-invasive brain stimulation (NIBS) combined with mirror therapy (MT) for patients with stroke.
Recent findings
This systematic review included eight RCTs; four of them were of high quality. The meta-analyses revealed a significant effect of NIBS with MT on improving the hand grip strength (P = 0.0010), and gross manual dexterity (P = 0.0002), while sensorimotor function and cortical excitability were not significant. The timing-dependent effect of the tDCS with MT on the sensorimotor function was non-significant.
Summary
Findings of this review emphasize the use of MT in stroke rehabilitation with NIBS to improve hand grip strength and gross manual dexterity. Moderate evidence is present for the effect of transcranial magnetic stimulation with MT on balance and walking, and limited evidence for temporal-spatial gait parameters improvement. More high quality RCTs with longer follow-up are needed to strengthen the present evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects and consequences of stroke are considered as major public health issues [1]. Guiraud et al., 2010 and Yaghi et al., 2017 stated that among the most common risk factors for stroke are smoking, hypertension, hypercholesterolemia, diabetes mellitus, and atrial fibrillation. They also stated that stroke resulting from undetermined etiology (Cryptogenic stroke) constitutes about one third of all stroke cases [2, 3]. One of the most common consequences of stroke is hemiplegia, in which paralysis affects muscles of the upper extremity more than those of the lower extremity [4], body functions [5] and patient’s ability to execute simple daily living tasks and, thereby reducing the overall quality of life [6].
Various physical therapy techniques are emerging to facilitate stroke rehabilitation that can redouble the motor recovery when combined [7]. One of these combined approaches is the use of non-invasive brain stimulation (NIBS) together with mirror therapy (MT). The NIBS technique has been an eminent technology used in to enhance post-stroke recovery. In stroke, they are used to reinforce “adaptive” plasticity after brain lesion and control “maladaptive” plasticity [8], therefore improve motor output when integrated with physical rehabilitation. Protocols of NIBS in rehabilitation are based on several models that explain the brain reorganization after an insult. Interhemispheric competition model that assumes increased inhibition of the affected hemisphere by reduced inhibition of the unaffected side [9]. Compensation for the impairment of the affected hemisphere by the unaffected hemisphere is called the vicariation model, subsequently adaptive changes occurs rather than maladaptive processes [9,10,11]. It is suggested that the bimodal balance model, which counts on the structural reserve, is more sufficient to explain the recovery after stroke [9]. In stroke, they are used to enhance cortical modifications triggered for recovery after brain lesion and so improve motor output when integrated with physical rehabilitation [8].
Transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) are the most common emerging approaches used in stroke rehabilitation. The tDCS is a neuromodulator paradigm; the stimulation is administered by weak electric current through cathodal and anodal application to induce change of brain polarity [12]. The rTMS is a painless brain stimulation that modulates cortical excitability at the stimulation site and trans-synaptically at distant sites [13]. This stimulation is applied via a magnetic field that induces an electric field in the brain [14].
Mirror therapy aids also in the rehabilitation of patients with stroke by placing a mirror in the patient's sagittal plane, reflecting the non-paretic side, while performing bilateral synchronized movements [15]. It makes the movement-related beta desynchronization, in the motor cortex, more symmetrical and normalized during bilateral movement, after the visual stimulation [16]. The activation of brain areas occurs through the illusion created by the mirror image of the non-paretic limb being superimposed on the affected limb behind the mirror [17].
Previous systematic reviews studied the therapeutic effect of either NIBS or MT and showed improvement of motor function [18•, 19]. The combination of NIBS with MT has shown additive effects on motor performance [20], however, the existing trials presented contradicted results [21, 22]. Therefore, the objectives of this review were to evaluate the evidence of using NIBS in combination with MT in patients with stroke.
Methods
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [23].
Database Search
Electronic databases; Pubmed, Cochrane library, Scopus, Web of science, EPSCOhost and PEDro, were searched for relevant studies using the keywords and Mesh terms shown in Appendix S1. Two authors searched the databases independently from the earliest available dates up to April 2023.
Eligibility Criteria
The eligibility criteria of the included studies were as the following:
-
a)
Randomized controlled studies that have been peer-reviewed and published in English with full text available; b) Participants were adult stroke survivors; c) Interventions used were non-invasive brain stimulation combined with mirror therapy (alone or with general exercises) and compared to sham therapy, non-invasive brain stimulation alone or mirror therapy alone; and d) Functional outcomes of upper and/or lower extremities were assessed.
Studies Selection
To remove any duplicates, all of the searched literature was exported to Mendeley software [24] and the remaining citations were uploaded to Abstract software [25] which allows for study organization and filtration. Then, a group of authors, independently, double-screened each title and abstract. Full text filtration was performed on potentially eligible or confusing abstracts by a group of independent authors who then extracted the data from the included studies and scored the methodological quality. Any disagreements between authors were discussed with senior author.
Quality Assessment
For methodological quality assessment, the included studies were graded using the Physiotherapy Evidence Database (PEDro) scale [26]. The PEDro scale has 11 items, the first of which is concerned with external validity and not included in the final score. Items 2–9 of PEDro help users in identifying trials with strong internal validity, while items 10–11 evaluates trials that present enough data to make their results interpretable. Based on the overall score; which ranges from 0 to 10, the quality is rated as high or low; high quality for articles ≥ 6 points (6–7 is good and 8–10 is excellent quality), low quality for articles < 6 points (4–5 is fair and < 4 is poor quality) [27].
Data Extraction
Data were extracted from every relevant study by two authors independently into an Excel spreadsheet and included: a) Study characteristics: authors and year of publication; b) Participants’ characteristics: stroke type, onset and severity; c) Intervention characteristics: intervention and control modalities, timing, intensity, duration and follow-up; and d) outcomes and results. Any disagreements were resolved by discussion with the senior author.
Data Synthesis
Data from the included studies were analyzed using Review Manager (RevMan – version 5.4.1, The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark, 2021), and Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA). A formal meta-analysis was conducted for all outcomes if the data were sufficient. Pooled continuous effect measures were expressed as the mean difference (MD) when the scale was unified or standardized mean difference (SMD) when the scales were different for the same outcome, with 95%CI and analyzed using Inverse-variance method. Between-study statistical heterogeneity was explored and quantified using the I2 test. By default, the fixed effect model was used in all analyses. If heterogeneity was statistically significant (p < 0.05) or I2 was > 50%, the Der Simonian and Laird random-effects model were used instead [28]. Subgroup analysis was conducted for latency and amplitude of cortical excitability. Publication bias could not been assessed because of the small number of included studies in each meta-analysis. We considered 2-sided statistical analysis testing setting the α-error level at 0.05.
The strength of evidence was determined using Levels of evidence adapted from Sackett Appendix S2, in which the levels are ranked from 1 to 5 depending on the PEDro scores; with 1 being the strongest evidence and 5 has no evidence [29, 30].
Results
Study Selection
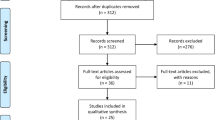
After initially searching the databases, 727 records were identified; of these records, 246 records were excluded as they were duplicated studies. Thus, 481 records were retrieved for title and abstract screening, which resulted in exclusion of 451 records which included different intervention, different population, or those, were reviews or protocols. Consequently, 30 records were retrieved for detailed evaluation. Full-text assessment yielded to exclusion of 22 records either for not meeting our inclusion criteria, as shown in Appendix S3, or because their full-text was not available. Eventually, eight records [21, 22, 31,32,33,34,35,36] were included in this review; five of them were involved in quantitative analysis [21, 22, 31,32,33]. D’Agata et al. [36] study was excluded from meta-analysis due to lack of sufficient data after emailing the corresponding author. The PRISMA flow chart of the systematic search and filtration was displayed in Fig. 1.
Study Characteristics
Quality Assessment
The PEDro score for each included study is illustrated in Table 1. After assessing the methodology of each study, four studies [21, 22, 34, 36] reached a good-quality score while the other four studies [31,32,33, 35] reached a fair-quality score. All studies showed randomization of subjects, however, the allocation concealment was achieved only in one study [22]. Blinding of subjects was achieved in two studies [21, 36], and blinding of assessors was achieved in five studies [21, 32,33,34, 36] while none of all achieved therapists blinding. In addition, subjects were similar at baseline assessment except in one study [33]. Three studies [21, 32, 36] did not obtain outcomes from 85% of their subjects, moreover, intention-to-treat analysis was not achieved in all of the included studies except in one study [22].
Participants
The included participants’ characteristics were shown in Tables 2, 3, and 4. In total, 240 participants were involved across the eight studies. The range of participants within the studies was 20–36, all participants were adults with mean age 58.02 years including both sexes with the majority of male participants (n = 145). Six studies [21, 22, 31, 33, 35, 36] included patients with chronic stroke (n = 184) while two studies [32, 34] included sub-acute stoke (n = 56). The causes of stroke were variable between ischemic (n = 125) and hemorrhagic (n = 71). The paretic side varied between left side (n = 97) and right side (n = 107) and the severity of the affection was mild to moderate as reported only in one study [21]. The majority of the studies assessed the motor function in the upper extremity [21, 22, 31,32,33, 36]. Balance was assessed in one study [34], and gait also in another one [35]. Three studies assessed the effect of tDCS [21, 22, 31] whereas four studies assessed the effect of rTMS [32,33,34,35] and one study assessed tDCS alongside with rTMS [36]; in combination with mirror therapy. Two studies used mirror box to deliver the mirror therapy [32, 36].
Interventions
The included interventions characteristics were demonstrated in Tables 2, 3, and 4. The used interventions were as follows:
-
a) Transcranial Direct Current Stimulation (tDCS) combined with Mirror Therapy (MT)
Three studies [21, 22, 31], carried out on 85 participants, examined the effect of tDCS combined with MT on the motor function of the upper extremity. Anodal tDCS was use in two studies [21, 31], while Jin et al. [22] used dual tDCS. The interventions were applied for 45 [31] or 90 [21, 22] minutes in total, 3 [31] or 5 [21, 22] days/week, for 2 [22], 4 [21] or 6 [31] weeks on chronic stroke survivors. Only Jin et al. [22] followed-up the participants after 2 weeks.
-
b) Repetitive Transcranial Magnetic Stimulation (rTMS) combined with Mirror Therapy (MT)
Four studies [32,33,34,35] reported the effect of rTMS on 121 patients with stroke. The high frequency rTMS combined with MT were applied on upper extremity in two studies [32, 33] that used the interventions 5 days/week, for 2 [32] or 6 [33] weeks, for 60 [33] or 65 [32] minutes on either sub-acute [32] or chronic [33] patients of stroke. The other two studies used either low [34] or high [35] frequencies rTMS combined with MT on the lower extremities of patients with stroke (> 6 months). They used the same protocol of intervention duration as follows; 5 days/week for 4 weeks, rTMS (20 min) and MT (20 min).
-
c) Both Repetitive Transcranial Magnetic Stimulation (rTMS) and Transcranial Direct Current Stimulation (tDCS) combined with Mirror Therapy (MT)
Lastly, D’Agata et al. [36] used two cycles of stimulation either rTMS or tDCS with MT applied on the upper extremity with 6 months washout period in between the two stimulation cycles. Each cycle was applied 5 days/week for 2 successive weeks; tDCS (20 min), MT (20 min) and rTMS (15 min), with multiple follow-up points; 3 and 6 months after both the 1st and 2nd stimulation cycle.
Outcomes
The measured outcomes and results were demonstrated in Tables 2, 3, and 4, with their level of evidence in Appendix S4.
-
1. Effect on Upper Extremities
-
a. Sensorimotor function of the upper extremity
The sensorimotor function of the upper extremity was evaluated by Fugl-Meyer Assessment Upper Extremity (FMA-UE) in four studies [21, 22, 31, 33]; that used NIBS with MT. The meta-analysis revealed a non-significant difference between groups post treatment (P = 0.06, MD = 4.94, 95%CI = -0.28, 10.16, I2 = 0%), as shown in Fig. 2. Moreover, three of these studies [21, 22, 31] used tDCS in combination with MT and showed also a non-significant effect on the sensorimotor function of the upper extremity compared to the control group (P = 0.06, MD = 4.94, 95%CI = -0.28, 10.16, I2 = 0%), as shown in Fig. 3.
-
b. Gross manual dexterity
The gross manual dexterity was assessed by Box and Block Test (BBT) in four studies [22, 31,32,33]; using either tDCS [22, 31] or rTMS [32, 33] in combination with MT. The meta-analysis demonstrated significant differences in favor of the NIBS, tDCS and rTMS when combined with MT, compared to the control group (P < 0.00001, MD = 11.21, 95%CI = 6.54, 15.89, I2 = 0%), (P = 0.003, MD = 9.52, 95%CI = 3.30, 15.74, I2 = 0%) and (P = 0.0002, MD = 13.42, 95%CI = 6.32, 20.52, I2 = 0%), respectively, as shown in Figs. 4, 5, 6.
-
c. Hand grip strength
The hand grip strength was assessed by hand dynamometer in two studies; [31, 32] the meta-analysis showed significant difference between groups post treatment in favor of the NIBS with MT group when compared with the control group (P = 0.0010, MD = 3.06, 95%CI = 1.24, 4.87, I2 = 0%), as shown in Fig. 7.
-
2. Effect on Lower Extremities
One study [34] administered rTMS with MT and found significant differences between groups in favour of the intervention group, for walking and balance results when assessed by the balance measurement system, while the Berg Balance Scale (BBS) result was not significant. The other study [35]; revealed significant differences between groups for the gait spatiotemporal parameters that included single support phase, step length, stride length and velocity in favour of the intervention group.
-
3. Effect on Cortical excitability
The meta-analysis of the cortical excitability in the two studies [32, 33], that used rTMS in combination with MT, showed non-significant differences between groups post treatment (P = 0.95, SMD = -0.03, 95%CI = -1.18, 1.12, I2 = 84%). For the subgroup analysis, regarding the latency and amplitude; meta-analysis revealed also a non-significant difference between groups post treatment (P = 0.44, SMD = -0.73, 95%CI = -2.56, 1.11, I2 = 87%), (P = 0.26, SMD = 0.65, 95%CI = -0.48, 1.77, I2 = 69%), respectively, as shown in Fig. 8.
-
4. Timing-depended effects
The timing-depended effects of the tDCS with MT were examined in two studies [21, 22]; with contradictory findings between outcomes. The results showed that there was a significant improvement in the Nottingham Extended Activities of Daily Living Scale (NEADL) scores in favor of the sequential group as compared to the concurrent [21]; however, the concurrent tDCS showed significant improvement in Action Research Arm Test (ARAT) in relation to sequential [22]. There were no significant differences between any of the studied groups regarding the Kinematic assessment scores [21].
Comparing the timing-dependent effect of the sequential and concurrent interventions on the FMA-UE, involved in two studies [21, 22]; revealed also non-significant difference between groups post treatment (P = 0.54, MD = 2.92, 95%CI = -6.33, 12.16, I2 = 0%), as shown in Fig. 9.
Discussion
The main objective of this systematic review and meta-analysis was to evaluate the effectiveness of NIBS technique combined with MT on the motor functions of both the upper and lower extremities in patients with stroke. Eight studies were included in this systematic review; half of them were of high quality, while the other studies were of low quality. Five studies were included in the meta-analysis and revealed that NIBS with MT have a significant effect on the hand grip strength and gross manual dexterity, whereas, the improvements in the sensorimotor function and cortical excitability were not significant. On the other hand, the timing-dependent effect of the tDCS with MT on the sensorimotor function, assessed by FMA-UE, was non-significant for both sequential and concurrent groups.
For better understanding of these results, it is important to know how this combination works. Normally, the transcallosal connections, between the two brain hemispheres, mediate a mutual inhibitory control which is reduced with brain injury. This inter-hemispheric imbalance could be improved by modulating cortical activity with NIBS that is used to increase cortical activity of the ipsilesional cortex or to decrease the activity of contralesional areas [37]. Depending on the rTMS frequency set during the application; using high frequency rTMS (> 1 Hz) on the ipsilesional hemisphere will facilitate cortical excitability, and controversy low frequency rTMS (≤ 1 Hz) on contralesional hemisphere will inhibit cortical excitability [38], however, applying high frequency rTMS over the contralesional hemisphere showed more positive effects than the conventional application of rTMS [39]. Such that, cerebral excitability is decreased by tDCS cathodal stimulation, which hyperpolarizes neurons, whereas it is increased by anodal stimulation, which depolarizes neurons [40]. Additionally, two hypotheses for MT mechanism of action are adopted. One of them is that MT potentially normalizes the asymmetrical pattern of movement-related beta desynchronisation in the primary motor cortex, while, the other is the motor neuron hypothesis which suggests that mirror neurons excitation during MT facilitates functional recovery [41]. Combining the non-specific NIBS effect with other techniques makes it more specific [42]; and in this case it is the MT.
The significant results for both hand gross manual dexterity and hand grip strength in favour of the NIBS combined with MT group are consistent with the results of the study reported by Yavuzer et al. [43] which revealed that MT improved functional recovery in the upper extremity and performance of daily living activities of stroke patients. Tosun et al. [44] demonstrated a significant motor enhancement of the affected upper extremities when using inhibitory rTMS and neuromuscular electrical stimulation. In addition, Aşkın et al. [45] reported that the combination of rTMS and physical therapy was considerably better to traditional physical therapy in terms of spasticity reduction and improvements in motor and cognitive skills. Moreover, Lee et al. [46•] stated that in stroke patients, tDCS paired with therapy can improve upper extremity function. Transcranial direct current stimulation paired with occupational/physical therapy, in particular, had a much larger effect on upper extremity function recovery in stroke patients with hemiplegia. These results were attributed to the optical illusion of movement of the non-paretic side activating the frontal or parietal lobe mirror neurons in the corresponding motor region through mirror reflection, thereby improving the movement of the paretic side and acts as a cognitive intervention to stroke patients [47]. NIBS of patients with stroke can elicit structural changes in neuroplasticity; thereby it helps them to recover the motor function of upper extremity [48].
In regard to the results of the FMA-UE; improvement of the sensorimotor function of the upper extremity was not significant between the groups post treatment. Moreover, receiving tDCS stimulation either followed by MT or concurrently with MT had a statistically significant difference when compared with the sham groups. In line with our results, systematic reviews of randomized controlled trials, used either tDCS [12] or added robot-assisted therapy to NIBS [49], also found a non-significant homogeneous summary effect size for FMA scores, but rather benefits of tDCS on enhancing ADL capacity [12]. The NIBS may serve as a priming stimulus to enhance the activity of the affected cortex. This stimulus would create an excitable environment of the brain which is beneficial for activating higher-order motor-cognitive processes during the consecutive MT [50]. By contrast, applying tDCS concurrently with MT might generate motor/cognitive interference during the MT practice, and consequently affect the cognitive-motor relearning processes and generalization of learned skills to daily activities [51].
Furthermore, the meta-analysis of cortical excitability showed non-significant differences between high frequency rTMS with MT and control groups post treatment for both latency and amplitude. In contrast, Kang et al. [52] revealed that the two sets of stimulation techniques, tDCS and rTMS, demonstrated substantial favourable effect sizes. Increasing cortical excitability in the ipsilesional hemisphere, through anodal tDCS and high frequency rTMS, and reducing cortical excitability in the contralesional hemisphere, through cathodal tDCS and low frequency rTMS, enhance paretic limb force generation. Navarro-López et al. [53] reported that single tDCS treatments set altered cortical excitability for a few minutes, but many treatment sessions altered cortical excitability for hours, even a day. This is therapeutically useful for usage in conjunction with physical therapy because a long-term state of enhanced (or decreased) excitability is required to promote neuroplastic brain changes.
According to this systematic review and meta-analysis, up to this moment; there are conflicting results between outcomes of upper extremity; sensorimotor function, hand grip strength, gross manual dexterity and cortical excitability, indicating a further need to explore the interaction effects and timing of NIBS with MT, to determine the optimal effective combination strategy.
Regarding the effect of NIBS on the lower extremities, only two studies of the same authors assessed the effects of rTMS with MT on walking, balance [34] and gait spatiotemporal parameters [35] and showed significant results in favor of the intervention groups. Vaz et al. [54] suggested that gait recovery can be achieved by employing NIBS to rebalance inter-hemispheric competition while the patient is undergoing another exercise-based therapy. In addition, Fan et al. [55] confirmed the valuable benefits of rTMS on stroke patients' lower limb motor skills (e.g., walking and balance). Furthermore, Navarro-López et al. [53] reported that in stroke patients, the application of tDCS in conjunction with PT improves gait metrics, static and dynamic balance, and lower limb functions. The settings that have showed benefits include 2 mA for at least 10 min with anodic or bihemispheric stimulation. These parameters have shown benefits at any stage of stroke in single or multiple session procedures.
The effects of NIBS techniques were comparable as reported by D’Agata et al. [36] but there are some advantages of using tDCS vs. rTMS in stroke rehabilitation. More than one NIBS cycle (2–4 weeks) should be employed in rehabilitation to achieve a clinical meaningful data after a washout period in responder patients only.
It is also important to consider the adverse effects of NIBS combined with MT. Only one study mentioned that none of the patients reported discomfort or severe side effects [22]. The other studies didn't report if adverse events occurred or not.
Limitations and Implications for Future Research
The current systematic review has some limitations, such as the small number of included studies with only half of them being of high quality. In addition, the included studies had a wide variety of the clinical characteristics of the stroke participants, intervention protocols, parameters of outcome measures, thus, the findings couldn't be generalized to the entire stroke population. Moreover, the absence of follow-up and adverse effects reporting in many studies, didn’t grant discussing the long-term effects of the current interventions combination. Furthermore, the results of NIBS with MT on lower extremities functions couldn't be affirmed due to the lack of the studies on the lower extremities.
The acquired results of this systematic review and meta-analyses could aid in improving the future trials on combined interventions, specifically NIBS and MT, to be implemented in routine stroke rehabilitation programs. More RCTs are recommended with higher quality, larger number of participants, longer duration and follow-up periods. Studies have to focus on the optimal aspects of the intervention protocols including location, duration timing and intensity. Standardized outcome measures should be used in addition to the neuroimaging outcomes to provide more precise result. Comparison of the intervention effects on different stroke population and different body functions, structures, activities and participation.
Conclusion
Our results emphasize that adding MT to NIBS has a beneficial effect on improving hand grip strength and gross manual dexterity of patients with stoke. Conversely, it has no effect on sensorimotor function of the upper extremity and cortical excitability. For the effect of NIBS with MT on the functions of lower extremities of the patients with stoke; there is moderate evidence on the effect of TMS with MT on balance and walking, and limited evidence on temporo-spatial gait parameters improvement. More high quality RCTs for longer follow-up duration are needed to ensure the effects of this combination on body functions, structures, activities and participation.
Registration and Protocol
The protocol of this systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with ID (CRD42021275368).
Data Availability
Data analyzed in this manuscript are available upon request to the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
Guiraud V, Ben AM, Mas JL, Touzé E. Triggers of ischemic stroke: A systematic review. Stroke. 2010;41(11):2669–77.
Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic stroke. Circ Res. 2017;120(3):527–40.
Lim KB, Lee HJ, Yoo J, Yun HJ, Hwang HJ. Efficacy of mirror therapy containing functional tasks in poststroke patients. Ann Rehabil Med. 2016;40(4):629.
Boukhennoufa I, Zhai X, Utti V, Jackson J, Mcdonald-Maier KD. Wearable sensors and machine learning in post-stroke rehabilitation assessment: a systematic review. Biomed Signal Process Control. 2022;71:103197.
Yumnam N, Akoijam JS, Singh LN, Oinam J. Effectiveness of mirror therapy in the motor recovery of upper extremity in the post stroke hemiplegic patients: a randomized controlled trial in a tertiary care hospital in Manipur, Northeast India. Int J Adv Med. 2019;6(5):1657.
Takeuchi N, Izumi SI. Combinations of stroke neurorehabilitation to facilitate motor recovery: perspectives on Hebbian plasticity and homeostatic metaplasticity. Front Hum Neurosci. 2015;9(JUNE):349.
Kubis N. Non-invasive brain stimulation to enhance post-stroke recovery. Front Neural Circuits. 2016;10(JUL2016).
Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608.
Riecker A, Gröschel K, Ackermann H, Schnaudigel S, Kassubek J, Kastrup A. The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp. 2010;31(7):1017–29.
Fleming MK, Rothwell JC, Sztriha L, Teo JT, Newham DJ. The effect of transcranial direct current stimulation on motor sequence learning and upper limb function after stroke. Clin Neurophysiol. 2017;128(7):1389–98.
Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J Neuroeng Rehabil. 2017;14(1):1–12.
Rastgoo M, Naghdi S, Nakhostin Ansari N, Olyaei G, Jalaei S, Forogh B, et al. Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. 2016;38(19):1918–26. https://doi.org/10.3109/0963828820151107780
Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord. 2019;12:1756286419878317.
Lee SA, Cha HG. The effect of motor imagery and mirror therapy on upper extremity function according to the level of cognition in stroke patients. Int J Rehabil Res. 2019;42(4):330–6.
Rossiter HE, Borrelli MR, Borchert RJ, Bradbury D, Ward NS. Cortical mechanisms of mirror therapy after stroke. Neurorehabil Neural Repair. 2015;29(5):444–52.
Zult T, Howatson G, Kádár EE, Farthing JP, Hortobágyi T. Role of the mirror-neuron system in cross-education. Sports Med. 2014;44(2):159–78.
• Vabalaite B, Petruseviciene L, Savickas R, Kubilius R, Ignatavicius P, Lendraitiene E. Effects of High-Frequency (HF) Repetitive Transcranial Magnetic Stimulation (rTMS) on upper extremity motor function in stroke patients: a systematic review. Med. 2021;57(11):1215. (This systematic review aimed to investigate the effect of high-frequency repetitive transcranial magnetic stimulation for upper extremity motor function recovery after stroke and concluded that HF-rTMS may increase impaired upper extremity motor function better than sham stimulation in stroke patients.)
Gandhi DBC, Sterba A, Khatter H, Pandian JD. Mirror therapy in stroke rehabilitation: current perspectives. Ther Clin Risk Manag. 2020;16:75.
von Rein E, Hoff M, Kaminski E, Sehm B, Steele CJ, Villringer A, et al. Improving motor performance without training: the effect of combining mirror visual feedback with transcranial direct current stimulation. J Neurophysiol. 2015;113(7):2383–9.
Wen LW, Chi CW, Chung LK, Yi WC, Ting LC, Wei HY, et al. Timing-dependent effects of transcranial direct current stimulation with mirror therapy on daily function and motor control in chronic stroke: a randomized controlled pilot study. J Neuroeng Rehabil. 2020;17(1).
Jin M, Zhang Z, Bai Z, Fong KNK. Timing-dependent interaction effects of tDCS with mirror therapy on upper extremity motor recovery in patients with chronic stroke: a randomized controlled pilot study. J Neurol Sci. 2019;405.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372.
Mendeley Reference Manager | Mendeley [Internet].
Byron CW, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an Evidence-based Practice Center: Abstrackr. IHI’12 - Proc 2nd ACM SIGHIT Int Heal Informatics Symp. 2012;819–23.
PEDro scale - PEDro [Internet].
Moseley AM, Elkins MR, Van der Wees PJ, Pinheiro MB. Using research to guide practice: the physiotherapy evidence database (PEDro). Brazilian J Phys Ther. 2020;24(5):384–91.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3–5.
Snider L, Majnemer A, Darsaklis V. Feeding interventions for children with cerebral palsy: a review of the evidence. 2011;31(1):58–77. https://doi.org/10.3109/019426382010523397
Shin CH, Gyu CH. Effect of mirror therapy with tDCS on functional recovery of the upper extremity of stroke patients. J Phys Ther Sci. 2015;27(4):1045.
Kim J, Yim J. Effects of high-frequency repetitive transcranial magnetic stimulation combined with task-oriented mirror therapy training on hand rehabilitation of acute stroke patients. Med Sci Monit. 2018;24:743.
Ji SG, Cha HG, Kim MK. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation combined with mirror therapy. J Magn. 2014;19(1):28–31.
Cha HG, Kim MK. Therapeutic efficacy of low frequency transcranial magnetic stimulation in conjunction with mirror therapy for sub-acute stroke patients. J Magn. 2015;20(1):52–6.
Cha HG, Kim MK. The effects of repetitive transcranial magnetic stimulation integrated mirror therapy on the gait of chronic stroke patients. J Magn. 2015;20(2):133–7.
D’Agata F, Peila E, Cicerale A, Caglio MM, Caroppo P, Vighetti S, et al. Cognitive and neurophysiological effects of non-invasive brain stimulation in stroke patients after motor rehabilitation. Front Behav Neurosci. 2016;10(Jun):135.
Biou E, Cassoudesalle H, Cogné M, Sibon I, De Gabory I, Dehail P, et al. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: a systematic review. Ann Phys Rehabil Med. 2019;62(2):104–21.
Du J, Yang F, Liu L, Hu J, Cai B, Liu W, et al. Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomized, double-blind clinical trial. Clin Neurophysiol. 2016;127(3):1907–13.
Wang Q, Zhang D, Zhao YY, Hai H, Ma YW. Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: a randomized clinical trial. Brain Stimul Basic, Transl Clin Res Neuromodulation. 2020;13(4):979–86.
Thair H, Holloway AL, Newport R, Smith AD. Transcranial direct current stimulation (tDCS): a beginner’s guide for design and implementation. Front Neurosci. 2017;11(NOV):641.
Hung GKN, Li CTL, Yiu AM, Fong KNK. Systematic review: effectiveness of mirror therapy for lower extremity post-stroke. 2015;26:51–9. https://doi.org/10.1016/j.hkjot201512003.
O’Brien AT, Bertolucci F, Torrealba-Acosta G, Huerta R, Fregni F, Thibaut A. Non-invasive brain stimulation for fine motor improvement after stroke: a meta-analysis. Eur J Neurol. 2018;25(8):1017–26.
Yavuzer G, Selles R, Sezer N, Sütbeyaz S, Bussmann JB, Köseoǧlu F, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89(3):393–8.
Tosun A, Türe S, Askin A, Yardimci EU, Demirdal SU, Incesu TK, et al. Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. 2017;24(5):361–7. https://doi.org/10.1080/1074935720171305644
Aşkın A, Tosun A, Demirdal ÜS. Effects of low-frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: a randomized controlled trial. 2017;34(2):102–7. https://doi.org/10.1080/0899022020171316254
• Lee JH, Jeun YJ, Park HY, Jung YJ. Effect of transcranial direct current stimulation combined with rehabilitation on arm and hand function in stroke patients: a systematic review and meta-analysis. Healthc (Basel, Switzerland). 2021;9(12). The findings of this meta-analysis provides an evidence that tDCS combined with rehabilitation, especially occupational therapy/physical therapy and virtual reality therapy, may benefit upper extremity function of the paretic upper limb in stroke patients.
Dohle C, Püllen J, Nakaten A, Küst J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. 2009;23(3):209–17.
Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kineses ZT, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–6.
Reis SB, Bernardo WM, Oshiro CA, Krebs HI, Conforto AB. Effects of robotic therapy associated with noninvasive brain stimulation on upper-limb rehabilitation after stroke: systematic review and meta-analysis of randomized clinical trials. Neurorehabil Neural Repair. 2021;35(3):256–66.
Christova M, Rafolt D, Gallasch E. Cumulative effects of anodal and priming cathodal tDCS on pegboard test performance and motor cortical excitability. Behav Brain Res. 2015;287:27–33.
Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128(11):2318–29.
Kang N, Summers JJ, Cauraugh JH. Non-invasive brain stimulation improves paretic limb force production: a systematic review and meta-analysis. Brain Stimul. 2016;9(5):662–70.
Navarro-López V, Molina-Rueda F, Jiménez-Jiménez S, Alguacil-Diego IM, Carratalá-Tejada M. Effects of transcranial direct current stimulation combined with physiotherapy on gait pattern, balance, and functionality in stroke patients. A systematic review. Diagnostics. 2021;11(4):656.
Vaz PG, Salazar AP da S, Stein C, Marchese RR, Lukrafka JL, Plentz RDM, et al. Noninvasive brain stimulation combined with other therapies improves gait speed after stroke: a systematic review and meta-analysis. 2019;26(3):201–13.https://doi.org/10.1080/1074935720191565696
Fan H, Song Y, Cen X, Yu P, Bíró I, Gu Y. The effect of repetitive transcranial magnetic stimulation on lower-limb motor ability in stroke patients: a systematic review. Front Hum Neurosci. 2021;15:620573.
Funding
The authors declare that they have no funding or grants, or equipment provided for the project from any source.
Author information
Authors and Affiliations
Contributions
N. A.: Conceptualization, Methodology, Formal analysis, Writing review, and editing A. T.: Investigation, Writing—Original Draft, H. S. A.: Methodology, Investigation, Data Curation, Writing - Original Draft, Project administration M. G. A.: Validation, Investigation, Writing, Review and Editing S. T. E.: Investigation, Writing, Original Draft A. G. M.: Investigation, Writing, Original Draft A. A. E.: Investigation, Writing, Original Draft P. S. G.: Investigation, Writing, Original Draft S. E.: Supervision, Conceptualization, Methodology, Validation, reviewing, and editing
Corresponding author
Ethics declarations
• They have no financial benefits to the authors.
• They have no previous presentation of the research, manuscript, or abstract in any form.
• This article does not contain any studies with human or animal subjects performed by any of the authors.
• The Tables and figure are original.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelhaleem, N., Tawfek, A., Abouamra, H.S. et al. Combined Effect of Non-Invasive Brain Stimulation with Mirror Therapy for Improving Motor Function in Patients with Stroke: a Systematic Review with Meta-Analysis. Curr Phys Med Rehabil Rep 12, 368–382 (2024). https://doi.org/10.1007/s40141-024-00448-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-024-00448-4