Abstract
Purpose of Review
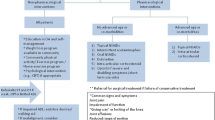
Osteoarthritis (OA) of the knee is a common disease with associated direct and indirect costs having a significant societal impact. This review is conducted to discuss various treatment options for knee OA, highlight the most recent evidence regarding efficacy, and devise clinical recommendations to meet the needs of the patients.
Recent Findings
Pharmacologically, options tend to be limited. Simple analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs are widely used, with efficacy and safety being questioned. Mild opioids can be considered, although strong opioids are discouraged and need to be incorporated with great caution. Therapeutic exercises and weight loss have been the cornerstone of knee OA management. Symptomatic relief can be achieved with intra-articular steroids, hyaluronic acid, and orthobiologics injections as well. More recently, genicular nerve neurotomy has been introduced to the treatment algorithm and shown promising results, although some refinement of the technique is likely needed in the future.
Summary
A proper and astute approach is adopting a comprehensive treatment program encompassing multimodal and multidisciplinary modalities. Future direction should focus on the clinical success of such effort in terms of pain and function improvement, patient satisfaction, and reduction in a financial burden to the health care system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogenesis of osteoarthritis (OA) is a complex process involving multiple factors rather than a simple byproduct of overuse. The intricate interplay of various inflammatory mediators causes cartilage degradation, synovitis, subchondral sclerosis, and meniscal degeneration leading to pain and functional limitations [1]. With the population aging and facing more obesity, OA is a serious public health concern with major financial implications. It is estimated that over 560 million people live with knee OA worldwide, and the cost to the healthcare system exceeds $27 billion annually in the USA [2]. A wide range of treatment modalities is available with varying degrees of success, yet many will ultimately require surgical interventions. The following article will discuss various pharmacological and non-pharmacological methods to manage painful knee OA.
Conservative Management for Knee OA
Exercise
A structured exercise program is a first-line treatment for knee OA supported by strong evidence for effectiveness and safety [3,4,5]. There are many forms of structured exercise programs, such as land-based therapy, aquatherapy [6], or mind-body exercise [7].
Most land-based therapy focuses on strengthening and stretching exercises of lower limbs, including the quadriceps, hip abductors, and hip adductors, to reduce compressive load at the knee joint by modifying the kinetic chain and reducing valgus or varus stress [8]. Balance training, in addition to a standard therapy regimen, has also shown pain relief and fall prevention [9]. Combining aerobic exercise, regardless of modes of exercise, also provides additional pain relief and functional improvement [10]. Aquatherapy has moderate quality evidence in pain reduction and functional improvement [11]. It is often done in heated water (32°C to 36°C), which helps to reduce pain and stiffness during exercise. Reduced joint loading due to the buoyancy of water is also advantageous to focus on strengthening without aggravating the pain [12] and is beneficial, especially for patients with arthritis in multiple joints. Mind-body exercises (Tai Chi or Yoga) can be considered a non-traditional land-based therapy option. Unlike a traditional program, mind-body exercises highlight the importance of psychological well-being. They are recommended as core treatment by Osteoarthritis Research Society International (OARSI) guideline and have shown promising results in pain relief and functional improvement [3, 13].
While they all showed improvement in pain and function, there is no strong evidence showing one is superior to the others, and optimal exercise protocol for knee OA is yet to be determined [6, 10, 1514••, ], hence combining different types of exercises based on individual conditions and preferences.
Braces and Orthoses
Knee braces or foot orthoses are often prescribed by physicians as an adjunctive treatment or requested by patients. It is hypothesized to reduce valgus or varus stress at the knee, stabilize the knee by providing external support, or improve proprioception. A medial unloader brace or lateral wedge insole is used to reduce loading at the medial compartment by creating a valgus effect at the knee. A lateral unloader brace or medial wedge insole is used to reduce stress at the lateral compartment via varus effect at the knee. A hinged knee brace and neoprene sleeve are thought to stabilize the knee [16]. However, only limited evidence is available to support their effectiveness [16]. Generally, compliances for knee braces are low compared to foot orthosis, and one study showed a 28% compliance rate for knee orthosis after the first year [16, 17].
Weight Loss
Weight loss is also one of the first-line treatments, along with structured exercise and topical NSAIDs recommended by OARSI and ESCEO [3, 4]. Many studies have shown that over 10% bodyweight reduction led to functional improvement, pain relief, and slower cartilage deterioration [12, 18, 19]. One study reported a reduction of 2.2 kg of joint loading with every 1 kg of weight loss [20].
Pharmacological Treatment
Pharmacological management to treat painful knee OA is harshly challenging since options for analgesics are scarce, and treating knee OA pain may necessitate chronic daily use of them. A long-term administration of these agents may be impossible due to adverse effects on multiple organ systems and physical dependency in the case of opioids. However, they can be beneficial and sometimes required, especially during periods of flare-ups.
Evidence for acetaminophen is poor, although it is considered the safest option. In a double-blinded randomized control trial, acetaminophen at 4g/day failed to show improvement in knee OA pain or function compared to placebo [21]. The Cochrane review in 2019 found that paracetamol provides only minimal improvements in pain and function for people with knee OA that are clinically insignificant and calls for a reconsideration of its position as a first-line agent [22]. However, acetaminophen may have synergistic effects when combined with other medications and still commands an important role in treatment strategy.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are another common and widely utilized analgesics. They work by inhibiting the action of cyclo-oxygenase (COX) enzymes and are classified based on the inhibition of either selectively COX-2 or non-selectively COX-1 and COX-2 enzymes. Evidence suggests this class of medications can provide significant pain relief and improve function. However, the main consideration is the safety profile. NSAIDs are associated with gastrointestinal and renal complications as well as an increase in cardiovascular risks. Therefore, careful consideration is warranted based on each individual’s underlying condition. Alternatively, topical formulations are available and considered to be a safer option.
In the era of the opioid epidemic, its use in treating chronic pain, such as painful knee OA, is strongly discouraged due to the development of tolerance and dependency. Also, the evidence suggests that benefit of opioids is minimal and clinically insignificant. Opioids are considered either strong or weak based on their potency. Weak opioids include codeine and tramadol, while strong opioids are agents like morphine, hydromorphone, fentanyl, oxycodone, and hydrocodone. Tramadol has a unique property of modulating spinal norepinephrine and serotonin levels in addition to being a weak opioid agonist, and it is conditionally recommended by the American College of Rheumatology in their latest guidelines [23]. Before chronic opioid therapy is initiated, it is critical that alternative options have been exhausted, the risks of such therapy are fully explained, and close monitoring of side effects, proper use, and aberrant behaviors are in place.
Supplements such as fish oil, vitamin D, bisphosphonates, and glucosamine are not recommended, given the lack of quality studies showing efficacy. In general, it is challenging to treat knee OA pain with pharmacological agents alone. They should be considered adjuncts to other treatment modalities. Also, its use should be personally tailored based on clinical presentations.
Corticosteroid Injection
Intra-articular corticosteroid injections (IACIs) are commonly performed to treat knee OA pain when refractory to other conservative treatments. The mechanism by which corticosteroids achieve pain relief is complex, but the main action is likely due to the anti-inflammatory effect, as well as a potential increase in the viscosity of intrinsic hyaluronic acid [24]. Evidence for IACIs improving pain and function short-term is robust, although the long-term benefit is more questionable. A meta-analysis of 23 studies found that IACI reduces pain and improves function short-term (6 weeks) [25••]. However, the difference between the treatment and control groups waned after that period. Another meta-analysis showed similar results. IACIs provided more pain relief relative to intraarticular hyaluronic acid in the short-term period (1 month) but found hyaluronic acid injection to be more efficacious in the long-term (6 months) [26].
Injections are performed using either a landmark-based or image-guided technique. The accuracy of landmark-guided injection is highly contingent on the approach and experience of performing physicians and is variable. One randomized trial found the modified anterolateral approach had a higher accuracy rate relative to the standard superolateral injection (89% vs. 58% p < 0.05) [27]. The ultrasound-guided technique is gaining more popularity as multiple studies show increased accuracy and efficacy. Ultrasound guidance was shown to have 48% less procedural pain compared to landmark-based (p < 0.001) and improved outcomes at 2 weeks [28]. Additionally, a meta-analysis of 12 clinical trials found ultrasound-guided injections to be superior across every anatomical needle injection site compared with blind injections [29]. Another frequently used equipment for image guidance is fluoroscopy. Although there are not many clinical trials comparing ultrasound- and fluoroscopic-guided knee injections, a meta-analysis that compared the accuracy of the two methods used in glenohumeral joint injections found no significant difference, concluding ultrasound-guided injections as the preferred modality due to lack of radiation exposure [30].
Available corticosteroids for IACIs are methylprednisolone, triamcinolone, dexamethasone, betamethasone, prednisolone, and hydrocortisone, which can be classified by the solubility and duration of action. Hydrocortisone is the most soluble, followed by methylprednisolone, prednisolone, triamcinolone acetonide, and triamcinolone hexanoate [31]. The estimated average duration of action of betamethasone is 9 days, methylprednisolone 7–84 days, triamcinolone acetate 14 days, and triamcinolone hexacetonide 8–90 + days [31]. When comparing single-dose injections of methylprednisone, betamethasone, and triamcinolone, they all had positive results on pain and function, but the benefit gradually decreased over a period of 12 weeks [32]. Additionally, they found methylprednisone to be a statistically more effective analgesic relative to the other agents at week 6 [32].
The two most commonly used corticosteroids clinically are triamcinolone and methylprednisolone. They are much preferred since their branched esters reduce the solubility of compounds, thus allowing them to remain in the joint space longer [31]. However, even though triamcinolone has a lower solubility relative to methylprednisone, an RCT comparing these two agents found methylprednisone to have longer-lasting effects, thus proving lower solubility does not always correlate with more sustained effects. In this study, triamcinolone was found to have quicker onset relative to methylprednisolone at week 3, but its effect was lost by week 8. Conversely, methylprednisolone had a slower onset of action but continued to show benefit at week 8 [33]. Another randomized trial that compared the efficacy of triamcinolone hexacetonide and methylprednisolone acetate found both formulations to be equally effective, and the improvement in pain and function can be sustained for up to 24 weeks [34].
IACIs are considered to be safe when the proper aseptic technique is followed. Some reported complications include infection, injection site pain, skin pigmentation, and skin or fat atrophy [31]. Septic arthritis is the most serious complication but rarely occurs. One retrospective cohort study of 22,370 intra-articular injections had only 11 patients developing septic arthritis (0.08%, 95% CI 0.03–0.12) [35]. Osteonecrosis has also been noted in just weeks to months after intra-articular injection of cumulative doses of 80–160 mg of methylprednisolone [36].
However, a more clinically relevant consideration should be the systemic side effects of corticosteroids. Hyperglycemia is most often observed and perhaps consequential for diabetic patients. A systematic review of 7 prospective observational studies that investigated the effect of IACIs in various intra-articular spaces on glucose levels in diabetic patients found a significant rise in glucose levels compared to the baseline [37]. One case series (n = 9) that investigated the effects of intraarticular knee injections in patients with controlled diabetes found increased blood glucose levels following an injection that lasted up to 5 days, which is in concordance with the duration of measurable serum methylprednisolone levels [38]. This effect is further explained by diabetics having significantly reduced cytochrome p450 3A4 expression leading to decreased clearance of glucocorticoids [39].
Studies have found IACIs to cause immunosuppression, ultimately increasing the risk of infection. One retrospective study that investigated the association between intra-articular injections and influenza vaccine effectiveness found that vaccinated patients that received an IACI were at increased risk for developing influenza compared to the vaccinated control group [40]. Therefore, special consideration is warranted for those at risk. As far as infection risk to patients undergoing total knee arthroplasty, evidence indicates the risk is greater in patients who received intra-articular injections within the last 3 months before surgery compared to the control group, while there was no significant difference beyond that period [41]. Commonly accepted practice is to delay surgery for 3 months following an IACI.
Lastly, IACIs are thought to accelerate the degeneration of cartilage, although evidence is somewhat conflicting. One study found no difference between treatment and placebo groups in joint space changes over two years of follow-up [42]. Another RCT, however, found significantly greater cartilage loss measured by MRI in patients who received 2 years of intra-articular triamcinolone compared to those that received intra-articular saline [43]. Therefore, IACIs should be performed judiciously only when needed rather than being established as a routine part of a treatment plan.
IACIs are considered to be safe and effective in treating painful knee OA, especially short-term. It is certainly an essential part of the treatment algorithm. Proper techniques and image guidance may improve safety and efficacy while carefully considering the side effects and potential complications when such therapy is offered.
Viscosupplementation
An alternative agent to corticosteroids for knee IACI is hyaluronic acid (HA) which can act as a lubricant or shock absorber by increasing the viscoelasticity of synovial fluid [44]. HA is an intrinsic component of the synovial fluid and cartilage. In addition to providing a cushion between the bones, it serves as a pathway for cell migration, especially for the chondrocytes, perhaps enhancing their proliferation and differentiation [45]. During a degenerative process, the quality and quantity of HA are compromised by diminished production, rapid fragmentation, and dilution by excessive effusion. It has been shown that the concentration and molecular weight of HA are decreased by 33% and 50%, respectively [46]. Therefore, its ability to protect the knee from mechanical stress is lessened, resulting in further inflammation and pain. The poor quality of HA is a key factor in the degeneration cascade.
The exact mechanism of exogenous HA in reducing pain is incompletely understood. The two most accepted concepts are HA restoration and anti-inflammatory action. Exogenous HA has been shown to improve the function of synovial fluid in terms of lubrication and stress distribution [47]. Anti-inflammatory effects are shown to be achieved by inhibition of phagocytosis by acting as a physical barrier, reducing inflammatory factors such as prostaglandins and fibronectin, and improving leukocyte function [48, 49]. More recent studies suggest a potential role of exogenous HA modifying disease progression of OA. Intra-articular HA has been shown to suppress chondrocyte apoptosis by reducing nitric oxide production, which is associated with chondrocyte death, and inhibit the enzymatic degradation of proteoglycan structure [50].
Many HA products are available and classified by material source, number of required treatments, and production technique, but the main differentiating factor is the molecular weight in terms of efficacy comparison. Hylan g-f20 (Synvisc and Synvisc-One) is derived from rooster comb and unique in its own class in terms of higher molecular weight (6000 kDa) [51]. Another group of HA preparations is sodium hyaluronate (SH) which is a salt form of HA. Many products within are Durolane, Hyalgan (high molecular weight), Euflexxa, Gelsyn (intermediate molecular weight), and Supartz (low molecular weight). SH shows additional anti-inflammatory action that may enhance analgesia. Lastly, formulations derived from hyaluronan include Orthovisc, Monovisc, and Hymovis [52]. Hyaluronan is a high molecular weight extracellular matrix, when injected into a knee, increases the viscoelasticity of synovial fluid, and modulates inflammatory reactions by suppressing gene expression and inhibiting cell migration and adhesion [53].
The clinical outcome of viscosupplementation varies so widely that it is difficult to draw a conclusion at the current time. Among different products, there is some evidence that the higher molecular weight formulations are superior to the ones with lower molecular weight [54]. When comparing single-injection HA to multi-injection products, the efficacy seems equivocal [55]. In a 2006 Cochrane Review, the authors suggested that viscosupplementation is superior to placebo, showing benefits in pain, function, and global assessment [56]. In a recent systemic review of randomized trials comparing HA injections and oral NSAIDs, HA injections showed statistically significant improvements in knee pain and function while having fewer adverse events [57]. However, just as many conflicting evidences exist as Rutjes et al. showed in their systemic review that viscosupplementation is associated with only small and clinically insignificant benefit [58]. Guidelines from various clinical societies also do not strongly support the use of viscosupplementation. While the American Academy of Orthopaedic Surgeons and Osteoarthritis Research Society International offer negative and uncertain recommendations, respectively, the American College of Rheumatology gives no recommendations on the use of HA [59,60,61].
Data from meta-analyses and systemic reviews are contradictory, and the current guidelines are uncertain at best. In addition, the types of HA agents, treatment numbers, and production mechanisms are immensely variable, so it is difficult to devise a consensus on the use and benefit of HA. However, it possesses properties that may counteract the degenerative process of an arthritic knee, provides physical support against mechanical stress, and certainly gives an alternative treatment option to manage knee OA without serious consequences.
Orthobiologics
As we learn more about the importance of cytokines and growth factors in the pathogenesis of OA, “Orthobiologics,” often called regenerative medicine, have been gaining popularity as an alternative to IACIs or viscosupplements as they are believed to slow down disease progression by intervening with inflammatory pathways of these cytokines. [62]. Orthobiologics include but are not limited to, platelet-rich plasma (PRP), bone marrow concentrate (BMAC), and mesenchymal stem cells (MSC) [63].
PRP is an autologous mixture of supraphysiologic concentration of platelets, associated growth factors, and cytokines, prepared by centrifuge of whole blood. PRP has been shown to affect the entire joint environment. Growth factors from PRP increase chondrogenesis and mesenchymal stem cell recruitment and differentiation by promoting matrix synthesis, cell growth and migration, and facilitating protein transcription. PRP also reduces inflammatory process and restore anabolic and catabolic balance in cartilage formation [64]. PRP can be further classified based on the dose of injected platelet (calculated by multiplying the platelet concentration in PRP by the volume of PRP), purity (pure, leukocyte-rich, leukocyte-poor), efficiency (percentage of platelet recovered in the PRP from the blood), and activation process (addition of calcium chloride or autologous thrombin) [64, 65].
PRP injection has shown significant clinical improvements in pain reduction, improved symptoms, and quality of life up to 12 months after PRP injection, supported by many level I clinical trials in all stages of knee OA [63, 64]. In many studies, PRP showed better outcomes compared to HA or placebo in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores and pain [63, 64]. There is limited evidence comparing PRP versus corticosteroid or between different purity PRPs. Despite the apparent benefit of PRP in knee OA, the optimal protocol, such as frequency, number of injections, or concentration, is yet to be determined.
The main difference between blood-derived forms such as PRP compared to stem cells (e.g., BMAC and adipose-derived MSC) is that the former stimulates pre-existing chondrogenic proliferation and differentiation by regulating intra-articular environment when the latter directly injects stem cells that have potential to differentiate into chondral tissue [66••]. Similar to PRP, stem cells also have paracrine and immune-modulating effects through growth factor and cytokine release [67]. Dulic et al. published a randomized controlled trial comparing clinical outcomes of BMAC, PRP, or HA in knee OA treatment. In 12 months, the BMAC group showed superior results in WOMAC, KOOS (Knee injury and Osteoarthritis Outcome Score), KOOS pain, and IKDC (International Knee Documentation Committee) scores [68]. While existing literatures supporting potential benefits of BMAC and adipose-derived MSC are fast-growing, the number of studies is far fewer than PRP and lacks high-quality trials.
Genicular Nerve Neurotomy/Radiofrequency Ablation
The first genicular nerve (GN) radiofrequency ablation (RFA) technique for treating painful knee OA was described by Choi et al. in 2011 [69]. Since then, it has been adopted by many practitioners, and substantial advancements in technique also have been made. Different types of ablation modalities, including thermal, cooled, and bipolar RFAs, are reported and available. In terms of image guidance, fluoroscopy and ultrasound provide benefits of precise target location but come with their own intrinsic limitations. Overall, GN RFA is gaining increasing attention due to its versatility, resulting in less pain and improved function without altering the physiology of the knee.
GN RFA is performed by targeting the nerves innervating the anterior knee joint capsule, the most common ones being the superior lateral genicular nerve (SLGN), superior medial genicular nerve (SMGN), and inferior medial genicular nerve (IMGN). When a needle tip lies in close proximity to the targeted nerve, an inserted electrode delivers a high-frequency current that results in complete denaturation of tissue by heating the surrounding tissue at 80-degree Celsius. While this poses a potential for surrounding tissue injury resulting in periarticular hematoma, damage to tendons, and skin injury, the reported complications from GN RFA are rare and considered safe [70].
A single-blind randomized control trial by El-Hakeim et al. reported that the conventional RFA group had a significant reduction in VAS score (3.13 vs. 5.73) and mean WOMAC scores (33.13 vs. 43.5) at the 6-month follow-up [70]. In a recent meta-analysis of randomized controlled trials, Liu et al. showed that the traditional RFA group compared to the control resulted in a significant reduction in pain score and improvement in knee function at 1–2 weeks, 4 weeks, 12 weeks, and 24 weeks [71]. Cooled RFA shows similar positive results in multiple randomized control trials. Compared to IACIs, 74.1% of cooled RFA subjects reported greater than 50% pain relief versus 16.2% in the injection group at 6 months [72]. Against HA injection, in a multicenter randomized trial, Chen et al. reported that 71% of cooled RF participants had a 50% reduction in NRS pain score while 38% of the HA group achieved the same results [73].
The evidence for GN RFA generally seems favorable. However, some inconsistencies surrounding proper target location and the number of nerves required to treat exist and need to be discussed further. The traditional targets using fluoroscopy initially described by Choi et al. have been challenged recently based on more sound anatomical studies [74, 75]. Also, techniques using ultrasound guidance have been described. Unlike fluoroscopy, ultrasound has the advantage of visualizing vascular structures, which may improve the safety of ablative procedures [76].
A recent anatomical study by Kim et al. illustrated that more than 3 conventionally ablated nerves have innervation to the knee [77]. In fact, some studies suggest as many as 10 nerves supply the anterior knee joint [75]. More recently, Fonkoue et al. showed that targeting additional nerves as in the recurrent fibular nerve and infrapatellar branch of the saphenous nerve resulted in greater pain relief and more number of subjects reporting greater than 50% pain reduction after diagnostic nerve blocks [78•]. While it is unknown whether ablation of more nerves would result in greater pain relief or even logistically possible, it certainly calls for further evaluation in the future refinement of GN RFA.
Conclusion
As the development of painful knee OA is complex, so is its treatment. The main goal of therapy should be focused on improving pain and function while decreasing disease progression. A successful outcome is dependent on many factors, including patient compliance, appropriate treatment algorithm, and proper expectation management. There is no one best modality for all patients with knee OA. It is likely that all available methods are applied in concert to achieve the best results. A synergistic multimodal and multidisciplinary approach is essential and perhaps the only way to meet the needs of knee OA patients presenting with a broad range of disease severity, symptoms, and expected progression.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Martel-Pelletier J, Boileau C, Pelletier J-P, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–84. https://doi.org/10.1016/j.berh.2008.02.001.
Ong KL, Runa M, Lau E, Altman R. cost-of-illness of knee osteoarthritis: potential cost savings by not undergoing arthroplasty within the first 2 years. Clin Econ Outcomes Res. 2019;11:245–55. https://doi.org/10.2147/ceor.s170119.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–89. https://doi.org/10.1016/j.joca.2019.06.011.
Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G, Martel-Pelletier J, Pelletier J-P, Rannou F, Rizzoli R, Roth R, Uebelhart D, Cooper C, Reginster J-Y. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Semin Arthritis Rheum. 2019;49(3):337–50. https://doi.org/10.1016/j.semarthrit.2019.04.008.
Raposo F, Ramos M, Lúcia Cruz A. Effects of exercise on knee osteoarthritis: a systematic review. Musculoskelet Care. 2021;19(4):399–435. https://doi.org/10.1002/msc.1538.
Wang T-J, Lee S-C, Liang S-Y, Tung H-H, Wu S-FV, Lin Y-P. Comparing the efficacy of aquatic exercises and land-based exercises for patients with knee osteoarthritis. J Clin Nurs. 2011;20(17–18):2609–22. https://doi.org/10.1111/j.1365-2702.2010.03675.x.
Wang C, Schmid CH, Iversen MD, Harvey WF, Fielding RA, Driban JB, Price LL, Wong JB, Reid KF, Rones R, McAlindon T. Comparative effectiveness of Tai Chi versus physical therapy for knee osteoarthritis. Ann Intern Med. 2016;165(2):77. https://doi.org/10.7326/m15-2143.
DeVita P, Aaboe J, Bartholdy C, Leonardis JM, Bliddal H, Henriksen M. Quadriceps-strengthening exercise and quadriceps and knee biomechanics during walking in knee osteoarthritis: a two-centre randomized controlled trial. Clin Biomech. 2018;59:199–206. https://doi.org/10.1016/j.clinbiomech.2018.09.016.
Liao C-D, Lin L-F, Huang Y-C, Huang S-W, Chou L-C, Liou T-H. Functional outcomes of outpatient balance training following total knee replacement in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2014;29(9):855–67. https://doi.org/10.1177/0269215514564086.
Kabiri S, Halabchi F, Angoorani H, Yekaninejad S. Comparison of three modes of aerobic exercise combined with resistance training on the pain and function of patients with knee osteoarthritis: a randomized controlled trial. Phys Ther Sport. 2018;32:22–8. https://doi.org/10.1016/j.ptsp.2018.04.001.
Bartels EM, Lund H, Hagen KB, Dagfinrud H, Christensen R, Danneskiold-Samsøe B. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2005. https://doi.org/10.1002/14651858.cd005523.
Lim WB, Al-Dadah O. Conservative treatment of knee osteoarthritis: a review of the literature. World J Orthop. 2022;13(3):212–29. https://doi.org/10.5312/wjo.v13.i3.212.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, MizusakiImoto A, Toupin-April K, Westby M, Álvarez Gallardo IC, Gifford W, Laferrière L, Rahman P, Loew L, de Angelis G, Cavallo S, Shallwani SM, Aburub A’, Bennell KL, van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clin Rehabil. 2017;31(5):612–24. https://doi.org/10.1177/0269215517691085.
•• Goh S-L, Persson MS, Stocks J, Hou Y, Welton NJ, Lin J, Hall MC, Doherty M, Zhang W. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: Systematic review and network meta-analysis. Sports Med. 2019;49(5):743–61. https://doi.org/10.1007/s40279-019-01082-0. This article reviewed the efficacy of different exercise programs for hip and knee OA.
Dong R, Wu Y, Xu S, Zhang L, Ying J, Jin H, Wang P, Xiao L Tong P. Is aquatic exercise more effective than land-based exercise for knee osteoarthritis?. Medicine 2018;97(52). https://doi.org/10.1097/md.0000000000013823
Duivenvoorden T, Brouwer RW, van Raaij TM, Verhagen AP, Verhaar JAN, Bierma-Zeinstra SMA. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.cd004020.pub3.
Squyer E, Stamper DL, Hamilton DT, Sabin JA, Leopold SS. Unloader knee braces for osteoarthritis: do patients actually wear them? Clin Orthop Relat Res. 2013;471(6):1982–91. https://doi.org/10.1007/s11999-013-2814-0.
Messier SP, Resnik AE, Beavers DP, Mihalko SL, Miller GD, Nicklas BJ, deVita P, Hunter DJ, Lyles MF, Eckstein F, Guermazi A, Loeser RF. Intentional weight loss in overweight and obese patients with knee osteoarthritis: is more better? Arthritis Care Res. 2018;70(11):1569–75. https://doi.org/10.1002/acr.23608.
Gersing AS, Schwaiger BJ, Nevitt MC, Zarnowski J, Joseph GB, Feuerriegel G, Jungmann PM, Guimaraes JB, Facchetti L, McCulloch CE, Link TM. Weight loss regimen in obese and overweight individuals is associated with reduced cartilage degeneration: 96-month data from the osteoarthritis initiative. Osteoarthr Cartil. 2019;27(6):863–70. https://doi.org/10.1016/j.joca.2019.01.018.
Aaboe J, Bliddal H, Messier SP, Alkjær T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthr Cartil. 2011;19(7):822–8. https://doi.org/10.1016/j.joca.2011.03.006.
Miceli-Richard C. Paracetamol in osteoarthritis of the knee. Ann Rheum Dis. 2004;63(8):923–30. https://doi.org/10.1136/ard.2003.017236.
Leopoldino AO, Machado GC, Ferreira PH, Pinheiro MB, Day R, McLachlan AJ, Hunter DJ, Ferreira ML. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev 2019;2019(8). https://doi.org/10.1002/14651858.cd013273
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Reston J. American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–33. https://doi.org/10.1002/art.41142.
Ostergaard M, Halberg P. Intra-articular corticosteroids in arthritic disease. BioDrugs. 1998;9(2):95–103. https://doi.org/10.2165/00063030-199809020-00002.
•• Najm A, Alunno A, Gwinnutt JM, Weill C, Berenbaum F. Efficacy of intra-articular corticosteroid injections in knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Joint Bone Spine. 2021;88(4):105198. https://doi.org/10.1016/j.jbspin.2021.105198. This study reviewed efficacy of IACI for knee OA.
He W, Kuang M, Zhao J, Sun L, Lu B, Wang Y, Ma J, Ma X. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg. 2017;39:95–103. https://doi.org/10.1016/j.ijsu.2017.01.087.
Chernchujit B, Tharakulphan S, Apivatgaroon A, Prasetia R. Accuracy comparisons of intra-articular knee injection between the new modified anterolateral approach and superolateral approach in patients with symptomatic knee osteoarthritis without effusion. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2019;17:1–4. https://doi.org/10.1016/j.asmart.2019.02.001.
Sibbitt WL, Kettwich LG, Band PA, Chavez-Chiang NR, DeLea SL, Haseler LJ, Bankhurst AD. Does ultrasound guidance improve the outcomes of ARTHROCENTESIS and corticosteroid injection of the knee? Scand J Rheumatol. 2011;41(1):66–72. https://doi.org/10.3109/03009742.2011.599071.
Fang WH, Chen XT, Vangsness CT. Ultrasound-guided knee injections are more accurate than blind injections: a systematic review of randomized controlled trials. Arthrosc Sports Med Rehabil 2021;3(4). https://doi.org/10.1016/j.asmr.2021.01.028
Amber KT, Landy DC, Amber I, Knopf D, Guerra J. Comparing the accuracy of ultrasound versus fluoroscopy in Glenohumeral injections: a systematic review and meta-analysis. J Clin Ultrasound. 2014;42(7):411–6. https://doi.org/10.1002/jcu.22154.
Cole BJ, Schumacher RH. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13(1):37–46. https://doi.org/10.5435/00124635-200501000-00006.
Yavuz U, Sökücü S, Albayrak A, Öztürk K. Efficacy comparisons of the intraarticular steroidal agents in the patients with knee osteoarthritis. Rheumatol Int. 2011;32(11):3391–6. https://doi.org/10.1007/s00296-011-2188-0.
Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone HEXACETONIDE and methylprednisolone acetate. Clin Rheumatol. 2004;23(2):116–20. https://doi.org/10.1007/s10067-003-0841-z.
Lomonte AB, de Morais MG, de Carvalho LO, Zerbini CA. Efficacy of triamcinolone hexacetonide versus methylprednisolone acetate intraarticular injections in knee osteoarthritis: a randomized, double-blinded, 24-week study. J Rheumatol. 2015;42(9):1677–84. https://doi.org/10.3899/jrheum.150297.
Petersen SK, Andreasen RA, Hansen IMJ. SAT0565 the frequency of septic arthritis after arthrocentesis and intra articular glucocorticoid injection is low. Poster Presentations. 2017. https://doi.org/10.1136/annrheumdis-2017-eular.1333.
Yamamoto T, Schneider R, Iwamoto Y, Bullough PG. Rapid destruction of the hip joint in osteoarthritis. Ann Rheum Dis. 2008;67(12):1783–4. https://doi.org/10.1136/ard.2007.085019.
Choudhry MN, Malik RA, Charalambous CP. Blood glucose levels following intra-articular steroid injections in patients with diabetes. JBJS Rev. 2016;4(3). https://doi.org/10.2106/jbjs.rvw.o.00029
Habib GS, Bashir M, Jabbour A. Increased blood glucose levels following intra-articular injection of methylprednisolone acetate in patients with controlled diabetes and symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2008;67(12):1790–1. https://doi.org/10.1136/ard.2008.089722.
Stout A, Friedly J, Standaert CJ. Systemic absorption and side effects of locally injected glucocorticoids. PM&R. 2019;11(4):409–19. https://doi.org/10.1002/pmrj.12042.
Sytsma TT, Greenlund LK, Greenlund LS. Joint corticosteroid injection associated with increased influenza risk. Mayo Clin Proc Innov Qual Outcomes. 2018;2(2):194–8. https://doi.org/10.1016/j.mayocpiqo.2018.01.005.
Yang X, Li L, Ren X, Nie L. Do preoperative intra-articular injections of corticosteroids or hyaluronic acid increase the risk of infection after total knee arthroplasty? A meta-analysis. Bone Joint Res. 2022;11(3):171–9. https://doi.org/10.1302/2046-3758.113.bjr-2021-0350.r1.
Raynauld J-P, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay J-L, Bertrand C, Pelletier J-P. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370–7. https://doi.org/10.1002/art.10777.
McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, Ward RJ. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis. JAMA. 2017;317(19):1967. https://doi.org/10.1001/jama.2017.5283.
Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32(1):10–37. https://doi.org/10.1053/sarh.2002.33720.
Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis. Am J Sports Med. 2009;37(8):1636–44. https://doi.org/10.1177/0363546508326984.
Watterson JR, Esdaile JM. Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg. 2000;8(5):277–84. https://doi.org/10.5435/00124635-200009000-00001.
Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9.
Forrester JV, Balazs EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40(3):435–46.
Ghosh P. The role of hyaluronic acid (hyaluronan) in health and disease: interactions with cells, cartilage and components of synovial fluid. Clin Exp Rheumatol. 1994;2(1):75–82.
Echigo R, Mochizuki M, Nishimura R, Sasaki N. Suppressive effect of hyaluronan on chondrocyte apoptosis in experimentally induced acute ostaoarthritis in dogs. J Vet Med Sci. 2006;68(8):899–902. https://doi.org/10.1292/jvms.68.899.
Medical Advisory Secretariat. Intra-articular viscosupplementation with hylan g-f 20 to treat osteoarthritis of the knee: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(10):1–66.
Peck J, Slovek A, Miro P, Vij N, Traube B, Lee C, Berger AA, Kassem H, Kaye AD, Sherman WF, Abd-Elsayed A. A comprehensive review of viscosupplementation in osteoarthritis of the knee. Orthopedic Rev 2021;13(2). https://doi.org/10.52965/001c.25549
Richardson C, Plaas A, Block JA. Intra-articular hyaluronan therapy for symptomatic knee osteoarthritis. Rheum Dis Clin North Am. 2019;45(3):439–51. https://doi.org/10.1016/j.rdc.2019.04.011.
Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158–65. https://doi.org/10.1177/0363546515609599.
Vincent P. Intra-articular hyaluronic acid in the symptomatic treatment of knee osteoarthritis: a meta-analysis of single-injection products. Curr Ther Res. 2019;90:39–51. https://doi.org/10.1016/j.curtheres.2019.02.003.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2005. https://doi.org/10.1002/14651858.cd005321.
Miller LE, Fredericson M, Altman RD. Hyaluronic acid injections or oral nonsteroidal anti-inflammatory drugs for knee osteoarthritis: systematic review and meta-analysis of randomized trials. Orthop J Sports Med. 2020;8(1):232596711989790. https://doi.org/10.1177/2325967119897909.
Rutjes AWS, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee. Ann Intern Med. 2012;157(3):180. https://doi.org/10.7326/0003-4819-157-3-201208070-00473.
McGrory BJ, Weber KL, Jevsevar DS, Sevarino K. Surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2016;24(8). https://doi.org/10.5435/jaaos-d-16-00159
McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22(3):363–88. https://doi.org/10.1016/j.joca.2014.01.003.
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–74. https://doi.org/10.1002/acr.21596.
Coşkun HS, Yurtbay A, Say F. Platelet rich plasma versus autologous conditioned serum in osteoarthritis of the knee: clinical results of a five-year retrospective study. Cureus. 2022. https://doi.org/10.7759/cureus.24500.
Crane DM, Oliver KS, Bayes MC. Orthobiologics and knee osteoarthritis. Phys Med Rehabil Clin N Am. 2016;27(4):985–1002. https://doi.org/10.1016/j.pmr.2016.07.004.
O’Connell B, Wragg NM, Wilson SL. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019;376(2):143–52. https://doi.org/10.1007/s00441-019-02996-x.
Magalon J, Chateau AL, Bertrand B, Louis ML, Silvestre A, Giraudo L, Veran J, Sabatier F. Depa classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exercise Med 2016;2(1). https://doi.org/10.1136/bmjsem-2015-000060
•• Delanois RE, Sax OC, Chen Z, Cohen JM, Callahan DM, Mont MA. Biologic therapies for the treatment of knee osteoarthritis: an updated systematic review. J Arthroplasty. 2022. https://doi.org/10.1016/j.arth.2022.05.031. This article reviewed efficacy of Orthobiologic injections for knee OA.
Pas HIMFL, Winters M, Haisma HJ, Koenis MJJ, Tol JL, Moen MH. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017;51(15):1125–33. https://doi.org/10.1136/bjsports-2016-096793.
Dulic O, Rasovic P, Lalic I, Kecojevic V, Gavrilovic G, Abazovic D, Maric D, Miskulin M, Bumbasirevic M. Bone marrow aspirate concentrate versus platelet rich plasma or hyaluronic acid for the treatment of knee osteoarthritis. Medicina. 2021;57(11):1193. https://doi.org/10.3390/medicina57111193.
Choi W-J, Hwang S-J, Song J-G, Leem J-G, Kang Y-U, Park P-H, Shin J-W. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;152(3):481–7. https://doi.org/10.1016/j.pain.2010.09.029.
Zachary L McCormick, MD, Jaymin Patel, MD, Aaron Conger, DO, Clark C Smith, MD, MPH The Spine Intervention 'Society’s Patient Safety Committee, The Safety of Genicular Nerve Radiofrequency Ablation, Pain Medicine. 2021;22(2)
El-Hakeim EH, Elawamy A, Kamel EZ, Goma SH, Gamal RM, Ghandour AM, Osman AM, Morsy KM. Fluoroscopic guided radiofrequency of genicular nerves for pain alleviation in chronic knee osteoarthritis: a single-blind randomized controlled trial. Pain Physician.
Liu J, Wang T, Zhu ZH. Efficacy and safety of radiofrequency treatment for improving knee pain and function in knee osteoarthritis: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2022;17(1):21.
Hunter C, Davis T, Loudermilk E, Kapural L, DePalma M. Cooled radiofrequency ablation treatment of the genicular nerves in the treatment of osteoarthritic knee pain: 18- and 24-month results. Pain Pract. 2020;20(3):238–46.
Chen AF, Khalouf F, Zora K, DePalma M, Kohan L, Guirguis M, Beall D, Loudermilk E, Pingree M, Badiola I, Lyman J. Cooled radiofrequency ablation compared with a single injection of hyaluronic acid for chronic knee pain. J Bone Joint Surg. 2020;102(17):1501–10. https://doi.org/10.2106/jbjs.19.00935.
Fonkoue L, Behets C, Steyaert A, Kouassi JEK, Detrembleur C, Cornu O. Anatomical study of the descending genicular artery and implications for image-guided interventions for knee pain. Clin Anat. 2020;34(4):634–43. https://doi.org/10.1002/ca.23680.
Tran J, Peng PWH, Lam K, Baig E, Agur AMR, Gofeld M. Anatomical study of the innervation of anterior knee joint capsule. Reg Anesth Pain Med. 2018;43(4):407–14. https://doi.org/10.1097/aap.0000000000000778.
Le PU. Is genicular nerve radiofrequency ablationsafe? A literature review and anatomicalstudy. July 2016. 2016; 5;19(5;19). https://doi.org/10.36076/ppj/2019.19.e679
• Kim JH, Shustorovich A, Arel AT, Downie SA, Cohen SP, Kim SY. Genicular nerve anatomy and its implication for new procedural approaches for knee joint denervation: a cadaveric study. Pain Med. 2021;23(1):144–51. https://doi.org/10.1093/pm/pnab238. This study showed the anatomic course of genicular nerves and proposed a new target for genicular RFA procedure.
Fonkoue L, Steyaert A, Kouame J-EK, Bandolo E, Lebleu J, Fossoh H, Behets C, Detrembleur C, Cornu O. A comparison of genicular nerve blockade with corticosteroids using either classical anatomical targets vs revised targets for pain and function in knee osteoarthritis: a double-blind, randomized controlled trial. Pain Med. 2021;22(5):1116–26. https://doi.org/10.1093/pm/pnab014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.H., Ugur, E. & Kim, S.Y. Non-surgical Treatment Recommendations for Knee Osteoarthritis. Curr Phys Med Rehabil Rep 11, 335–343 (2023). https://doi.org/10.1007/s40141-023-00408-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-023-00408-4