Abstract
Dysphagia is common in patients with dementia of varying types and often results in serious health consequences, including malnutrition, dehydration, aspiration pneumonia, and even death. Due to progressive cognitive and functional decline, patients with dementia experience difficulties throughout the eating process which encompasses all aspects of self-feeding and swallowing function. Variations in underlying neuropathology and disease severity may influence specific swallow disorders observed. New functional neuroimaging modalities offer exciting possibilities to increase understanding of neural control of swallowing that will lead to design of novel treatments. Current treatment approaches include a combination of compensatory strategies for swallowing, cueing from caregivers, and modifications to the dining experience that maximize independence during mealtime. A new focus on development of treatment regimens, possibly involving taste and smell receptor stimulation and rehabilitative exercise that may be implemented during the prodromal stages of dementia, is necessary to prevent or delay further swallowing decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is a broad term for a progressive decline in cognitive function, including memory, reasoning, and problem-solving, that results in an inability to perform activities of daily living [1]. Neurodegeneration in the form of a variety of underlying pathologies leads to the collection of symptoms that comprise dementia. There are various types of dementia, with Alzheimer’s disease (AD), vascular dementia (VaD), dementia with Lewy bodies (DLB), and frontotemporal lobe dementia (FTLD) as the most common [1–3]. Mild cognitive impairment (MCI) refers to changes in cognitive status, which are beyond what is expected with normal aging but not severe enough to impact activities of daily living [4]. This condition may be a prodromal stage of AD [5]. Following a systematic review and meta-analysis, it was estimated that approximately 35.6 million people in 2010 were living with dementia world-wide [1]. Its prevalence is known to increase exponentially with age [1, 2, 6], doubling with every 5.5 year increment in North America [1]. Due to population growth and an increasing aging demographic, projections for the total number of individuals with dementia are nearly to double every 20 years, increasing to 65.7 million in 2030 and 115.4 million in 2050 globally [1].
There is a growing concern over the pervasiveness of dysphagia, or swallowing disorders, in patients with dementia [7, 8••, 9]. Dysphagia refers to the difficulty in moving food, liquid, secretions, or medications through the mouth and throat, and into the esophagus. This debilitating condition often leads to serious negative health-related sequelae, including aspiration pneumonia, malnutrition, and dehydration [10–12]. Horner et al. [13] estimated that up to 45 % of institutionalized patients with dementia suffer from dysphagia. Pneumonia is the second most common cause for death in patients with AD [14], and aspiration pneumonia specifically has been found to be significantly and independently associated with dementia [15]. Along with other factors including mobility, nutritional status, and host immune response, aspiration has been identified as a risk factor for discontinuation of oral intake as well as the development of pneumonia in patients diagnosed with AD and DLB [16, 17].
Patients with dementia experience difficulties throughout the entire eating process which includes self-feeding (transferring food from the table to the mouth) in addition to swallowing (transferring food from the mouth into the stomach) [7]. With approximately 50 % of patients with AD losing the ability to feed themselves within 8 years following their diagnosis [18], this inability to eat safely and efficiently is the most life-threatening of all functional impairments for these patients [19–23]. The purpose of this paper is to (1) provide a current overview of the physiological and neurophysiological changes in swallowing that occur with dementia, (2) improve understanding of the neuropathology underlying such changes, and (3) suggest optimal current and future treatment approaches based on this information.
Eating and Swallowing Difficulties in Patients with Dementia
The process of eating encompasses cognitive awareness of the eating situation, visual recognition of food, and physiologic responses to the smell and presence of food that all lead up to the act of swallowing [24]. Self-feeding, in terms of recognition of appropriate items to eat, planning the execution of what should be transported to the mouth, how it will be transported, and in what amount comprises a critical skill set within this eating process [7, 25••]. Normal swallowing consists of a patterned sensorimotor process or response governed by a complex neural network involving both somewhat automatic and volitional systems that respond to sensory inputs [26]. Abnormalities in the swallow may develop as a result of sensory and/or motor damage [26, 27]. Additionally, impairments in cognitive flexibility and attention have been shown to affect swallowing safety [28]. Patients not oriented to person, place, or time are at a 31 % increased risk for liquid aspiration and those unable to follow single-step verbal commands have a 57 and 48 % increased risk for liquid and puree aspiration, respectively [29].
Age-Related Changes in Swallowing
Age-related changes in swallow function, a.k.a. presbyphagia [30], are partially due to the effects of sarcopenia, the degenerative loss of skeletal muscle mass, quality, and strength with aging, of the head and neck musculature [31•]. Given the increase in prevalence of dementia with age, it might be assumed that swallowing changes occur as part of this normal aging process [27]. However, the prevalence of swallowing impairment has been shown to be higher in elderly patients with AD than in normal elderly individuals [8••, 13, 18]. Eating behavior in patients with dementia can be influenced by disorders of attention [27], initiation [32], orientation [29, 33], recognition, executive function [34], decision-making [27], and apraxia [25••, 35].
Eating Dependency
The presence of dysphagia, aspiration, and eating dependency is negatively associated with the severity of dementia [13, 18, 36]. In order to improve understanding of the specific deficits in eating that occur in early stage dementia, Priefer and Robbins [7] designed a study to examine differences in the eating process between those diagnosed with mild AD (10 patients) and a group of normal elderly subjects (15 controls). A standard meal was developed, designed to represent everyday challenges, and videotaped for each subject and his/her spouse to assess self-feeding behaviors. Self-feeding dependency was determined through the frequency of successful cueing behaviors and/or direct assistance from the spouse. Results showed AD subjects to receive significantly more self-feeding cues or direct assistance from their eating partner enabling dependency, than control subjects. Patients with moderate and severe AD have been found to display passivity and distraction during meals as well as inappropriate feeding velocity and refusal of food [37]. Factors that have been found to influence independence in eating among elderly patients with AD include difficulty in beginning a meal, severity of dementia, and the presence of dysphagia signs [25••].
It is known that those with dementia who need to be fed or cued during meals are at greater risk of illness and mortality than those who can feed themselves [38, 39]. A patient’s degree of functional dependency has been shown to be an important predictor for the occurrence of aspiration pneumonia in institutionalized patients [40, 41]. When a caregiver is initiating and continuing the feeding, the patient lacks control over the coordination with his/her own swallow timing which he or she may not be able to communicate [37]. Refusal of food may be related to a lack of understanding of the feeding situation or could even be an oral apraxia resulting in an inability to open the mouth that is misinterpreted by a caregiver as refusal [37].
Swallowing Changes by Dementia Type
Changes in swallowing physiology that occur with dementia have been analyzed through recordings of videofluoroscopic swallowing examinations (VFSE) in patients with varying types of dementia [7] (see Table 1). Findings for a heterogeneous group of patients with dementia [AD, VaD, Parkinson’s disease dementia (PDD)] included aspiration of liquids, incomplete laryngeal closure, and impaired inversion of the epiglottis [42]. However, the specific swallowing disorders observed depend upon the type and severity of dementia. In those patients with mild AD, specifically, Preifer and Robbins [7] found significant group differences in the oral transit duration (OTD) for solid boluses and in the pharyngeal response durations (PRD) and total swallow durations (TSD) for liquid boluses. Since the OTD measure is under voluntary control and represents posterior movement of the bolus in the oral cavity, a longer duration of OTD may reflect difficulties with initiation of oral bolus preparation and of the swallow [7]. Humbert et al. [8••] found patients with mild AD to have reduced hyolaryngeal movement as compared to an age-matched control group, although swallow safety was intact with no aspiration observed. As the disease progresses, patients with moderate AD have been found to develop inadequate clearance of the pharynx, reduced upper esophageal opening, and occurrences of airway penetration and/or aspiration [13].
Different patterns of swallowing changes with AD patients as compared to VaD patients have been demonstrated [43]. AD patients have been found to have significantly longer oral transit delays with liquids, while VaD patients experience more difficulty with formation and mastication of semi-solid boluses, movement of the hyolaryngeal complex, and inversion of the epiglottis [43]. The authors hypothesized that these differences are due to the specifics of the neuropathology and location of brain lesions, with sensory impairment in AD patients due to temporoparietal damage versus motor impairment in VaD patients due to corticobulbar tract disruption [43].
DLB patients have been shown to have a higher incidence of self-reported eating and swallowing problems as compared to AD patients [44]. Londos et al. [45•] examined subjective complaints of dysphagia as swallow dysfunction in a group of 82 patients with either DLB or PDD. Thirty-two percent of the patients in this study reported symptoms of dysphagia and 92 % of those patients had documented swallowing dysfunction on VFSE. In contrast to those patients with AD, pharyngeal abnormalities, specifically delayed pharyngeal initiation, residue, and penetration/aspiration, were more common than oral dysfunction in this group [45•].
Patients with frontotemporal lobar dementia (FTLD) also demonstrate unique changes in eating and swallowing. A small group of patients were found to eat rapidly and compulsively and to take large bolus sizes [46]. Results of flexible endoscopic examinations of swallowing (FEES) also showed these patients to have more frequent occurrences of early leakage of food into the pharynx during mastication as well as incomplete clearance of the bolus from the pharynx [46].
Salivary Alterations and Oral Health in Patients with Dementia
The development of aspiration pneumonia in elders has been shown to be multifactorial with poor oral health status as an important contributor [41]. Adequate production of saliva is necessary to ensure gingival integrity and to protect against bacterial overgrowth in the oral cavity [47]. Along with dysphagia, patients with AD have been found to produce significantly reduced amounts of saliva as compared to healthy control subjects [48], which may be due to autonomic nervous system dysfunction in these patients [49]. In a study of 134 geriatric patients, Terpenning et al. [50] confirmed the presence of pneumonia in 36 % of inpatients and 25 % of long-term care patients with hyposalivation; 11 % of long-term care patients with adequate saliva production developed pneumonia. This link between inadequate saliva production and pneumonia is likely due to higher levels of oral bacteria in saliva and altered composition of salivary flora in patients [50, 51]. Elders with dementia have been found to have worse oral hygiene and health than those without dementia [52•, 53]. In dysphagic patients with dementia, saliva itself or in combination with food or liquid may be aspirated leading to repeated entry of these bacteria into the lungs [41, 54].
In addition to maintenance of oral health and tissue integrity, an adequate balance in the production and composition of saliva is necessary for mastication and lubrication of oral structures during swallowing [55]. In contrast to those patients with AD who experience hyposalivation, some patients who are treated with cholinesterase inhibitors, such as donepezil, have been reported to experience sialorrhea, or hypersalivation. Either direction of change in amount of saliva produced will impact salivary composition negatively, affecting oral sensation, specifically taste and texture perception [56]. Alterations in saliva that occur in elderly patients are thought to affect the ability to initiate a swallow [55] which may result in avoidance of certain foods or decreased tolerance for certain consistencies or flavors of food and/or liquid. Interestingly, recent research on saliva levels of amyloid beta peptides [57] and salivary tau species [58] in patients with AD and healthy controls presents some support for the utility of salivary biomarkers in the diagnosis of dementia.
Neuroanatomical Correlates of Dementia
As alluded to previously, the specific changes in the eating process, including self-feeding and swallowing, depend upon the underlying neurophysiologic changes characteristic of each dementia type as well as stage in the progression of the disease. All dementia types are characterized by neuroanatomical changes. These changes typically occur in different brain regions and involve different abnormalities identified across the dementias. Most of the evidence on these neuroanatomical correlates stems from research involving either neuropathological/autopsy findings [59, 60] or, more recently, neuroimaging research [61]. Table 2 presents evidence of the main brain areas involved in the most common types of dementia and the primary abnormalities identified in each type.
Swallowing Neural Network—Possible Implications in Dementias
From a series of in vivo swallowing neuroimaging studies in healthy adults, a swallowing neural network has been identified [62–64, 65••]. This network most commonly includes areas such as the primary sensorimotor cortex, the supplementary motor area, the insula, the frontal operculum, and the anterior cingulate gyrus [62–64, 65••].
Although, the swallowing network does not appear to be implicated in the initial stages of most dementia types, as mentioned previously, swallowing and feeding challenges may be present in patients with dementia early on and typically become more severe as the disease progresses. This is partially because eating encompasses both functions of self-feeding and swallowing, which depend on intact sensorimotor pathways and spared cognitive and executive functions. Although the specific sensorimotor pathways remain unaffected until the later stages of the disease, the cognitive and executive function limitations can influence swallowing and self-feeding in earlier stages. For example, a patient with early AD with temporoparietal cortex neuropathology may experience spatial and perceptual difficulties and may forget how to use a utensil during meals; in comparison, a patient with early FTLD with frontotemporal involvements may exhibit social and behavioral symptoms, such as impulsivity, agitation, or apathy during eating. In later stages when diffuse brain lesions are seen across dementia types, the sensorimotor aspects of the swallowing sequence also are influenced.
Neuroimaging of Swallowing in Dementia
To date only a handful of studies have investigated the neural control of swallowing in patients with dementia. Humbert and colleagues [8••] studied the swallowing and neural physiology of a group of 13 patients with early AD and a group of 11 age-matched controls using task-fMRI. Results suggested that the AD group had significantly decreased fMRI signal intensity during swallowing in important swallowing network areas, such as the primary sensorimotor cortex, and the Rolandic and frontal opercula bilaterally [8••]. Swallowing physiology was minimally affected in the AD group, possibly suggesting that sub-clinical swallowing neural changes may be present before clinically significant swallowing changes are observed.
In a similar paradigm, the same group of investigators studied the neural activity elicited during the command to not swallow (swallowing inhibition) in an early AD group and a group of healthy young and older adults. The AD group showed increased activation of two swallowing network areas, the frontal operculum and the insula, during this swallowing inhibitory task compared to the other two groups [66]. According to the authors, this finding may indicate the need for increased neural “effort” to turn off the swallowing network areas in these patients.
Challenges and Promising Directions of Swallowing Neuroimaging in Dementia
The use of task-fMRI and other task-related neuroimaging modalities to identify the neural control of swallowing in dementia has been limited for several reasons. First, in swallowing fMRI protocols, the task is often cued by visual or audio stimuli and the subject must perform the task in strict compliance with the stimulus, which may be challenging for a patient with cognitive limitations. Additionally, one of the biggest challenges in completing motor task-fMRI paradigms is the fact that the signal is prone to motion artifacts. In task-fMRI studies of the neural control of human swallowing, subjects are required to swallow in the supine position many times during an experiment. The lingual, mandibular, and hyolaryngeal movements during the completion of a swallow can cause significant head movement, which may interfere with the brain activity signal [67]. Furthermore, monitoring devices including surface electrodes, pneumographic belts placed around the neck, or oral pressure tubing are needed to ensure that the subjects’ swallows comply with the stimuli. Such devices may result in sensory feedback, interfering with normal muscle function, motor planning, and even brain activations [68] and may not be tolerated by patients with dementia.
In recent years, other MR modalities have emerged as useful in investigating neural correlates of dementia and have provided important clinical insights in the diagnosis of dementia and MCI and could offer promising solutions in the neural investigation of swallowing in these populations. Two of the most widely used include resting-state functional connectivity MRI (fcMRI) and diffusion tensor imaging (DTI).
Resting-state fcMRI allows for investigation of brain activity at rest when individuals are not required to perform a task. Thus, it is not dependent on task completion or performance in the magnet. This allows us to use fMRI to investigate the brain networks of clinical populations, such as patients with dementia, for whom it would be challenging or impossible to complete a task-related fMRI paradigm due to cognitive or behavioral limitations. Functional connectivity MRI has revealed that patients with AD and MCI at high risk for developing AD have decreased default mode network (DMN) connectivity [69], i.e., a network of brain areas that are typically active at rest in healthy individuals and represent our internal thoughts and representation. Functional connectivity of the DMN appears to be linked with several mental and motor disorders and appears to be a promising and easily obtained clinical marker of disease.
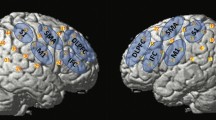
Regarding swallowing, we recently completed a pilot resting-state fcMRI study on a group of healthy elders and two patients with mild AD in order to indirectly examine the swallowing network areas functional connectivity in correlation with measures of swallowing function [70]. Figure 1 presents the functional connectivity results for the two AD participants (without and with dysphagia) with the left primary motor area as the seed/focus of the analysis. For the patient without dysphagia, the left primary motor cortex (significant motor area for swallowing) showed significant and high intensity positive temporal correlations (red and yellow) with the primary motor and sensory cortices, the cingulate gyrus, and the insula bilaterally (all of which are swallowing-related areas). Anti-correlations (blue) are seen primarily with areas of the prefrontal, temporal, and anterior cingulate cortex. These results were similar with the results seen in the group of healthy elders. For the AD patient with dysphagia, however, the analogous positive correlations appeared to be weaker and more widespread, while anti-correlations were primarily seen with few areas of the prefrontal and the visual cortices. These results are qualitative at this time, but show preliminary evidence that the functional connectivity of an important swallowing area is altered in a patient with AD and dysphagia.
Functional connectivity of left primary motor area (seed area) and other brain areas at rest; images are shown in radiological convention (right is shown on the left); A anterior, R right [70]
Diffusion tensor imaging allows for the study of white matter tract integrity and structural connectivity of brain areas and has been widely used in the study of AD and MCI. It also does not require the completion of a task. DTI studies have shown white matter bundles degeneration and correlations of white matter degeneration and disease severity in patients with AD as well as MCI [71]. DTI has not yet been applied in the investigation of the structural connectivity of swallowing-related areas in patients with dementia. The combination of resting-state fMRI and DTI methods could provide a critical non-invasive way of investigating the neural control of swallowing in the clinical and preclinical stages of the dementias and remains to be explored. A more thorough and accurate understanding of changes in the neural control of swallowing in patients with dementia is critically important for the development of more effective treatment techniques.
Approaches to Management of Dysphagia in Patients with Dementia
When determining the most effective approach to management of dysphagia in patients suffering from dementia, it is critical to consider which recommendations and strategies are most likely to encourage independence in eating. As mentioned previously, the degree of a patient’s dependence is a predictor for the development of aspiration pneumonia [40, 41]. Following completion of a comprehensive swallowing evaluation, including a thorough clinical bedside assessment and an instrumental swallowing examination during which specific swallowing disorders are identified, a variety of currently available behavioral interventions can be utilized to improve the safety of the swallow, while new innovative treatments are emerging from brain imaging methods and findings that require testing for effectiveness.
Compensatory and Postural Strategies
Compensatory approaches to treatment are designed to alter the biomechanics of the swallow by redirecting bolus flow but do not alter swallow physiology itself [27, 72]. These techniques include postural adjustments, diet modification, and sensory enhancement. Postural adjustments include the chin tuck, head rotation, head tilt, head back, and side-lying positions of the head that use gravity to influence bolus flow [72]. Their use is required with each swallow which may create problems for patients with dementia suffering from attentional issues and memory loss. Diet modifications typically include alterations in dietary textures and fluid viscosities [73] that may influence the quality of life. Patients with dysphagia and their families may reject these alterations and, given that many patients with dementia already experience changes in taste and smell that affect their appetite [74–76], this rejection may be likely.
In a randomized clinical trial of 711 patients diagnosed with either dementia or Parkinson’s disease, Logemann and colleagues [77] found immediate (during videofluoroscopic swallow assessment) elimination of aspiration on thin liquids to occur most frequently with honey-thickened liquids followed by nectar-thickened liquids and chin-down posture. However, patients preferred the chin-down posture over the thickened liquids. Furthermore, for a subset of these patients followed for 3 months, Robbins et al. [78] reported a more than two-fold hospital admission increase likely due to increased frequency of pneumonia in those who received a honey-thick liquid (3,000 centipoise) versus nectar-thick liquid (300 centipoise) [78]. In addition, the patients who were hospitalized and drinking honey-thick liquids spent three times as many days in the hospital. Therefore, while honey-thick liquids resulted in the most immediate remedy for aspiration, they were not preferred by patients and, when aspirated, resulted in the highest likelihood for developing pneumonia in the long term, as thicker viscosities may be more difficult to eject from the airway. If recommending a modified diet for patients with dementia, specification of the fluid thickness needs to be communicated clearly to the nursing staff, patient, and patient’s family as varying terms are used to describe different types of modified diets [79].
Sensory Techniques
Sensory enhancement techniques, such as alterations to the bolus, oral stimulation, or olfactory enhancement may be effective in eliciting faster oral and pharyngeal initiation of the swallow. Changes to the bolus with regards to taste, temperature, volume, or viscosity may be useful as sensory impairments, including impaired taste perception, are common in patients with dementia [74, 75]. This may involve incorporation of more spicy, sweet, or sour foods into the diets of these patients [27]. Also thermal-tactile stimulation has been used to stimulate the afferent receptors of swallowing but its effects are thought to be short-lived [27, 80].
Environmental and Cognitive Strategies
Other strategies that may be employed to improve the eating process for patients with dementia involve environmental changes along with certain eating and food preparation strategies. The minimization of distractions during mealtime has been found to result in improved nutrition and caloric intake in patients with dementia [81]. Avoiding interruptions and conversations during mealtime, limiting items on the plates or trays to only food items and removing condiments, presenting finger foods for which utensils are not needed, and delaying the presentation of desserts until the end of the meal are all strategies to limit distractions and influence nutritious eating habits [27]. Presenting one food item at a time can be useful and cutting food into bite-sized pieces can encourage pacing of the meal. Patients with dementia also may require a longer time frame to complete their meals [82] and should be encouraged to eat slowly. They may benefit from more frequent, smaller meals or snacks throughout the day [27]. Providing a consistent mealtime routine with the same caregivers in a calm environment is important as well. The use of music mostly of the relaxing type also may provide positive effects on various behavioral symptoms of dementia during mealtime [83].
The encouragement and support of self-feeding is critically important in supporting independence with eating in patients with dementia [27]. As reported in the study by Priefer and Robbins [7] described previously, patients with dementia are likely to receive self-feeding cues or direct assistance from their caregiver or eating partner, referred to as “enabling disability”. These cues were directed mostly to food preparation tasks that are thought to require more conscious thought than the more automatic tasks of transporting food into the mouth from the hand or utensil [7]. With difficulty in beginning a meal as a strong risk factor for the hindrance of eating independence [25••], aiding the patient with cues to begin and restart eating will help prevent confusion and interruption of eating [84]. While cueing and direct assistance (partial to complete assistance) may be helpful in maintaining independence and are frequently recommended for patients with dementia, it is important not to provide assistance beyond what is needed as this can result in dependence [7]. Demonstrating a task or providing hand-over-hand assistance can be effective if necessary.
Several recent studies have supported potential benefits of spaced-retrieval (SR) therapy for increasing food intake in patients with dementia [85••, 86]. SR consists of asking patients to recall presented information at increasing time intervals [85••]. A correct response results in doubling of the time interval to the next trial; while an incorrect response results in sharing of the correct answer and asking to recall it again at the previous time interval resulting in a correct response [85••, 86]. Wu et al. [85] and Lin et al. [87] combined Montessori-based activities (breaking daily activities down into continuous sequential procedures that are repetitively practiced) with SR therapy and found decreased frequency of eating difficulties during meals along with an increase in food intake and body weight over a 6-month follow-up time frame.
Oral Hygiene
The importance of oral hygiene in the care of patients with dementia cannot be over-emphasized. Since it is common for patients with dementia to suffer from poor oral health along with salivary alterations, regular and consistent mouth care is critical in preventing bacterial overgrowth and subsequent development of pneumonia due to aspiration of secretions. These patients will likely require assistance in the implementation of such care.
Tube Feeding
For some patients with advanced dementia, the decision may be made to provide nutrition and hydration via tube feedings [17, 27]. While many speech-language pathologists treating dementia patients for dysphagia believe that feeding via a percutaneous gastrostomy tube (PEG) decreases the risk of aspiration pneumonia [88], several studies have shown that patients with dementia who receive a PEG tube are at continued risk for respiratory disease [89, 90]. Additionally, the presence of a nasogastric tube has been shown to reduce survival in elderly patients with advanced dementia [91]. This decision is complex so it is critically important that benefits and risks concerning tube feeding are discussed thoroughly with the patient and caregivers [17, 27, 88].
Preventative Approaches
Rehabilitative exercises consist of regimens that enact changes in muscle strength and range of motion, and thereby in many cases restore age appropriate “normal” underlying swallowing physiology [92–95]. Some examples include use of the Mendelsohn maneuver, effortful swallow, Shaker exercises, and lingual strengthening [26]. We do not currently have evidence to support the role of such regimens in patients with dementia. However, the focus of current research has shifted from diagnosing and treating dementia in the clinical stages of the disease to identifying preclinical and prodromal signs of dementia in order to provide treatment as early as possible and to delay progression of the disease. In that respect, studying swallowing changes early in the disease process or at preclinical stages may reveal the need to use or create new treatment modalities, including rehabilitative regimens that may prevent or delay the devastating sequelae of swallowing decline.
Conclusions
While dementia has tormented the lives of many individuals and their loved ones, its effect on swallowing and eating only recently is being understood, particularly from a neuropathological standpoint, due to its increasing global prevalence and recent advances in the availability of technology. Diagnostic methods and treatments are found in practice, while evidence-based literature is emerging. Much more innovation in both diagnostic methods and carefully designed intervention studies are promising the new and creative likelihood of prevention and effectiveness of early interventions implemented even at sub-clinical stages.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement J Alzheimers Assoc. 2013;9(1):63–75 e2.
Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old Results from the Canadian Study of Health and Aging. Neurology. 1994;44(9):1593–9.
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7.
Duron E, Vidal J-S, Bounatiro S, Ben Ahmed S, Seux M-L, Rigaud A-S, et al. Relationships between personality traits, medial temporal lobe atrophy, and white matter lesion in subjects suffering from mild cognitive impairment. Front Aging Neurosci. 2014;6:195.
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92.
Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–32.
Priefer BA, Robbins J. Eating changes in mild-stage Alzheimer’s disease: a pilot study. Dysphagia. 1997;12(4):212–21.
•• Humbert IA, McLaren DG, Kosmatka K, Fitzgerald M, Johnson S, Porcaro E, et al. Early deficits in cortical control of swallowing in Alzheimer’s disease. J Alzheimers Dis JAD. 2010;19(4):1185–97. While dysphagia has been traditionally viewed as a late consequence of Alzheimer’s disease, this study was the first to identify changes in swallowing neurophysiology that occur in the preclinical phases of the disease. A group of patients with early stage Alzheimer’s disease were found to demonstrate lower levels of cortical activation in areas traditionally involved in normal swallowing along with reduced hyolaryngeal movement observed on videofluoroscopic recordings.
Alagiakrishnan K, Bhanji RA, Kurian M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: a systematic review. Arch Gerontol Geriatr. 2013;56(1):1–9.
Clavé P, Rofes L, Carrión S, Ortega O, Cabré M, Serra-Prat M, et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestlé NutrInst Workshop Ser. 2012;72:57–66.
Berlinger WG, Potter JF. Low Body Mass Index in demented outpatients. J Am Geriatr Soc. 1991;39(10):973–8.
Watson R. Undernutrition, weight loss and feeding difficulty in elderly patients with dementia: a nursing perspective. Rev Clin Gerontol. 1997;7(04):317–26.
Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1994;8(3):177–89.
Beard CM, Kokmen E, Sigler C, Smith GE, Petterson T, O’Brien PC. Causeofdeath in Alzheimer’s disease. Ann Epidemiol. 1996;6(3):195–200.
Wada H, Nakajoh K, Satoh-Nakagawa T, Suzuki T, Ohrui T, Arai H, et al. Risk factors of aspiration pneumonia in Alzheimer’s disease patients. Gerontology. 2001;47(5):271–6.
Yamamoto T, Kobayashi Y, Murata M. Risk of pneumonia onset and discontinuation of oral intake following videofluorography in patients with Lewy body disease. Parkinsonism Relat Disord. 2010;16(8):503–6.
Chouinard J. Dysphagia in Alzheimer disease: a review. J Nutr Health Aging. 2000;4(4):214–7.
Volicer L, Seltzer B, Rheaume Y, Karner J, Glennon M, Riley ME, et al. Eating difficulties in patients with probable dementia of the Alzheimer type. J Geriatr Psychiatr Neurol. 1989;2(4):188–95.
Sitzmann JV, Mueller R. Enteral and parenteral feeding in the dysphagic patient. Dysphagia. 1988;3(1):38–45.
Bucht G, Sandman PO. Nutritional aspects of dementia, especially Alzheimer’s disease. Age Ageing. 1990;19(4):S32–6.
Ciocon JO. Indications for tube feedings in elderly patients. Dysphagia. 1990;5(1):1–5.
Litchford MD, Wakefield LM. Nutrient intakes and energy expenditures of residents with senile dementia of the Alzheimer’s type. J Am Diet Assoc. 1987;87(2):211–3.
Singh S, Mulley GP, Losowsky MS. Why are Alzheimer patients thin? Age Ageing. 1988;17(1):21–8.
Logemann J. Evaluation and treatment of swallowing disorders. 2nd ed. Austin: PRO-ED, Incorporated; 1998.
•• Edahiro A, Hirano H, Yamada R, Chiba Y, Watanabe Y, Tonogi M, et al. Factors affecting independence in eating among elderly with Alzheimer’s disease. Geriatr Gerontol Int. 2012;12(3):481–90. Results of this study identified difficulty in beginning a meal, presence of signs of dysphagia, and dementia severity as factors that hinder independence in eating in patients with Alzheimer’s disease. The authors suggest eliminating environmental factors that could affect ability to begin a meal and providing assistance with this process.
Robbins J, Butler SG, Daniels SK, Diez Gross R, Langmore S, Lazarus CL, et al. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res JSLHR. 2008;51(1):S276–300.
Easterling CS, Robbins E. Dementia and dysphagia. Geriatr Nur (Lond). 2008;29(4):275–85.
Troche MS, Okun MS, Rosenbek JC, Altmann LJ, Sapienza CM. Attentional resource allocation and swallowing safety in Parkinson’s disease: a dual task study. Parkinsonism Relat Disord. 2014;20(4):439–43.
Leder SB, Suiter DM, Lisitano Warner H. Answering orientation questions and following single-step verbal commands: effect on aspiration status. Dysphagia. 2009;24(3):290–5.
Cite [Internet]. Wikipedia, the free encyclopedia. 2014 [cited 2014 Aug 22]. Available from: http://en.wikipedia.org/w/index.php?title=Special:Cite&page=Presbyphagia&id=593363161.
• Buehring B, Hind J, Fidler E, Krueger D, Binkley N, Robbins J. Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J Am Geriatr Soc. 2013;61(3):418–22. This study revealed positive correlations between isometric tongue pressure and grip strength, jump height, and power in older adults with and without sarcopenia. These results support inclusion of oropharyngeal functional decline as part of the sarcopenia syndrome.
Watson R, Deary IJ. Measuring feeding difficulty in patients with dementia: multivariate analysis of feeding problems, nursing intervention and indicators of feeding difficulty. J Adv Nurs. 1994;20(2):283–7.
Gray GE. Nutrition and dementia. J Am Diet Assoc. 1989;89(12):1795–802.
Tully MW, LambrosMatrakas K, Musallam K. The eating behavior scale: a simple method of assessing functional ability in patients with Alzheimer’s disease. J Nutr Health Aging. 1998;2(2):119–21.
LeClerc CM, Wells DL. Use of a content methodology process to enhance feeding abilities threatened by ideational apraxia in people with Alzheimer’s-type dementia. Geriatr Nurs NYN. 1998;19(5):261–7 quiz 268.
Sato E, Hirano H, Watanabe Y, Edahiro A, Sato K, Yamane G, et al. Detecting signs of dysphagia in patients with Alzheimer’s disease with oral feeding in daily life. Geriatr Gerontol Int. 2014;14(3):549–55.
de Correia SM, Morillo LS, Jacob Filho W, Mansur LL. Swallowing in moderate and severe phases of Alzheimer’s disease. Arq Neuropsiquiatr. 2010;68(6):855–61.
Sonies BC. Oropharyngeal dysphagia in the elderly. Clin Geriatr Med. 1992;8(3):569–77.
Bosch X, Formiga F, Cuerpo S, Torres B, Rosón B, López-Soto A. Aspiration pneumonia in old patients with dementia. Prognostic factors of mortality. Eur J Intern Med. 2012;23(8):720–6.
Langmore SE, Skarupski KA, Park PS, Fries BE. Predictors of aspiration pneumonia in nursing home residents. Dysphagia. 2002;17(4):298–307.
Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13(2):69–81.
Feinberg MJ, Ekberg O, Segall L, Tully J. Deglutition in elderly patients with dementia: findings of videofluorographic evaluation and impact on staging and management. Radiology. 1992;183(3):811–4.
Suh MK, Kim H, Na DL. Dysphagia in patients with dementia: Alzheimer versus vascular. Alzheimer Dis Assoc Disord. 2009;23(2):178–84.
Shinagawa S, Adachi H, Toyota Y, Mori T, Matsumoto I, Fukuhara R, et al. Characteristics of eating and swallowing problems in patients who have dementia with Lewy bodies. Int Psychogeriatr IPA. 2009;21(3):520–5.
• Londos E, Hanxsson O, Alm Hirsch I, Janneskog A, Bülow M, Palmqvist S. Dysphagia in Lewy body dementia—a clinical observational study of swallowing function by videofluoroscopic examination. BMC Neurol. 2013;13:140. This article defines swallowing dysfunction as observed with videofluoroscopic swallowing evaluations (VFSE) in a group of patients diagnosed with dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD). Results revealed that almost all DLB or PDD patients with subjective reports of dysphagia had abnormalities in their swallowing on VFSE, majority of the pharyngeal type.
Langmore SE, Olney RK, Lomen-Hoerth C, Miller BL. Dysphagia in patients with frontotemporal lobar dementia. Arch Neurol. 2007;64(1):58–62.
Nederfors T, Isaksson R, Mörnstad H, Dahlöf C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population–relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211–6.
Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
Affoo RH, Foley N, Rosenbek J, Kevin Shoemaker J, Martin RE. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer’s disease: a scoping review of the evidence. J Am Geriatr Soc. 2013;11:2203–13.
Terpenning M, Bretz W, Lopatin D, Langmore S, Dominguez B, Loesche W. Bacterial colonization of saliva and plaque in the elderly. Clin Infect Dis Off Publ Infect Dis Soc Am. 1993;16(Suppl 4):S314–6.
Shay K, Scannapieco FA, Terpenning MS, Smith BJ, Taylor GW. Nosocomial pneumonia and oral health. Spec Care Dent Off Publ Am Assoc Hosp Dent Acad Dent Handicap Am Soc Geriatr Dent. 2005;25(4):179–87.
• Zenthöfer A, Schröder J, Cabrera T, Rammelsberg P, Hassel AJ. Comparison of oral health among older people with and without dementia.Community Dent Health. 2014;31(1):27–31. Results of this study reveal several aspects of oral health, including amount of plaque, and periodontal status, that are worse in a group of older institutionalized individuals with dementia as compared to a group without dementia. These findings in combination with known swallowing dysfunction that occurs in patients with dementia support the importance of regular, thorough oral hygiene for prevention of pneumonia.
Ribeiro GR, Costa JLR, Ambrosano GMB, Garcia RCMR. Oral health of the elderly with Alzheimer’s disease. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(3):338–43.
Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–63.
Loesche WJ, Bromberg J, Terpenning MS, Bretz WA, Dominguez BL, Grossman NS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401–7.
Engelen L, van den Keybus PAM, de Wijk RA, Veerman ECI, Amerongen AVN, Bosman F, et al. The effect of saliva composition on texture perception of semi-solids. Arch Oral Biol. 2007;52(6):518–25.
Bermejo-Pareja F, Antequera D, Vargas T, Molina JA, Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol. 2010;10:108.
Shi M, Sui Y-T, Peskind ER, Li G, Hwang H, Devic I, et al. Salivary tau species are potential biomarkers of Alzheimer’s disease. J Alzheimers Dis JAD. 2011;27(2):299–305.
Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex NYN. 1991;1(1):103–16.
Braak H, Braak E. Neuropathologicalstageing of Alzheimer-related changes. Acta Neuropathol (Berl). 1991;82(4):239–59.
Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H. Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease. Med Clin North Am. 2013;97(3):399–424.
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, et al. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277(1 Pt 1):G219–25.
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85(2):938–50.
Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, et al. Neurophysiology of swallowing: effects of age and bolus type. NeuroImage. 2009;44(3):982–91.
•• Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30(10):3209–26. This article presents a summary of research studies that have used functional Magnetic Resonance Imaging (fMRI) to study the neural control of swallowing in normal subjects and patients with dysphagia. Methodologic challenges and caveats are discussed along with future directions for use of fMRI in swallowing-related research and clinical practice.
Humbert IA, McLaren DG, Malandraki G, Johnson SC, Robbins J. Swallowing intentional off-state in aging and Alzheimer’s disease: preliminary study. J Alzheimers Dis JAD. 2011;26(2):347–54.
Malandraki GA, Johnson S, Robbins J. Functional MRI of swallowing: from neurophysiology to neuroplasticity. Head Neck. 2011;33(Suppl 1):S14–20.
Paine TL, Conway CA, Malandraki GA, Sutton BP. Simultaneous dynamic and functional MRI scanning (SimulScan) of natural swallows. Magn Reson Med. 2011;65(5):1247–52.
Hafkemeijer A, van der Grond J, Rombouts SARB. Imaging the default mode network in aging and dementia. Biochim Biophys Acta. 2012;1822(3):431–41.
Malandraki GA, Nair VA, Hind J, Prabhakaran V, Lye YH, Robbins J. Resting-state functional connectivity MRI of swallowing network areas: preliminary differences between healthy elders and patients with mild Alzheimer’s disease. Annual Dysphagia Research Society Meeting in Seattle, WA; 2013.
Hess CP. Update on diffusion tensor imaging in Alzheimer’s disease. Magn Reson Imaging Clin N Am. 2009;17(2):215–24.
Rogus-Pulia N, Robbins J. Approaches to the rehabilitation of dysphagia in acute poststroke patients. Semin Speech Lang. 2013;34(3):154–69.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke J Cereb Circ. 2005;36(12):2756–63.
Behrman S, Chouliaras L, Ebmeier KP. Considering the senses in the diagnosis and management of dementia. Maturitas. 2014;77(4):305–10.
Aliani M, Udenigwe CC, Girgih AT, Pownall TL, Bugera JL, Eskin MNA. Aroma and taste perceptions with Alzheimer disease and stroke. Crit Rev Food Sci Nutr. 2013;53(7):760–9.
Roqué M, Salvà A, Vellas B. Malnutrition in community-dwelling adults with dementia (NutriAlz Trial). J Nutr Health Aging. 2013;17(4):295–9.
Logemann JA, Gensler G, Robbins J, Lindblad AS, Brandt D, Hind JA, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech Lang Hear Res JSLHR. 2008;51(1):173–83.
Robbins J, Gensler G, Hind J, Logemann JA, Lindblad AS, Brandt D, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509–18.
Hughes SM. Management of dysphagia in stroke patients. Nurs Older People. 2011;23(3):21–4.
Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia. 1996;11(4):225–33.
Durnbaugh T, Haley B, Roberts S. Assessing problem feeding behaviors in mid-stage Alzheimer’s disease. GeriatrNurs NYN. 1996;17(2):63–7.
Burns A, Jacoby R, Luthert P, Levy R. Cause of death in Alzheimer’s disease. Age Ageing. 1990;19(5):341–4.
Whear R, Abbott R, Thompson-Coon J, Bethel A, Rogers M, Hemsley A, et al. Effectiveness of mealtime interventions on behavior symptoms of people with dementia living in care homes: a systematic review. J Am Med Dir Assoc. 2014;15(3):185–93.
Watanabe T, Kobayashi R, Katahira N, Bessho Y. Cues to facilitate eating behaviors in elderly with dementia—a study at a health care home for the elderly. J Jpn Acad Community Health Nurs. 2006;8:58–64.
•• Wu HS, Lin LC, Wu SC, Lin KN, Liu HC. The effectiveness of spaced retrieval combined with Montessori-based activities in improving the eating ability of residents with dementia. J AdvNurs. 2014;70(8):1891–901. The authors of this study employed spaced retrieval therapy combined with Montessori-based activities to improve the eating abilities of ninety patients with dementia. Statistically significant increases in amount of oral intake and body weight following the intervention support its potential for amelioration of eating difficulties in patients with dementia.
Wu H-S, Lin L-C, Su S-C, Wu S-C. The effects of spaced retrieval combined with errorless learning in institutionalized elders with dementia: recall performance, cognitive status, and food intake. Alzheimer Dis Assoc Disord. 2014;28(4):333–9.
Lin L-C, Huang Y-J, Su S-G, Watson R, Tsai BW-J, Wu S-C. Using spaced retrieval and Montessori-based activities in improving eating ability for residents with dementia. Int J Geriatr Psychiatr. 2010;25(10):953–9.
Sharp HM, Shega JW. Feeding tube placement in patients with advanced dementia: the beliefs and practice patterns of speech-language pathologists. Am J Speech Lang Pathol. 2009;18(3):222–30.
Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA J Am Med Assoc. 1999;282(14):1365–70.
Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–71.
Alvarez-Fernández B, García-Ordoñez MA, Martínez-Manzanares C, Gómez-Huelgas R. Survival of a cohort of elderly patients with advanced dementia: nasogastric tube feeding as a risk factor for mortality. Int J Geriatr Psychiatr. 2005;20(4):363–70.
Steele CM, Bailey GL, Polacco REC, Hori SF, Molfenter SM, Oshalla M, et al. Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. Int J Speech Lang Pathol. 2013;15(5):492–502.
Clark HM, O’Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. J Speech Lang Hear Res JSLHR. 2009;52(4):1034–47.
Stierwalt JAG, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–56.
Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–8.
Teipel SJ, Pruessner JC, Faltraco F, Born C, Rocha-Unold M, Evans A, et al. Comprehensive dissection of the medial temporal lobe in AD: measurement of hippocampus, amygdala, entorhinal, perirhinal and parahippocampal cortices using MRI. J Neurol. 2006;253(6):794–800.
Younes L, Albert M, Miller MI. Inferring change point times of medial temporal lobe morphometric change in preclinical Alzheimer’s disease. NeuroImage Clin. 2014;5:178–87.
Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: differential-STAND. NeuroImage. 2011;55(2):522–31.
Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain J Neurol. 2007;130(Pt 3):708–19.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40.
Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59(11):931–45.
Joachim CL, Morris JH, Selkoe DJ. Clinically diagnosed Alzheimer’s disease: autopsy results in 150 cases. Ann Neurol. 1988;24(1):50–6.
Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol (Berl). 2009;117(1):15–8.
Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol (Berl). 2006;112(3):253–60.
Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kövari E. Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(10):864–8.
Acknowledgments
Dr. Malandraki's work has been supported by a pilot grant from the University of Wisconsin-Madison's Alzheimer's Disease Research Center, P50 AG033514-Wisconsin Alzheimer's Disease Research Center; Administrative core, Clinical core. This manuscript was partially prepared at the William S. Middleton Veteran Affairs Hospital in Madison, WI; GRECC manuscript #2014-029. The views and content expressed in this article are solely the responsibility of the authors and do not necessarily reflect the position, policy, or official views of the Department of Veteran Affairs or the U.S. government.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Swallowing Disorders.
Rights and permissions
About this article
Cite this article
Rogus-Pulia, N., Malandraki, G.A., Johnson, S. et al. Understanding Dysphagia in Dementia: The Present and the Future. Curr Phys Med Rehabil Rep 3, 86–97 (2015). https://doi.org/10.1007/s40141-015-0078-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-015-0078-1