Abstract
Purpose of Review
To summarize recent evidence that discusses the potential benefits and challenges of using new technology and virtual care models for post-operative patient monitoring after ambulatory surgery.
Recent Findings
• Artificial intelligence (AI) systems can be integrated into practice to play an important role in perioperative risk mitigation.
• Remote monitoring and wearable technology can work synergistically to improve clinical outcomes after surgery.
• Novel care models may result in a high level of care and patient satisfaction compared to inpatient stays.
Summary
AI can be a useful tool to identify patients at increased surgical risk. The integration of AI with remote monitors and wearables holds promise for improving patient outcomes. Concerns associated with data privacy and security, along with clinician reluctance, are challenges to overcome. New models such as virtual care at home and care hotels are options that may provide ways to improve clinical monitoring after discharge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
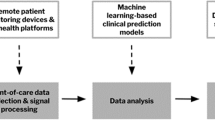
Outpatient surgery has become increasingly more complex in the past decade. Surgeries that previously required post-operative hospital admission are now completed in either hospital-attached outpatient surgery areas or stand-alone ambulatory surgical centers. With the correct protocols, the performance of these surgeries on an outpatient basis can result in higher patient satisfaction without a decrease in safety, despite increasing patient comorbidities. Financial pressures have also contributed to a drive towards not only outpatient surgery, but also same-day discharge after these more complex procedures. Safe post-operative discharge depends upon the ability to (1) predict which patients represent a low risk of having an adverse event after discharge, (2) provide adequate monitoring for certain higher risk patients as needed, and (3) utilize flexible clinical models that can accomplish these goals. This article will discuss these aspects of care, recognize current limitations to implementation, and examine how technology can aid the anesthesiologist caring for these patients in innovative ways.
Perioperative Risk Mitigation and Artificial Intelligence
Artificial intelligence can be defined as “a group of diverse computational techniques such as machine learning, deep learning, and natural language processing” [1•]. While the intricacies of these systems are beyond the scope of this paper, the clinician should be aware that these systems exist and that they can provide robust analyses of enormous amounts of data in the realms of predictive analytics as well as early detection of medical conditions or complications. For example, one study used ECG information from 180,922 patients to train and validate an AI-enabled ECG algorithm that can identify patients at risk of atrial fibrillation even in the presence of normal sinus rhythm and without the need for cumbersome traditional approaches such as Holter monitoring [2]. Another study of over 7 million patients found that an AI-enabled ECG can reliably identify 3-, 5-, and 10-year risk for acute coronary events and mortality based on signs of elevated coronary calcium, obstructive coronary disease, and left ventricular akinesis detected in the ECG by the algorithm [3]. Deep learning models have also been successful at identifying patients with hyperkalemia solely by analyzing two-lead ECGs [4].
As the complexity of outpatient surgery increases and the number of comorbidities of patients undergoing these procedures rises, identification of which patients are at higher risk of post-operative complications such as unanticipated post-operative admission is key to optimizing safety. While some studies identify items such as length of surgery greater than 3 h, ASA score of III and IV, advanced age, higher body mass index (BMI), chronic obstructive pulmonary disease, renal disease, and cardiac arrhythmias as risk factors for unanticipated admission [5], the impact of these factors can vary based on the type of procedure involved or other causes that may not be included in the statistical model utilized. Thus, use of advanced, dynamic, and patient-specific predictive modeling can be advantageous to provide an individual risk assessment for each patient [6]. Clinical risk stratification tools that utilize artificial intelligence (AI) show promise in this area. For example, AI applications that predict which patients are more likely to be discharged home on the day of surgery and not require emergent care in the next 24 h can be of great benefit to surgeons and anesthesiologists alike. Machine learning models have been able to predict a patient’s risk for readmission within days or weeks after surgery [6, 7] and need for extended post-operative opioid use [8,9,10] with fairly good reliability. Another study successfully used a machine learning model to reorder surgical cases in an effort to reduce the number of days per week where after-hours post-anesthesia care unit (PACU) staffing would be needed (12% vs 41%, p < 0.0001) [11].
Likewise, AI could aid post-discharge decision-making by being integrated into real-time electronic symptom monitoring systems [12] to alert the physician of altered recovery trajectory. Perhaps the most pragmatic application of AI within this area is its implementation into medical early warning systems, where an AI framework could track a patient’s recovery trajectory in real time [13•]. Patient-reported outcomes, where a patient self-reports pain, quality of life, and satisfaction metrics, could also be key to these efforts. These self-reported outcomes can then be analyzed by AI both to predict which patients are likely to be at higher risk of post-operative complications and to identify those patients who begin to lag behind in their recovery trajectory [14]. One advantage of this approach is that the use of AI can result in an individualized analysis for each patient, compared to an estimated likelihood of success assessed from population-based data.
Real-time electronic symptom monitoring systems can also include AI conversational agents such as chatbots to facilitate communication with patients and decrease post-operative follow-up burden on health staff. Using clinician-vetted algorithms, these chatbots can be trained via neural networks to respond to common post-operative questions while still retaining a high level of patient satisfaction [15]. Crucially, the use of natural language processing has accelerated the accuracy of information delivered by the AI system and its ability to risk-stratify queries and identify situations that require assessment by a clinician.
Unfortunately, implementation of these systems is still in its relative infancy. Despite some of the first studies describing the capability of neural networks to predict post-operative outcomes being published over 20 years ago [16], the implementation of AI systems into daily clinical practice has been slow and limited. Reasons for this slow adoption include high initial hardware and software cost, potential need for health information system infrastructure change, low-quality datasets to train the algorithms, associated clinical workflow changes, and poor understanding of AI among physicians. This latter point is important given the potential fear that sometimes exists among clinicians of AI systems replacing physicians as decision-makers. Such clinician bias could limit the impact of AI algorithms. For example, in a study by Rushlow et al. assessing the use of AI-enabled ECG to diagnose left ventricular dysfunction, clinicians who were high-adopters of the AI recommendations were twice as likely to identify low ejection fraction as low-adopters in patients with an AI-positive ECG [17]. Physicians should understand both the advantages and limitations of AI systems and likewise recognize that AI holds significant promise as an aid in clinical decision-making and as a guidance system for patient safety.
Wearable Technology
While some post-discharge follow-up such as pain, nausea, and wound healing can be accomplished via phone calls, electronic surveys [18], apps [19], or self-reported data, the use of these mechanisms does not allow for the collection of continuous physiologic data. Wearable devices can allow for the collection of vital signs and physical activity that would otherwise go unrecorded or unrecognized [20]. Technology companies such as CloudDX, Withings, Fitbit, and Masimo offer equipment that can be worn and/or used by the patient at home to collect vital signs and other data with little effort. Some evidence exists that the use of these devices at home can have a tangible impact on patient outcomes. One study [21•] reported that patients who underwent virtual care at home with remote automated monitoring after hospital discharge had a higher rate of detection of drug errors, with no difference in the percentage of patients requiring acute hospital care. Another study reported an association between a drop in mobility scores as measured by a wearable device and onset of post-operative complications [22]. A small trial among hospital patients in the UK suggested that use of continuous vital sign devices could lead to faster administration of antibiotics after recognition of sepsis; however, it is unclear whether this is because of faster recognition of signs of sepsis or faster action after such recognition [23]. Regardless, continuous monitoring after acute or surgical care could help identify negative outcomes that may not otherwise be recognized. For example, in a multicenter randomized clinical trial in Canada, post-cardiac surgery patients with no history of atrial fibrillation who wore a patch-based continuous cardiac rhythm monitor were more likely to have atrial fibrillation detected after discharge (19.6% vs 1.7%, p < 0001) [24]. In perhaps one of the most interesting studies thus far on the use of AI and wearables, van den Eijnden et al. investigated the use of a machine learning model and wearables to characterize post-operative recovery. The model classified patients as fast-recovering or slow-recovering, and for some patients the model showed low recovery scores days before post-operative complications presented [25].
Unfortunately, research on the use of wearable devices for post-operative monitoring is still quite limited. In one recent review, only 24 studies were identified that addressed the use of wearables for this purpose [20]. Most of these studies assessed measures of physical activity with little or no collection of physiologic measures such as oxygen saturation that would be key for formulation of likely post-operative trajectory. Other factors limiting the use of wearables are the variable accuracy of these devices and lack of research about inter- and intra-device reliability [20]. The adoption of advanced post-operative monitoring of higher risk patients in the home setting depends upon high levels of device accuracy and reliability and the integration of these devices with predictive algorithms.
Implications for Practice
Over the course of time, technological advances have been associated with advances in the field of anesthesia. Novel achievements such as the development of endotracheal intubation, automated monitoring, and programmable anesthesia delivery systems have given rise to modern anesthesia methods. Practice standards now consist of perioperative anesthetic care which includes preoperative, intra-operative, and post-operative considerations in the care of a patient. Advances in intra-operative monitoring and anesthesia delivery technology have made caring for a patient under anesthesia the safest it has ever been in the history of the practice. The ability to provide safe, efficient, and scalable anesthetics has led to the ability to care for more complex patients and has expanded the ability for more complex procedures to be performed in an outpatient environment. Likewise, improvements in surgical techniques have led to a significant reduction in post-surgical hospital length of stay [26], with an increasing number of procedures now performed using minimally invasive approaches that facilitate same-day or next-day discharge. Minimization or complete avoidance of hospitalization after surgery, while being advantageous for patients, may also create challenges in the care of said patients. For example, without a robust system for ensuring adequacy for discharge or remote follow-up if needed, the risk of hospital readmissions or other negative outcomes may inadvertently be increased. Post-operative mortality, although much more rare now compared to in times past, is still a significant safety concern throughout the world. In fact, one analysis suggests that, worldwide, 30-day post-operative is the third greatest contributor to death [1•, 27]. While advances in healthcare technology have enabled safer post-operative care, much work remains to be done to improve post-operative safety around the globe and identify the ideal monitoring plan for each individual patient. The use of electronic devices capable of monitoring a variety of physiological data, combined with artificial intelligence, holds promise to elucidate this path. Anticipated challenges with use of emerging post-discharge technology include the typical concerns associated with privacy, security, and validity of data, along with barriers to clinician usage and adaptability to provide care based on these novel devices.
The use of technology that is already highly integrated into daily life may be key to utilizing post-operative monitoring at home. Ownership of smartphones, which often are equipped with high-resolution camera and video capability, is greater than 90% in the 18- to 49-year-old United States population [28]. The accessibility of telecommunication technology along with the explosion of wearable devices capable of collecting a host of physiologic data has driven estimates of telemedicine market growth potential to exceed USD 280 billion by 2030 [29]. Despite this vast potential found in merging consumer technology with medical practice, challenges such as maintaining compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), ensuring high levels of device accuracy, and protecting against cyberattacks present challenges to widespread implementation. How third-party companies store and ensure security of the data being stored can impact healthcare providers and health systems from a liability perspective. In addition to the potential breach of HIPAA compliance, state and local regulations also impact how and if the abovementioned technologies are used. Technology may be exponentially advancing, but the ability for lawmakers to remain in lockstep has proven to be challenging. Varying state and inter-state licensing regulations for telemedicine and/or tele-prescribing can be difficult to navigate. The ability to capture billing for services provided in a virtual or remote format is only now beginning to be less nebulous. Despite the ease with which the click of a button on an application can make the transmission of wearable device data to a medical professional possible, obtaining informed consent for evaluation and management remains a key component for practicing medicine. Whether an electronic, verbal, or written informed consent is the standard of practice to participation in a telemedicine encounter varies by state [30]. Yet despite these challenges, early research and use of AI and wearable devices has shown a significant impact on patient care and medical practice patterns.
The potential for improved patient outcomes is the cornerstone of the zeal behind the integration of AI and wearable devices into clinical care. Post-operative inpatient use of wearable devices can decrease the incidence of hypotension and hypoxemia [31], while use of remote home monitoring can reduce both emergency department visits and hospital readmissions while improving multiple metrics on quality-of-life assessments [26]. One challenge in the use of these devices, however, is consistency of use. In one study, while upwards of 75% of patients showed interest in using remote monitoring, fewer than one-third actually signed up for or consistently used wearable devices [26]. Additionally, patient access to this technology can limit widespread use. Older populations have a lower likelihood of having a wearable device, and underserved communities may lack the resources to take advantage of the technology or be less willing to engage with wearable device technology for a host of reasons [32]. Lastly, clinical decision-making has also been shown to be affected by having access to additional data from wearable devices. A study by Linton et al. assessed whether wearable data availability during simulated telephone calls on discharged pediatric patients and patient families about post-operative concerns affected the clinical decisions made by clinicians. The study showed that wearable devices did indeed affect clinicians’ decision-making [33]. Big data resulting in data overload or being inundated with wearable device data reporting requirements is a potential drawback of AI and wearable device usage, and clinician reluctance to using the technology can stem from these concerns. Most, if not all, technological advances are fraught with a mixture of excitement and concern, and the use of AI and wearable devices is not exempted from this conflict.
Examples from the Real World
Increasing healthcare costs nationwide, exacerbated by the COVID pandemic, have placed significant capacity and financial constraints on healthcare systems. This strain is very likely accelerating the amount of both surgical and non-surgical medicine that is being performed in the outpatient setting rather than within the hospital space. A particular challenge for physicians has been to determine how to shift the perioperative care of patients to outside the hospital without a negative impact on outcomes. Monitoring from a distance may hold promise in this area as a way to facilitate out-of-hospital care for complex patients and procedures. In one 2022 study assessing the feasibility of virtual monitoring among complex oncology patients discharged from the hospital, reporting of both remotely collected (vital signs, step count) and patient-reported health outcomes (quality of life) remained at 61% even 30 days post-discharge [34]. Thirteen of the 21 patients viewed this reporting as helpful in their recovery. Of note, patients with higher levels of acuity also showed significant worsening in the reported metrics, suggesting that data collection systems such as these may allow for early intervention. Another recent study demonstrated that, 5 days post-discharge after colorectal surgery, 98% of patients continued to report vital sign data via the use of wireless monitors [35]. Smartphone technology can also be utilized for remote monitoring. One 2023 study reported that 83% of abdominal surgery patients used a smartphone app successfully for post-operative wound monitoring; many of these patients also reported high satisfaction with app utilization and feasibility [36].
Advanced-care-at-home models may have an impact on outcomes. A multicenter randomized control trial of 9058 patients examined quality outcomes after non-elective surgery. One cohort included a virtual component with virtual physician assessments and nurse wound checks, along with remote vital sign monitoring, while the other cohort received standard post-operative care. The authors reported that while days alive at home post-operatively was unchanged, patients in the virtual care cohort did have some improvement in pain management and, importantly, decreased incidence of drug errors of patient-administered medications. A post hoc analysis demonstrated a reduction in readmission and emergency department visits [21•]. Smaller studies have also supported the utility of at-home monitoring. One randomized trial among patients undergoing breast surgery compared post-operative care utilizing in-person follow-up versus a mobile application. No significant difference in complication rates was observed; moreover, patient convenience scores were higher in the mobile application group [37]. Another RCT in patients undergoing breast or gynecologic surgery reported improved quality of recovery in patients who had post-operative follow-up via a mobile application compared to standard follow-up [38].
The medical hotel model has been proposed as a unique means to monitor patients post-operatively, simultaneously avoiding hospitalization while also increasing the comfort and satisfaction of patients during their early recovery. Medical hotel models typically utilize on-site or virtual nursing resources with the ability to escalate care as needed [39]. Compared to inpatient care, a hotel care model may result in equally high level of care and patient satisfaction [40]. The Care Hotel at the Mayo Clinic is a care model that began in 2020 and utilizes a hybrid in-person and virtual care nursing model that allows for patients to be in a more private environment, removed from the inpatient wards, while still being able to connect with a clinical professional at all times. Select patients with mild to moderate disease have the option to utilize the Care Hotel after hospital discharge or as a post-surgical option after complex surgery that typically requires an inpatient overnight stay (Table 1). Early data from the Care Hotel experience has demonstrated that participating patients can achieve high levels of satisfaction with care during their post-operative recovery [41, 42]. This model can be particularly useful for magnet centers where patients travel great distances for their medical or surgical care.
Common limitations of these studies include small patient cohorts, unclear assessments of the exact effect of virtual care on high-impact clinical outcomes, varied health systems with wide-ranging resources and infrastructure, and assorted health policy environments. The latter two limitations in particular call into question the applicability of specific virtual care interventions across wide-ranging surgical patient populations and healthcare environments throughout the world. Nevertheless, consistent themes found throughout the available studies suggest that (1) clinical outcomes are likely not worse with virtual care interventions, (2) advanced care outside of the hospital setting is highly accepted by patients, and (3) both clinicians and patients can benefit financially from this type of model. Future research should investigate the applicability of virtual care across a variety of healthcare systems and examine safety and quality-of-recovery outcomes. Perhaps by showing enough value to the physician and patient, models that provide high-quality advanced care outside of the hospital will eventually become the standard of care for the mild-to-moderate risk patient undergoing complex surgery that has traditionally required an overnight stay.
Conclusion
The use of technologies such as AI and wearable devices can be beneficial for perioperative risk mitigation and improvements in post-operative safety. The combined use of AI and wearable technology could be particularly beneficial in facilitating complex surgery to be performed on an outpatient basis. While some barriers to implementation exist, the implications for clinical practice are significant. Patient care models such as virtual care at home and medical hotels are well-positioned to be integrated into post-discharge monitoring.
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Maheshwari K, Cywinski JB, Papay F, Khanna AK, Mathur P. Artificial intelligence for perioperative medicine: perioperative intelligence. Anesth Analg. 2023;136(4):637–45. Extensive article on the implications of artificial intelligence for perioperative care..
Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394(10201):861–7.
Awasthi S, Sachdeva N, Gupta Y, Anto AG, Asfahan S, Abbou R, et al. Identification and risk stratification of coronary disease by artificial intelligence-enabled ECG. EClinicalMedicine. 2023;65:102259.
Galloway CD, Valys AV, Shreibati JB, Treiman DL, Petterson FL, Gundotra VP, et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol. 2019;4(5):428–36.
Ardon AE, Nimma S, Nin OC. Twenty-three-hour stays in the ambulatory surgical center: benefits, pathways and protocols. Curr Opin Anesthesio. 2023;36(6):617–23.
Bignami EG, Cozzani F, del Rio P, Bellini V. The role of artificial intelligence in surgical patient perioperative management. Minerva Anestesiol. 2021;87(7):817–22.
Dong ST, Zhu YH, Yang H, Tang NY, Huang GY, Li J, Tian K. Evaluation of the predictors for unfavorable clinical outcomes of degenerative lumbar spondylolisthesis after lumbar interbody fusion using machine learning. Front Public Health. 2022;10
Klemt C, Harvey MJ, Robinson MG, Esposito JG, Yeo I, Kwon YM. Machine learning algorithms predict extended postoperative opioid use in primary total knee arthroplasty. Knee Surg Sport Tr A. 2022;30(8):2573–81.
Lu YN, Forlenza E, Wilbur RR, Lavoie-Gagne O, Fu MC, Yanke AB, et al. Machine-learning model successfully predicts patients at risk for prolonged postoperative opioid use following elective knee arthroscopy. Knee Surg Sport Tr A. 2022;30(3):762–72.
Katakam A, Karhade AV, Schwab JH, Chen AF, Bedair HS. Development and validation of machine learning algorithms for postoperative opioid prescriptions after TKA. J Orthop. 2020;22:95–9.
Tully JL, Zhong WL, Simpson S, Curran BP, Macias AA, Waterman RS, Gabriel RA. Machine learning prediction models to reduce length of stay at ambulatory surgery centers through case resequencing. J Med Syst. 2023;47(1)
Avery KNL, Richards HS, Portal A, Reed T, Harding R, Carter R, et al. Developing a real-time electronic symptom monitoring system for patients after discharge following cancer-related surgery. Bmc Cancer. 2019;19
• Feinstein M, Katz D, Demaria S, Hofer IS. Remote monitoring and artificial intelligence: outlook for 2050. Anesth Analg. 2024;138(2):350–7. Discusses the implications of remote monitoring within the context of artificial intelligence.
Hassan AM, Biaggi-Ondina A, Rajesh A, Asaad M, Nelson JA, Coert JH, et al. Predicting patient-reported outcomes following surgery using machine learning. Am Surg. 2023;89(1):31–5.
Dwyer T, Hoit G, Burns D, Higgins J, Chang J, Whelan D, et al. Use of an artificial intelligence conversational agent (chatbot) for hip arthroscopy patients following surgery. Arthrosc Sports Med Rehabil. 2023;5(2):e495–505.
Kim WO, Kil HK, Kang JW, Park HR. Prediction on lengths of stay in the postanesthesia care unit following general anesthesia: preliminary study of the neural network and logistic regression modelling. J Korean Med Sci. 2000;15(1):25–30.
Rushlow DR, Croghan IT, Inselman JW, Thacher TD, Friedman PA, Yao XX, et al. Clinician adoption of an artificial intelligence algorithm to detect left ventricular systolic dysfunction in primary care. Mayo Clin Proc. 2022;97(11):2076–85.
Tokita H, Twersky R, Laudone V, Levine M, Stein D, Scardino P, Simon BA. Complex cancer surgery in the outpatient setting: the Josie Robertson Surgery Center. Anesth Analg. 2020;131(3):699–707.
Semple JL, Sharpe S, Murnaghan ML, Theodoropoulos J, Metcalfe KA. Using a mobile app for monitoring post-operative quality of recovery of patients at home: a feasibility study. Jmir Mhealth Uhealth. 2015;3(1)
Amin T, Mobbs RJ, Mostafa N, Sy LW, Choy WJ. Wearable devices for patient monitoring in the early postoperative period: a literature review br. Mhealth. 2021;7(3)
McGillion MH, Parlow J, Borges FK, Marcucci M, Jacka M, Adili A, et al. Post-discharge after surgery virtual care with remote automated monitoring-1 (PVC-RAM-1) technology versus standard care: randomised controlled trial. BMJ. 2021;374:n2209. Provides evidence that virtual postoperative care can provide advantages over traditional care.
Ghomrawi HMK, Baumann LM, Kwon S, Hebal F, Hsiung G, Williams K, et al. Using accelerometers to characterize recovery after surgery in children. J Pediatr Surg. 2018;53(8):1600–5.
Downey C, Randell R, Brown J, Jayne DG. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J Med Internet Res. 2018;20(12)
Ha ACT, Verma S, Mazer CD, Quan A, Yanagawa B, Latter DA, et al. Effect of continuous electrocardiogram monitoring on detection of undiagnosed atrial fibrillation after hospitalization for cardiac surgery: a randomized clinical trial. JAMA Netw Open. 2021;4(8):e2121867.
van den Eijnden MAC, van der Stam JA, Bouwman RA, Mestrom EHJ, Verhaegh WFJ, van Riel NAW, Cox LGE. Machine learning for postoperative continuous recovery scores of oncology patients in perioperative care with data from wearables. Sensors-Basel. 2023;23(9)
Dawes AJ, Lin AY, Varghese C, Russell MM, Lin AY. Mobile health technology for remote home monitoring after surgery: a meta-analysis. Br J Surg. 2021;108(11):1304–14.
Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A. Res NIHRGH Global burden of postoperative death. Lancet. 2019;393(10170):401.
Kamdar N, Jalilian L. Telemedicine: a digital interface for perioperative anesthetic care. Anesth Analg. 2020;130(2):272–5.
Insights FB. Telemedicine market size, share and COVID-19 impact analysis, by type (products and services), by modality (store-ard-forward (asynchronous), real-time (synchronous), and others), by application (teleradiology, telepathology, teledermatology, telecardiology, telepsychiatry, and others), by end-use (healthcare facilities, homecare, and others), and regional forecast, 2023–2030. 2023. Afortunebusinessinsights.com/industry-reports/telemedicine-market-101067.
Bridges KH, McSwain JR, Wilson PR. To infinity and beyond: the past, present, and future of tele-anesthesia. Anesth Analg. 2020;130(2):276–84.
Flick M, Saugel B. Continuous ward monitoring: the selection, monitoring, alarms, response, treatment (SMART) road map. Brit J Anaesth. 2021;127(5):675–7.
Abelson JS, Symer M, Peters A, Charlson M, Yeo H. Mobile health apps and recovery after surgery: what are patients willing to do? Am J Surg. 2017;214(4):616–22.
Linton SC, De Boer C, Tian Y, Alayleh A, Bouchard ME, Figueroa A, et al. Effect of consumer-grade wearable device data on clinician decision making during post-discharge telephone calls after pediatric surgery. J Pediatr Surg. 2022;57(9):137–42.
Melstrom LG, Zhou XK, Kaiser A, Chan K, Lau C, Raoof M, et al. Feasibility of perioperative remote monitoring of patient-generated health data in complex surgical oncology. J Surg Oncol. 2023;127(1):192–202.
Leenen JPL, Ardesch V, Patijn G. Remote home monitoring of continuous vital sign measurements by wearables in patients discharged after colorectal surgery: observational feasibility study. JMIR Perioper Med. 2023;6:e45113.
McLean KA, Sgro A, Brown LR, Buijs LF, Daines L, Potter MA, et al. Evaluation of remote digital postoperative wound monitoring in routine surgical practice. NPJ Digit Med. 2023;6(1):85.
Armstrong KA, Coyte PC, Brown M, Beber B, Semple JL. Effect of home monitoring via mobile app on the number of in-person visits following ambulatory surgery: a randomized clinical trial. JAMA Surg. 2017;152(7):622–7.
Temple-Oberle C, Yakaback S, Webb C, Assadzadeh GE, Nelson G. Effect of smartphone app postoperative home monitoring after oncologic surgery on quality of recovery: a randomized clinical trial. JAMA Surg. 2023;158(7):693–9.
Leyendecker JYN, Prasse T, Eysel P, Bredow J, Hofstetter CP. Outpatient fully endoscopic cervical unilateral laminotomy for bilateral decompression with virtual postoperative monitoring. J Minim Invasive Spine Surg Tech. 2023;8(1):28–35.
Huzell MAFJ, Dalberg K. Randomized clinical trial comparing perioperative care for breast cancer patients at a patient hotel versus a general surgical ward. Patient Exp J. 2015;2(2):153–63.
Chadha RM, Paulson MR, Avila FR, Torres-Guzman RA, Maita KC, Garcia JP, et al. A virtual hybrid care hotel model supports the recovery of post-procedural patients with mild to severe systemic diseases. Am Surg. 2023;89(6):2247–53.
Chadha RM, Paulson MR, Avila FR, Torres-Guzman RA, Maita K, Garcia JP, et al. Surgical patient satisfaction with a virtual hybrid care hotel model: a retrospective cohort study. Ann Med Surg (Lond). 2022;74:103251.
Author information
Authors and Affiliations
Contributions
Alberto E Ardon: This author contributed to the literature search, reviewed articles, and helped write and edit the manuscript.
Ryan Chadha: This author contributed to the literature search, reviewed articles, and helped write and edit the manuscript.
John George III: This author contributed to the literature search, reviewed articles, and helped write and edit the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors have no financial relationships to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ardon, A., Chadha, R. & George, J. Post-discharge Care and Monitoring: What’s new, What’s Controversial. Curr Anesthesiol Rep 14, 299–305 (2024). https://doi.org/10.1007/s40140-024-00627-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-024-00627-y