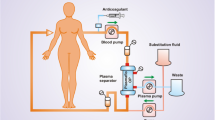
Abstract
Purpose of Review
This review summarizes the American Society for Apheresis (ASFA) recommendations for apheresis indications in the perioperative setting. It reviews pathophysiology of these indications, highlights the key rationales for apheresis, and examines the current evidence behind the recommendations. This concise review will allow readers to learn about indications and available evidence behind apheresis in the perioperative setting.
Recent Findings
Encountered indications in the perioperative setting include heparin induced thrombocytopenia (HIT), desensitization/rejection prophylaxis or treatment of antibody-mediated rejection in solid organ transplants, sickle cell anemia, and thyroid storm.
Summary
Apheresis can be utilized as an adjunct therapy for many perioperative indications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapeutic apheresis is a procedure in which a component of patient’s blood is selectively removed for the purpose of removing pathogenic substances. The patient’s whole blood is removed and separated into components using centrifugation or membrane filtration [1]. Therapeutic plasma exchange (TPE) is a type of apheresis in which patient’s plasma is removed and replaced with either donor plasma or 5% albumin. There are advantages and disadvantages of both replacement fluids – for example, donor plasma contains coagulation factors and proteins that replace those lost in the removal process, however, transfusion of plasma carries the risk of possible blood-product related risks, such as transfusion-associated lung injury (TRALI), anaphylactic reactions in IgA-deficient patients, pathogen transmission, and transfusion-related hypocalcemia. Albumin does not carry blood-product related risks, however it does not contain coagulation factors. Pathogenic substances removed via TPE are most commonly immunoglobulins, immune complexes, or toxins. While TPE removes these harmful substances, it is non-selective and therefore also removes beneficial substances such as albumin-bound medications, biologic medications, and coagulation proteins. In the peri-operative setting, the removal of coagulation factors can be of concern due to risk of bleeding. The removal of 1.0 plasma volume exchange is estimated to decrease coagulation factors by up to 50% and fibrinogen up to 60% [2]. The coagulation factors can rebound to pre-TPE levels in one to two days in a patient with normal hepatic function, except fibrinogen which only recovers partially [3]. Therefore, in a peri-operative patient, using some plasma as part of thereplacement fluid might be better than using just albumin. The effectivity of the target substance removal can vary based on certain characteristics, such as concentration in the blood, degree bound to protein, and amount of distribution between the intravascular and extravascular space. Typically, in one session of TPE, 1.0 to 1.5 plasma volumes are replaced. The amount of the removed component is most effectively removed with the first session, with exponentially decreasing amounts removed thereafter, due to dilution of the substance in the intravascular space with replacement fluid [4]. Typically a 1.0 plasma volume exchange removes 62% of the native plasma along with the intravascular pathogenic substance. Vascular access for the purpose of apheresis may be in the form of peripheral or central venous access. The recommended size for peripheral access is optimally at least a 17-gauge needle for blood withdrawal and at least an 18-gauge needle for return [5]. However, smaller needles may be used in certain settings, e.g. pediatrics with reduced flow rates. Adverse reactions to TPE can be related to infusion of plasma or fluid shifts, the most common of which include hypocalcemia, hypotension, fever, urticaria, and pruritis [6]. Hypotension can also be severe in a patient taking an angiotensin-converting enzyme (ACE) inhibitor, possibly due to increased kinin production. Red blood cell exchange (RCE) is another type of apheresis in which the patient’s abnormal red blood cells (e.g. sickled red blood cells) are removed and replaced with donor red cells. The American Society for Apheresis (ASFA) routinely reviews clinical evidence behind apheresis performed for diseases and assigns a category and grade for each entity [7••]. The assigned category defines the strength of the TPE role in treatment (I-IV), and the assigned grade (1A-2C) defines the strength of the quality of evidence behind the category. Definitions of each category and grade are summarized in Table 1. In this review, we will discuss the assigned ASFA category and grade along with current literature for diseases encountered in the perioperative setting, which are summarized in Table 2.
Indications for Therapeutic Apheresis in Perioperative Setting
Heparin Induced Thrombocytopenia (HIT)
Heparin-induced thrombocytopenia (HIT) is a complication typically resulting from exposure to unfractionated or low molecular weight heparin. An antibody is formed against a heparin molecule complexed with endogenous platelet factor 4 (PF4), resulting in thrombocytopenia and prothrombotic venous and arterial pathology. The “4Ts” score is a pretest probability scoring system used to predict likelihood of HIT. Points are given for thrombocytopenia, timing of thrombocytopenia, thrombosis, and if there are no other causes for thrombocytopenia. Higher scores correlate to a stronger likelihood of HIT [8]. There are two categories of assays available for the diagnosis of HIT: 1) functional, or platelet activation assays and 2) PF4-dependent immunoassays. The gold standard assay is the serotonin release functional assay. Briefly, patient serum containing the antibody is incubated with donor platelets containing radiolabeled serotonin. Heparin is added, which activates the platelets in the presence of the antibody and releases the labeled serotonin, which is then measured. The immunoassays detect the PF4-heparin complex antibody (anti-HPF4) using an enzyme-linked immunosorbent assay.
The general treatment for HIT is to cease any heparin administration and administer anticoagulation with a parenteral non-heparin agent, due to high risk for thrombosis and mortality. If a patient with acute HIT requires elective cardiothoracic surgery, it is optimal to delay surgery until the platelet count has recovered and the antibody is undetectable via either the antigen or functional assay [9, 10, 11••]. In patients who urgently need cardiothoracic surgery, a diagnosis of HIT poses a challenge. Indeed cardiothoracic surgery may involve cardiopulmonary bypass (CPB) and/or extracorporeal membrane oxygenation (ECMO) which requires anticoagulation to prevent thrombosis in the circuit. Alternative anticoagulants to fractionated heparin have been used in this situation, such as the direct thrombin inhibitor bivalirudin, which is not optimal due to a delayed reversal effect, a lack of standardization approach to rapid monitoring with an increased risk of bleeding [6, 12, 13•, 14]. Due to the increased risk of bleeding with bivalirudin, it may be preferable to utilize peri-operative TPE with heparin re-exposure in the circuit rather than bivalirudin in patients undergoing high-risk cardiothoracic procedures (i.e. re-do sternotomy, transitioning off mechanical cardiac support).
The rationale of peri-operative TPE for HIT is removal of anti-HPF4. ASFA assigns pre-procedural (CPB, ECMO, and pre-transplantation) TPE a Category III, Grade 2C recommendation (Table 2). The largest systematic review examining the role of TPE for HIT examined 113 cases treated with TPE and/or IVIG [15••]. 91% of cases were diagnosed via immunoassay and/or serotonin release assay. Of all cases examined, 30 involved patients with HIT received IVIG and/or TPE prior to CPB surgery. 87% of these patients were treated with TPE only, and 13% with TPE and IVIG. The cases had an average number of three TPE sessions each. Almost all patients (29 of 30) received heparin re-exposure during CPB, with one patient with bivalirudin as an alternative anticoagulant. Plasma was the most common replacement fluid utilized. Post-operative outcomes were overall positive, with only two patients experiencing bleeding and no HIT-related thrombotic events. As previously discussed, the use of plasma rather than albumin as the replacement fluid has the advantage of providing valuable coagulation factors and some fibrinogen lost in the pheresis process, especially in a perioperative patient. In some clinical contexts cryoprecipitate may be used to replace lost fibrinogen, however practice has not been studied in the perioperative patient[16]. In the cases that had post-operative bleeding complications, a combination of albumin and plasma was used for one patient, and the replacement fluid for the other patient was not listed [17, 18]. The largest case series examining the role of TPE in cardiac surgery patients (included in the systematic review by Onuoha et al.), examined 11 patients who received a single session of intraoperative TPE due to HIT diagnosed via immunoassay and clinical findings[19]. Plasma was used as the replacement fluid for all cases. While heparin was utilized in the CPB circuit, anti-HPF4 antibody titer decreased significantly (50%–84%) post-TPE. Of these 11 patients, two died of thrombotic events, reported as unrelated to HIT. Functional assays for HIT, which may not be logistically feasible in certain clinical settings, were not performed for any of these patients. Additional smaller case series have reported the successful use of peri-operative TPE in the setting of HIT with variations in the approach – such as the number of sessions and timing of TPE performed prior to surgery, antibody reactivity levels deemed acceptable for surgery, and the types of anticoagulants used in CPB (for example heparin versus bivalirudin) [9, 20,21,22]. Due to the small sample size of these studies and lack of randomized control trials (RCTs), the evidence for peri-operative TPE in the setting of HIT is overall weak. Larger studies are needed to strengthen evidence for this indication.
Kidney Transplant
When evaluating a kidney transplant recipient, ABO and human leukocyte antigen (HLA) compatibility are evaluated. Both ABO and HLA incompatible organs can be successfully transplanted, however preoperative desensitization strategies such as plasmapheresis and immunosuppressants are utilized to prevent or treat antibody-mediated rejection (AMR).
ABO Incompatible Kidney Transplant
A major ABO incompatible (ABOi) transplantation is defined as a recipient presenting with naturally occurring blood group antibodies/isoagglutinins (anti-A and/or anti-B) directed against the ABO type of the donor organ. ABOi kidney transplants performed without desensitization result in severe AMR and hyperacute graft rejection; however, with pre-transplant desensitization the outcomes might be comparable to ABO compatible transplants. The pool of available organs for patients with end-stage kidney disease can be greatly widened by allocating ABOi organs which is more widely performed in other countries, such as Japan [23]. Comparatively, in the United States allocation of ABOi kidney transplants is much lower, accounting for 1.5% of all kidney transplants [24•]. Of note, subtype A2 or subtype A2B donor kidneys express a low level of the A1 antigen on the kidney and endothelium, and these kidneys can be successfully transplanted into blood group non-A recipients without TPE [25,26,27]. Prior to transplantation, the recipient’s isoagglutinin (anti-A) titer is measured. The premise of TPE is to reduce circulating isoagglutinins in the serum to promote a successful transplantation. Immunoadsorption can also be used for this purpose but is not used in the United States. The goal set for post-desensitization titer determines the number of sessions and varies between institutions, but is typically less than 8 to 32 at the IgG phase [28, 29•, 30, 31]. Post-transplantation, titers are monitored, and a significant rise may indicate a need for TPE or kidney biopsy, due to associated risk of rejection [32]. Although there is an association with AMR and high post-transplant isoagglutinin, this is not always true, and some patients with clinical AMR do not have elevated titers [32]. Routine TPE is not performed. TPE may also be used to reduce the rise in isoagglutinin titers post-transplantation. It is important to note that other desensitization strategies are used in conjunction to TPE, including intravenous immune globulin (IVIG), B cell depletion rituximab, and splenectomy.
ASFA assigns TPE for desensitization of an ABOi living donor transplant a Category I, Grade 1B recommendation (Table 2). Multiple controlled studies have been published denoting success of ABOi renal transplants using TPE as an adjunct to other conditioning regimens [28, 29•, 30, 31, 32]. The adjunctive conditioning regimens varied between studies. Masterson et al. examined twenty ABOi renal transplants performed in recipients low baseline isoagglutinin titer (defined as ≤ 16) and the use of routine immunosuppression, without TPE, with a relative success—only one patient experienced AMR [33•]. ASFA has not defined the titer to be achieved with TPE for desensitization. One important factor to consider in examining the available evidence of TPE use in this context is the methodology of titer measurement. Titers between laboratories show significant variation due to difference in methods and reagents [34]. Most studies consider the titers performed at the anti-human globulin (AHG) phase (the IgG titers), to be the most significant, however titers measured at the room temperature (the IgM phase) and their effect on graft survival and outcomes is uncertain [32].
TPE for the indication of AMR due to ABOi transplant receives a Category II, Grade 1B recommendation (Table 2). There is no recommendation on number of sessions, duration, or ideal titer. Isoagglutinin levels are monitored after transplantation, and significant rise in titer may trigger a renal biopsy or preemptive TPE. Currently, there is no guideline regarding a specific titer or rise in titer in which TPE is indicated, as AMR is diagnosed based on the combination of multiple clinical and serologic findings. In some studies, a low titer may have a negative predictive value for AMR, but the positive predictive value is lower and patients with AMR may not have elevated titers [32]. TPE appears to play a significant role along with other modalities of immunosuppression in AMR, however, overall there is less data examining its role in preventing AMR rather than in desensitization.
HLA Incompatible Kidney Transplant
In addition to the ABO antigens, HLA antigen compatibility is also important in kidney transplant patients. Recipients may present with preformed antibodies to HLA antigens on the donor kidney, also referred to as donor specific antibodies (DSA), previously developed via transfusions, pregnancy, or previous transplants. During pre-transplant work-up, recipients undergo HLA typing and screening for HLA antibodies. If the patient has HLA antibodies, the percent of reactive cells from the panel (PRA) is obtained. Next, the calculated panel of reactive antibody (cPRA) is obtained based on the known HLA frequency in the population. A crossmatch is also performed using the patients DSA and donor T cells. A high cPRA presents a significant barrier to kidney transplantation, as it makes it harder to find an eligible living kidney donor. Specifics of HLA incompatible transplantation eligibility and pre-transplant protocols vary between institutions. HLA incompatible transplantation performed with desensitization of the DSA via TPE and IVIG has shown increased patient survival compared to dialysis alone or remaining on the waiting list and then receiving an HLA compatible transplant [35••, 36•].
TPE may be used as a method of desensitization as well as a treatment for AMR in these settings, with the intent of removing DSA. ASFA assigns TPE for AMR and desensitization from a living donor a category I, grade 1B recommendation (Table 2). ASFA recommends pre-operative TPE is typically performed daily to every other day for one to five sessions, or until a negative crossmatch is achieved. In addition, post-operative TPE is continued for at least three sessions. TPE is not recommended for deceased donor transplants due to the transient effects and logistical barriers with deceased donor transplants [7••]. When a deceased donor organ is made available, the allocation process is rapid due to time-sensitive nature of the quality of organ (cold ischemia time). In addition, the effects of TPE are transient. Lefaucher et al. showed that treatment of AMR with TPE in combination with IVIG and rituximab is superior to treatment using IVIG alone [37•]. TPE combined with IVIG is the most commonly used modality for treatment of AMR and is considered the standard of care, however the specifics of treatment such as number of sessions and dosing is not defined. Overall, the use of TPE for these indications in combination with other antibody-depleting strategies appears effective, with moderate quality evidence. TPE may also be used for pre-transplant desensitization or treatment of AMR post-transplant due to non-HLA antibodies (for e.g. angiotensin type 1 receptor antibody) [38].
Liver Transplant
Similar to kidney transplantation, AMR of the transplanted liver may be due to ABO antibodies or HLA (DSA) antibodies, although the role of DSA antibodies is less clearly defined [39, 40]. ABO incompatible liver transplantation may be performed from either living or deceased donors [41•, 42•, 43, 44]. TPE can be used for either desensitization or to treat rejection with the goal of removing antibodies. The regimens for these purposes vary between institutions and there is no consensus guideline on dosing, combination of therapies, or goal titers in TPE.
Living Donor Liver Transplant (LDLT)
ASFA assigns desensitization for an ABO incompatible living donor liver transplant (LDLT) a category I, grade 1C recommendation (Table 2). Although TPE is considered a mainstay method of desensitization in ABOi transplants, there is also a retrospective study showing rituximab monotherapy as an appropriate desensitization strategy [45, 46]. There have not been any studies utilizing TPE monotherapy for desensitization. Overall, while the quality of evidence for TPE use for desensitization in LDLT is weak, it is still used as a main adjunct, given the significant risk of hyperacute rejection.
Deceased Donor Liver Transplant (DDLT)
ASFA assigns TPE for ABOi deceased donor liver transplant (DDLT) desensitization and AMR a category III, grade 2C recommendation (Table 2). TPE is either performed immediately pre-transplant, or both immediately before and after transplantation, to prevent hyperacute rejection. There is overall less evidence for the use of TPE in ABOi DDLT than in LDLT, likely due to rarity of ABOi DDLT. Mysore et al. proposed the use of a titer-based management for desensitization in pediatric ABOi DDLT in a retrospective study [47]. Patients with pre-transplant titers of ≥ 32 received TPE, rituximab, IVIG, and mycophenolate, while patients with titers ≤ 16 received steroids and tacrolimus. Outcomes were similar to ABO compatible liver transplants over the three-year follow-up period. It should also be noted that ASFA specifically states that TPE is not an indication for desensitization of group A2 into group O DDLT due to decreased expression of the A antigen in the donor endothelium, similar to subtype A2 donor transplants in DDKT [48]. Additionally, in DDLT, ASFA specifies the recommendation for AMR to include rejection from ABO and anti-HLA antibodies.
Heart Transplant
TPE may be used as an adjunct for DSA desensitization and treatment for AMR in heart transplantation. ABO incompatible heart transplants are not performed in adults, therefore the principle of TPE in these settings is to remove donor specific HLA antibodies. Similar to other organ transplants, the regimens for desensitization and rejection treatment are not standardized and vary between institutions [49•]. ASFA assigns TPE for desensitization as a category II, grade 1C recommendation. Per ASFA, one to three sessions alongside TPE are recommended prior to transplant, with three to five sessions post-transplant.
One retrospective study compared sensitized heart transplant recipients who received IVIG and TPE for desensitization and unsensitized recipients who did not receive desensitization, and found similar outcomes for an average follow-up of 22 months[50]. Another retrospective study showed similar results, with a desensitization protocol utilizing TPE, rituximab, tocilizumab, and post-operative IVIG. The available studies regarding TPE for desensitization have important limitations, including varying definitions of a sensitized recipient, relatively short follow-up times, and small sample sizes. TPE for AMR receives a category III, grade 2C recommendation (Table 2). ASFA recommends TPE performed daily or every other day for one week, adjusted based on clinical and other laboratory response. The evidence behind this indication also lacks strength – studies have been retrospective with small sample sizes and varying definitions of AMR and various treatment regimens [51]. For example, one case series utilized TPE exchanging twice the patient blood volume for five days with methylprednisone rescue and change in immunosuppressants [42•]. Another case report of hyperacute rejection was successfully treated using biventricular ventricular assist devices, TPE, IVIG, and rituximab. The lack of standardization in the diagnosis of AMR plays a significant role in the available evidence [52]. For example, at a consensus conference involving 67 heart transplant centers across the world, 53% of centers diagnosed AMR based on clinical findings with a negative biopsy [53••]. Criteria that can be used include endomyocardial biopsy findings such as histologic findings or immunohistochemistry, circulating DSA, and graft dysfunction, however institutions reported varied use of these criteria in diagnosis (i.e., centers use unique criteria) [53••].
Lung Transplant
TPE may be used to treat AMR or for desensitization in lung transplantation. ABO incompatible lung transplants are not routinely performed; TPE is performed to remove DSA. ASFA assigns both indications a category III, grade 2C recommendation (Table 2). Three to six sessions are recommended for treatment of AMR, however there are no specific recommendations for desensitization. Although there are now standardized diagnostic criteria for antibody-mediated rejection in lung transplants, it is still a diagnosis of exclusion and can be challenging at times [54]. Further complicating available evidence in these transplants is a lack of standardization for treatment or desensitization. Evidence for TPE in combination with other immunosuppressants for these indications has been retrospective with varying results. For example, one group utilized TPE alongside IVIG and antithymocyte globulin as a desensitization protocol for recipients with DSA and found no difference in long-term allograft and chronic lung allograft dysfunction (CLAD)-free survival against patients without DSA [55]. Another study treated AMR with TPE, rituximab, high-dose corticosteroids, and IVIG with poor clinical responses. A conflicting study showed successful treatment of antibody mediated rejection with TPE and IVIG [56]. Overall, TPE is commonly used alongside other modalities for AMR and desensitization, albeit with weak evidence.
Sickle Cell Disease
Sickle cell disease (SCD) is a genetic disorder caused by an amino acid substitution in the beta globin chain, resulting in an abnormally shaped hemoglobin S (HgbS). HgbS causes a host of acute and chronic morbidity, such as vaso-occlusive complications (crisis, acute chest syndrome, stroke), infection, anemia, avascular necrosis, and pulmonary hypertension. Treatment of SCD includes hydroxyurea, red cell simple transfusion and exchange transfusions, and preventative measures. The principle of red blood cell exchange (RCE) is to remove HgbS and to replace with normal healthy red cells, lowering the HgbS percentage. It has the advantage over simple transfusions in more efficient removal of HgbS, and decreased risk of blood hyperviscosity viscosity and iron overload. Although there have not been RCTs comparing simple versus exchange transfusions in numerous specific complications of SCD, RCE is generally preferred for acutely ill patients over simple transfusions [57,58,59].
Sickle cell patients experience more perioperative complications than the general population, and commonly undergo surgeries due to complications of the disease such as cholecystectomies and arthroplasties. The American Society of Hematology (ASH) recommends preoperative transfusion in patients undergoing low to moderate-risk procedures greater than one hour with general anesthesia, with a goal hemoglobin of greater than or equal to 9 g/dL [57]. Importantly, the guidelines state transfusion should be individualized based on factors such as the patient’s disease severity, risk of surgery, and prior transfusion complications, as the overall evidence for pre-operative transfusion versus no transfusion is varied [60, 61•]. Although there are no RCTs examining transfusions in high-risk surgeries (i.e. neurosurgical or cardiothoracic), these patients are thought to benefit from transfusions, specifically RCE. The studies examining pre-operative simple exchange versus RCE are varied, and only one RCT exists. ASH recommends pre-operative RCE for patients who require transfusion but already have a hemoglobin level of > 9–10 g/dL to prevent viscosity-related complications. ASFA assigns RCE for pre-operative management in sickle cell patients a category III, 2A recommendation (Table 2), with a target HgbS of less than 30%. One group compared simple transfusion and RCE in the pre-operative setting and showed simple transfusion as effective as RCE, with fewer transfusion-associated complications [62]. In this study, one group was transfused to a goal hemoglobin of 10 g/dL and received RCE to achieve a HgbS level to less than 30%, and the other received only simple transfusions to a goal of 10 g/dL. Of note, none of these patients were critically ill requiring emergent surgery, and further studies would be helpful to examine the benefit of RCE versus simple transfusion in this setting.
Thyroid Storm
Thyroid storm is a form of severe thyrotoxicosis that is precipitated by medications (amiodarone-induced), untreated hyperthyroidism, thyroidal or non-thyroidal surgery, parturition, or other causes of severe stress such as trauma or infection [63]. It is suspected in patients with clinical features of hyperthyroidism coupled with elevated free T4 and/or T3 with decreased thyroid stimulating hormone (TSH). Standard management includes supportive care and medical treatment with beta blockers, antithyroidal drugs, and glucocorticoids. In refractory cases or cases in which antithyroidal drugs are not tolerated, TPE alone or TPE as a bridge to thyroidectomy or radioactive iodine ablation are considered [64]. The principle of TPE in thyrotoxicosis is to remove plasma protein-bound T3 and T4. In addition, the replacement fluid dilutes the pool of the free hormone [65]. TPE has additional benefit in removal of the elevated catecholamines, amiodarone in amiodarone-induced cases, and autoantibodies in Graves’ disease cases [2]. This effect is transient and retrospective studies have shown a rise in levels the next day [65]. ASFA assigns TPE for thyroid storm a category II, grade 2C recommendation (Table 2). The largest case series to date examined 11 patients with thyrotoxicosis who underwent TPE prior to thyroidal or non-thyroidal surgery [66]. All patients showed improvement in thyrotoxicosis symptoms and decreased levels of free T4 and T3, however the decrease was not statistically significant. Other case series have also demonstrated clinical improvement after TPE, with varying effects of the decrease in levels of free T3/T4 [67,68,69]. It has been hypothesized that the varying percentage of decrease in free hormone is due to shifts from the extracellular compartment [69]. Although there have been multiple case series/reports of the success of TPE for thyroid storm, more work is necessary to determine its true safety and efficacy via controlled trials. Furthermore, there are even fewer reports examining TPE for thyroid storm in the perioperative setting, which is another area that necessitates more work.
Conclusion
The most common indications for TPE in the perioperative setting are HIT in the setting of urgent cardiothoracic surgery and desensitization and treatment of AMR for solid organ transplants. RCE may be used for patients with sickle cell disease in the pre-operative setting, especially in those undergoing high-risk surgery or already have a high hemoglobin level. The role of TPE for HIT is not established due to weak evidence available. Within solid organ transplants, recommendations and evidence for TPE effectiveness vary between organ transplant type. The strongest recommendations (category I) with the strongest evidence are for desensitization of ABOi living donor kidney transplantation and treatment of AMR in ABO compatible living donor kidney transplants. Desensitization for an ABOi LDLT also receives a category I recommendation, but with evidence from observational studies only. More quality studies on the role of TPE in all organ transplants will be essential, as this information will help expand the pool of available organs for patients in need. A current significant challenge in gathering evidence for TPE in organ transplants is the variation of transplant protocols between institutions. There is an overall lack of standardization in acceptability of donors, diagnosis, and treatment of rejection. The recommendation for pre-operative RCE in sickle cell patients is not established and clinical decisions are individualized within each case. TPE for thyroid storm in refractory cases have shown overall positive clinical results, but with case series-level evidence only.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sloan CY, Yi D, Chen A, Collard CD. Perioperative therapeutic plasmapheresis. Anesthesiology. 2013;118:722–8. https://doi.org/10.1097/ALN.0b013e3182835192.
Orlin JB, Berkman EM. Partial plasma exchange using albumin replacement: Removal and recovery of normal plasma constituents. Blood. 1980;56:1055–9.
Flaum MA, Cuneo RA, Appelbaum FR, et al. The hemostatic imbalance of plasma-exchange transfusion. Blood. 1979;54:694–702.
Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American society for apheresis guidelines. Hematology Am Soc Hematol Educ Program. 2012;2012:7–12. https://doi.org/10.1182/asheducation-2012.1.7.
Cohn CS, Delaney MJ, Katz ST, Louis M. Technical Manual, 20th edition. https://ebooks.aabb.org/pdfreader/technical-manual-20th-edition50155278. Accessed 1 May 2024.
Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. J Clin Apher. 2007;22(5):270–6. https://doi.org/10.1002/jca.20143.
•• Connelly-Smith L., Alquist, CR., Aqui, NA., Hofmann, JC., Klingel R., Onwuemene OA, Patriquin CJ, Pham HP, Sanchez AP, Schneiderman J, Witt V, Zantek ND, Dunbar NM. (2023). Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American Society for Apheresis: The Ninth Special Issue. J Clin Apheresis. 2023;38(2):77–278. https://doi.org/10.1002/jca.22043. Updated ASFA guidelines on use of therapeutic apheresis.
Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759–65. https://doi.org/10.1111/j.1538-7836.2006.01787.x.
Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286–92. https://doi.org/10.1056/NEJM200104263441704.
Pötzsch B, Klövekorn WP, Madlener K. Use of heparin during cardiopulmonary bypass in patients with a history of heparin-induced thrombocytopenia. N Engl J Med. 2000;343(7):515. https://doi.org/10.1056/NEJM200008173430718.
•• Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S-e530S. https://doi.org/10.1378/chest.11-2303. Most recent guidelines for treatment of HIT by CHEST.
Moreno-Duarte I, Ghadimi K. heparin induced thrombocytopenia for the perioperative and critical care clinician. Curr Anesthesiol Rep. 2020;10(4):501–11. https://doi.org/10.1007/s40140-020-00405-6.
• Shore-Lesserson L, Baker RA, Ferraris VA, Greilich PE, Fitzgerald D, Roman P, Hammon JW. The society of thoracic surgeons, the society of cardiovascular anesthesiologists, and the american society of extracorporeal technology: clinical practice guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650–662. https://doi.org/10.1016/j.athoracsur.2017.09.061. Practice recommendations for anticoagulation choice during cardiopulmonary bypass.
Naqvi SY, Jawaid A, Dao B, Falvey J, Vidula H, Gosev I, Thomas S. Management of heparin-induced thrombocytopenia using plasmapharesis in patients undergoing heartmate 3 left ventricular assist device. ASAIO J. 2022;68(9):e152–5. https://doi.org/10.1097/MAT.0000000000001631.
•• Onuoha C, Barton KD, Wong ECC, Raval JS, Rollins-Raval MA, Ipe TS, Kiss JE, Boral LI, Adamksi J, Zantek ND, Onwuemene OA. Therapeutic plasma exchange and intravenous immune globulin in the treatment of heparin-induced thrombocytopenia: a systematic review. Transfusion. 2020;60(11):2714–2736. https://doi.org/10.1111/trf.16018. Largest systematic review to date examining IVIG and TPE for HIT.
Pham HP, Williams LA 3rd. Plasma vs. cryoprecipitate for fibrinogen replacement in therapeutic plasma exchange procedures. J Clin Apher. 2015;30(6):382–3. https://doi.org/10.1002/jca.21392.
Cho JH, Parilla M, Treml A, Wool GD. Plasma exchange for heparin-induced thrombocytopenia in patients on extracorporeal circuits: a challenging case and a survey of the field. J Clin Apher. 2019;34(1):64–72.
Maffei SR, Lamba HK, Mensah CK, et al. Plasmapheresis in patients with heparin-induced thrombocytopenia requiring ventricular assist device. Ann Thorac Surg. 2020;109(6):e439–40.
Welsby IJ, Um J, Milano CA, Ortel TL, Arepally G. Plasmapheresis and heparin re-exposure as a management strategy for cardiac surgical patients with heparin-induced thrombocytopenia. Anesth Analg. 2010;110(1):30–5. https://doi.org/10.1213/ANE.0b013e3181c3c1cd.
Jaben EA, Torloni AS, Pruthi RK, Winters JL. Use of plasma exchange in patients with heparin-induced thrombocytopenia: a report of two cases and a review of the literature. J Clin Apher. 2011;26(4):219–24. https://doi.org/10.1002/jca.20289.
Kajitani M, Aguinaga M, Johnson CE, Scott MA, Antakli T. Use of plasma exchange and heparin during cardiopulmonary bypass for a patient with heparin induced thrombocytopenia: a case report. J Card Surg. 2001;16(4):313–8. https://doi.org/10.1111/j.1540-8191.2001.tb00527.
Voeller RK, Melby SJ, Grizzell BE, Moazami N. Novel use of plasmapheresis in a patient with heparin-induced thrombocytopenia requiring urgent insertion of a left ventricular assist device under cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2010;140:e56–8.
Tanabe K, Tokumoto T, Ishida H, Toma H, Nakajima I, Fuchinoue S, Teraoka S. ABO-incompatible renal transplantation at Tokyo Women's Medical University. Clin Transpl. 2003;175–81. https://pubmed.ncbi.nlm.nih.gov/15387109/.
• Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93(6):603–9. https://doi.org/10.1097/TP.0b013e318245b2af. Large multicenter matched control study comparing outcomes of ABO-compatible and ABO-incompatible kidney transplantations.
Breimer ME, Brynger H, Le Pendu J, Oriol R, Rydberg L, Samuelsson BE, Vinas J. Blood group ABO-incompatible kidney transplantation biochemical and immunochemical studies of blood group A glycolipid antigens in human kidney and characterization of the antibody response (antigen specificity and antibody class) in O recipients receiving A2 grafts. Transplant Proc. 1987;19(1 Pt 1):226–30.
Sorensen JB, Grant WJ, Belnap LP, Stinson J, Fuller TC. Transplantation of ABO group A2 kidneys from living donors into group O and B recipients. Am J Transplant. 2001;1(3):296–9. https://doi.org/10.1034/j.1600-6143.2001.001003296.x.
Nelson PW, Helling TS, Pierce GE, Ross G, Shield C, Beck ML, Blake C, Cross DE. Successful transplantation of blood group a2 kidneys into non-a recipients. Transplantation. 1988;45(2):316–9.
Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA, Melancon JK, Ratner L, Zachary AA, Haas M. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87(8):1246–55. https://doi.org/10.1097/TP.0b013e31819f2024.
• Toki D, Ishida H, Setoguchi K, Shimizu T, Omoto K, Shirakawa H, Iida S, Horita S, Furusawa M, Ishizuka T, Yamaguchi Y, Tanabe K. Acute antibody-mediated rejection in living ABO-incompatible kidney transplantation: long-term impact and risk factors. Am J Transplant. 2009;9(3):567–77. https://doi.org/10.1111/j.1600-6143.2008.02538.x. Retrospective study examining factors associated with acute antibody-mediated rejection in ABO-incompatible kidney transplantation.
Parolo A, Silvestre C, Neri F, Rigotti P, Furian L. Therapeutic apheresis in kidney AB0 incompatible transplantation. Transfus Apher Sci. 2017;56(4):506–9. https://doi.org/10.1016/j.transci.2017.07.006.
Hamano I, Hatakeyama S, Fujita T, Murakami R, Hamaya T, Togashi K, Suzuki Y, Yamamoto H, Yoneyama T, Yoneyama T, Hashimoto Y, Narumi S, Tomita H, Ohyama C. Outcome of ABO blood type-incompatible living-related donor kidney transplantation under a contemporary immunosuppression strategy in Japan. Transplant Proc. 2020;52(6):1700–4. https://doi.org/10.1016/j.transproceed.2020.01.152.
Tobian AA, Shirey RS, Montgomery RA, Cai W, Haas M, Ness PM, King KE. ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant. 2010;10(5):1247–53. https://doi.org/10.1111/j.1600-6143.2010.03103.x.
• Masterson R, Hughes P, Walker RG, Hogan C, Haeusler M, Robertson AR, Millar R, Suh N, Cohney SJ. ABO incompatible renal transplantation without antibody removal using conventional immunosuppression alone. Am J Transplant. 2014;14(12):2807–13. https://doi.org/10.1111/ajt.12920. Prospective study examining outcomes of conventional immunosuppression without TPE in ABO incompatible renal transplantation.
Thorpe SJ, Fox B, Sharp G, White J, Milkins C. A WHO reference reagent to standardize haemagglutination testing for anti-A and anti-B in serum and plasma: international collaborative study to evaluate a candidate preparation. Vox Sang. 2016;111(2):161–70. https://doi.org/10.1111/vox.12399.
•• Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–26. https://doi.org/10.1056/NEJMoa1012376. Matched control study comparing outcomes of HLA-incompatible kidney transplantation with desensitization versus dialysis alone.
• Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, Van Arendonk KJ, Stegall MD et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N Engl J Med. 2016 ;374(10):940–50. https://doi.org/10.1056/NEJMoa1508380. Matched control study comparing survival benefit between patients receiving an HLA-incompatible live donor kidney versus remaining on the waiting list.
• Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9(5):1099–107. https://doi.org/10.1111/j.1600-6143.2009.02591.x. Comparison of antibody-mediated rejection treatment using TPE and other modalities versus IVIG alone.
Carroll RP, Deayton S, Emery T, Munasinghe W, Tsiopelas E, Fleet A, Lake M, Humphreys I, Jalalonmuhali M, Coates P. Proactive treatment of angiotensin receptor antibodies in kidney transplantation with plasma exchange and/or candesartan is safe and associated with excellent graft survival at 4 years: a single centre Australian experience. Hum Immunol. 2019;80(8):573–8. https://doi.org/10.1016/j.humimm.2019.04.005.
O’Leary JG, Demetris AJ, Friedman LS, Gebel HM, Halloran PF, Kirk AD, Knechtle SJ, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14(4):779–87. https://doi.org/10.1111/ajt.12667.
Komagome M, Maki A, Nagata R, Masuda W, Kogure R, Mitsui T, et al. Refractory acute antibody mediated rejection in liver transplant after desensitization of preformed donor specific antibody-validity of bortezomib and everolimus: a case report. Transplant Proc. 2022;54(1):147–52. https://doi.org/10.1016/j.transproceed.2021.11.022.
• Hsu SC, Thorat A, Jeng LB, Li PC, Chen TH, Yang HR, Poon KS. ABO-incompatible living donor liver transplantation with reduced rituximab dose: a retrospective analysis of 65 patients - can we fast-track liver transplant surgery and improve long-term survival? Ann Transplant. 2020;25:e923502. https://doi.org/10.12659/AOT.923502. Large analysis of ABO-incompatible liver transplantation outcomes with use of TPE and low-dose rituximab.
• Maitta RW, Choate J, Emre SH, Luczycki SM, Wu Y. Emergency ABO-incompatible liver transplant secondary to fulminant hepatic failure: outcome, role of TPE and review of the literature. J Clin Apher. 2012;27(6):320–9. https://doi.org/10.1002/jca.21244. Role and outcome of TPE use in an ABO-incompatible liver transplant.
Lee CF, Cheng CH, Wang YC, Soong RS, Wu TH, Chou HS, et al. Adult living donor liver transplantation across ABO-incompatibility. Medicine (Baltimore). 2015;94(42):e1796. https://doi.org/10.1097/MD.0000000000001796.
Rummler S, Bauschke A, Bärthel E, Jütte H, Maier K, Ziehm P, Malessa C, Settmacher U. Current techniques for AB0-incompatible living donor liver transplantation. World J Transplant. 6(3):548–55. https://doi.org/10.5500/wjt.v6.i3.548.
Yamamoto H, Uchida K, Kawabata S, Isono K, Miura K, Hayashida S, et al. Feasibility of monotherapy by rituximab without additional desensitization in ABO-incompatible living-donor liver transplantation. Transplantation. 2018;102(1):97–104. https://doi.org/10.1097/TP.0000000000001956.
Lee TB, Ko HJ, Shim JR, Choi BH, Ryu JH, Yang K. ABO-incompatible living donor liver transplantation with a simplified desensitization and immunosuppression protocol: a single-center retrospective study. Exp Clin Transplant. 2021;19(7):676–85. https://doi.org/10.6002/ect.2021.0025.
Mysore KR, Himes RW, Rana A, Teruya J, Desai MS, Srivaths PR, Zaruca K, et al. ABO-incompatible deceased donor pediatric liver transplantation: novel titer-based management protocol and outcomes. Pediatr Transplant. 2018;22(7):e13263. https://doi.org/10.1111/petr.
Kluger MD, Guarrera JV, Olsen SK, et al. Safety of blood group A2-to-O liver transplantation: an analysis of the United Network of Organ Sharing database. Transplantation. 2012;94:526–31.
•Velleca A, Shullo MA, Dhital K, et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J Heart Lung Transplant : Off Publ Int Soc Heart Transplant. 2023;42(5):e1-e141. https://doi.org/10.1016/j.healun.2022.10.015. Updated ISHLT guidelines for management of heart transplant patients.
Pisani BA, Mullen GM, Malinowska K, Lawless CE, Mendez J, Silver MA, Radvany R, Robinson JA. Plasmapheresis with intravenous immunoglobulin G is effective in patients with elevated panel reactive antibody prior to cardiac transplantation. J Heart Lung Transplant. 1999;18(7):701–6. https://doi.org/10.1016/s1053-2498(99)00022-4.
Wang SS, Chou NK, Ko WJ, Chi NH, Hung SC, Hsu RB, Yu HY, Chen YS, Chu SH, Tsao CI, Shun CT. Effect of plasmapheresis for acute humoral rejection after heart transplantation. Transplant Proc. 2006;38(10):3692–4. https://doi.org/10.1016/j.transproceed.2006.10.060.
Kaczorowski DJ, Datta J, Kamoun M, Dries DL, Woo YJ. Profound hyperacute cardiac allograft rejection rescue with biventricular mechanical circulatory support and plasmapheresis, intravenous immunoglobulin, and rituximab therapy. J Cardiothorac Surg. 2013;8:48. https://doi.org/10.1186/1749-8090-8-48.
•• Kobashigawa J, Crespo-Leiro MG, Ensminger SM, Reichenspurner H, Angelini A, Berry G et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2011; 30: 252–269. International consensus guidelines on treatment and prevention of antibody-mediated rejection in heart transplantation.
Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: a consensus report of the international society for heart and lung transplantation. J Heart Lung Transplant. 2016;35(4):397–406. https://doi.org/10.1016/j.healun.2016.01.1223.
Aversa M, Martinu T, Patriquin C, Cypel M, Barth D, Ghany R, Ma J, Keshavjee S, Singer LG, Tinckam K. Long-term outcomes of sensitized lung transplant recipients after peri-operative desensitization. Am J Transplant. 2021;21(10):3444–8. https://doi.org/10.1111/ajt.16707.
Daoud AH, Betensley AD. Diagnosis and treatment of antibody mediated rejection in lung transplantation: a retrospective case series. Transpl Immunol. 2013;28:1–5.
Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 Guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4:327–55.
Hulbert ML, Scothorn DJ, Panepinto JA, Scott JP, Buchanan GR, Sarnaik S, et al. Exchange blood transfusion compared with simple transfusion for first overt stroke is associated with a lower risk of subsequent stroke: a retrospective cohort study of 137 children with sickle cell anemia. J Pediatr. 2006;149(5):710–2. https://doi.org/10.1016/j.jpeds.2006.06.037.
Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med. 1994;96(2):155–62. https://doi.org/10.1016/0002-9343(94)90136-8.
Al-Samak ZM, Al-Falaki MM, Pasha AA. Assessment of perioperative transfusion therapy and complications in sickle cell disease patients undergoing surgery. Middle East J Anaesthesiol. 2008;19(5):983–95.
• Howard J, Malfroy M, Llewelyn C, et al. The transfusion alternatives preoperatively in sickle cell disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet. 2013;381(9870):930–938.RCT examining role of preoperative transfusion in sickle cell patients.
Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, Pegelow C, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The preoperative transfusion in sickle cell disease study group. N Engl J Med. 1995;333(4):206–13. https://doi.org/10.1056/NEJM199507273330402.
De Almeida R, McCalmon S, Cabandugama PK. Clinical review and update on the management of thyroid storm. Mo Med. 2022;119(4):366–71.
Chiha M, Samarasinghe S, Kabaker AS. Thyroid storm: an updated review. J Intensive Care Med. 2015;30(3):131–40. https://doi.org/10.1177/0885066613498053.
Muller C, Perrin P, Faller B, Richter S, Chantrel F. Role of plasma exchange in the thyroid storm. Ther Apher Dial. 2011;15(6):522–31. https://doi.org/10.1111/j.1744-9987.2011.01003.x.
Ezer A, Caliskan K, Parlakgumus A, Belli S, Kozanoglu I, Yildirim S. Preoperative therapeutic plasma exchange in patients with thyrotoxicosis. J Clin Apher. 2009;24(3):111–4. https://doi.org/10.1002/jca.20200.
Binimelis J, Bassas L, Marruecos L, Rodriguez J, Domingo ML, Madoz P, et al. Massive thyroxine intoxication: evaluation of plasma extraction. Intensive Care Med. 1987;13(1):33–8. https://doi.org/10.1007/BF00263555.
Puy H, Lamoril J, Marcelli JM, Lalau JD, Debussche X, Quichaud J, Desmet G. Thyroid hormone extraction by plasma exchange: a study of extraction rate. Biomed Pharmacother. 1992;46(9):413–7. https://doi.org/10.1016/0753-3322(92)90046-a.
Schlienger JL, Faradji A, Sapin R, Blickle JF, Chabrier G, Simon C, Imler M. Traitement de l’hyperthyroïdie grave par échange plasmatique. Efficacité clinique et biologique. Huit observations [Treatment of severe hyperthyroidism by plasma exchange. Clinical and biological efficacy. 8 cases]. Presse Med. 1985;14(23):1271–4 (French).
Author information
Authors and Affiliations
Contributions
P.S. reviewed the topic and drafted the manuscript, P.L. advised P.S. in the process and critically reviewed the paper; M.F. conceptualized the review, and critically reviewed the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shih, P.A., Fontaine, M.J. & Lokhandwala, P.M. Apheresis Indications in the Perioperative Setting. Curr Anesthesiol Rep 14, 366–375 (2024). https://doi.org/10.1007/s40140-024-00636-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-024-00636-x