Abstract
Purpose of Review
To present recent developments in pediatric regional anesthesia (RA) including safety, complications, special populations, evidence-based trends, and ultrasound guidance. The data presented should be used to improve outcomes of children receiving RA.
Recent Findings
Current data demonstrates a very low occurrence of serious complications and validates the use of GA for block placement in children. Fewer neuraxial blocks are performed in favor of peripheral blocks as the use of ultrasound guidance has made new blocks possible and old blocks better and safer. Neonates and infants have more RA options. Adjuvant medications and ambulatory catheters increase the duration of RA and facilitate cost-effective, low-risk hospital discharge. The local anesthetic systemic toxicity checklist reinforces new guidelines.
Summary
Increasing pediatric-specific RA data shows increased analgesic choices and improving patient care. Large well-designed studies to precisely define the risks and benefits and appropriate applications of novel ideas and technologies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regional anesthesia (RA) is an important therapeutic modality for pain management in children. RA provides excellent pain relief and allows pediatric caregivers to use multimodal analgesic techniques and decrease opioids as the primary pain treatment modality [1•, 2•]. Considerable progress has been made in the practice of pediatric RA over the past few years with abundant new literature on safety, shifts away from neuraxial blocks, novel RA techniques, incorporation of ultrasound guidance, and new understanding of local anesthetic systemic toxicity (LAST) and treatment. Increasing RA options are available to children including neonates and small infants. This paper is not a comprehensive review of pediatric RA and will focus on recent advances and developments.
Safety
Patient safety is always the first consideration when choosing a pain management plan for children. Increasing amounts of data attest to the efficacy and safety of pediatric RA [3••, 4••, 5•]. Several large pediatric-specific RA databases from Europe and North America provide evidence from large numbers of children that delineate trends in practice and clarify safety concerns [3••, 4••]. The Pediatric Regional Anesthesia Network (PRAN) reported on the safety profile of 104,393 regional anesthetics in children under 18 years of age and the Association des Anesthésistes Réanimateurs Pédiatriques d’Expression Française (ADARPEF) reports on 31,132 children. The combined results from both databases, 135,525 children, demonstrate the rarity of complications. There was zero mortality and no complications resulted in permanent sequelae. The most common serious complication was transient neurologic deficit which occurred at a rate of 1.3–2.4/10,000 with the highest risk group being children 10 and older. All transient deficits, however, were sensory in nature and resolved by 6 months. LAST occurred in 0.67–5/10,000 patients and more commonly in infants. Data on indwelling continuous catheters demonstrate that, aside from technical issues related to the catheter itself, placing indwelling perineural catheters do not increase the incidence of serious complications, with the exception of deep infections [3••, 4••, 6•, 7]. There was one epidural abscess and four deep infections in the epidural catheter group. Although rare, infections are directly proportional to the duration of using the catheter. Catheters should be discontinued as soon as they are no longer necessary [8]. Miscellaneous complications included post-dural puncture headaches (n = 11), epidural hematoma (n = 1), colonic punctures (n = 2), pneumothorax (n = 1), wrong-sided blocks (n = 2), broken needles requiring surgical removal (n = 2), intra-abdominal catheter (n = 1), and total spinals with apnea (n = 3). Overall, serious complications occurred more often with neuraxial blocks than peripheral nerve blocks (PNBs) [1•, 2•, 3••]. The ADARPEF researchers report the incidence of serious complications following neuraxial and PNBs as 0.26 and 0.04%, respectively, making a compelling argument for the use of PNBs, when feasible.
Studies on hypospadias repair document a 4–13-fold increase in the incidence of urologic complications when a caudal block was used compared with a penile block or no block [9••]. This raises concerns about the use of caudals in this population. Confounding factors such as severity of the hypospadias deformity were adjusted for in the analysis. Potential explanations include penile engorgement from sympathetic blockade and vasodilation of sinuses. Therefore, the benefits of caudal analgesia must be weighed against the risk in these patients.
RA in pediatric patients > 6 months is almost always administered after the induction of general anesthesia (GA) (93.7–95.9%) or under heavy sedation due to safety concerns of performing RA in an anxious, awake, and uncooperative patient [3••, 8]. Data shows no difference in the occurrence of LAST between awake and asleep patients but placing blocks in awake or lightly sedated patients was associated with an increased incidence of transient neurologic symptoms in the PRAN study [4••, 10••]. Studies that focused on interscalene blocks specifically, considered a greater risk block in adults, reports no increased risk of complications when they were performed on children who were already anesthetized [11, 12]. Performing RA on children under GA is, thus, associated with good safety and should be viewed as the standard of care.
Overall, data indicate that PNBs confer added safety compared with neuraxial techniques [3••, 4••, 9••].
LAST and Treatment
The incidence of LAST is greater in infants compared with older children and adults [3••, 13••]. Data from the PRAN database indicates that 71.4% of reports of LAST occurred in infants < 12 months even though they comprised only 22.8% of the total number of children [4••]. LAST occurred in 5/23,706 (.02%) children < 12 months old while the overall reported rate was 7/104,393 (0.0076%). Another recent publication reviewed all reports of adult and pediatric LAST in the literature from 2014 to 2016 and indicated that 20% of all reported cases of LAST (n = 10) occurred in children < 12 months of age [13••]. Combining the two reports reveals some patterns. LAST occurred most often during bolus administration of local anesthetic (LA) with unrecognized intravascular injection (13/14), and was infrequently associated with LA drug overdose (2/14). Infants receiving penile nerve block appear to be uniquely susceptible to LAST accounting for over half of all reports (9/14) [13••, 14]. When children were anesthetized, cardiac symptoms were the most common manifestation of LAST (9/10) whereas if the children were awake, seizure activity was the most common manifestation (3/4) [13••, 14].
The best course regarding LAST is to prevent it. Given the reduced myocardial toxicities of ropivacaine and levobupivacaine, these local anesthetics are preferred in clinical situations when available [15, 16]. When LAST is suspected, malignant cardiac arrhythmias and cardiac collapse are treated different from other forms of cardiac emergencies [17••, 18]. Guidelines recommend early aggressive airway management to prevent hypoxia, acidosis, and hypercarbia which potentiate LAST and make resuscitation more difficult [17••]. Low-dose epinephrine should be titrated as needed to support hemodynamic parameters, starting with a dose of 1 mcg/kg IV. Evidence supports a critical role for intralipid emulsion (ILE) in reversal of LAST [17••, 19, 20••]. The current understanding of the mechanism of action of ILE in the treatment of LAST supports a multifactorial mechanism, which includes the creation of a lipid compartment to segregate the LA and remove it. In addition, ILE also provides direct vasoconstrictive and inotropic benefits [17••, 20••]. Case reports support the use of ILE to treat LAST in children [4••, 19] and positive responses immediately after the administration of ILE favor early administration. Current recommendations suggest administering a bolus of 1.5 ml/kg ILE over 2–3 min at the first suggestion of LAST such as arrhythmia, seizure, or progressive clinical deterioration [17••]. An infusion of 0.25 ml/kg/min should follow and continue for 10 min following attainment of hemodynamic stability. The maximum recommended therapeutic dose of ILE has recently been increased to 12 ml/kg but resolution of symptoms is usually achieved with smaller doses. And, in the era of checklists, a checklist approved by the ASRA Committee on treatment of LAST is available [21••].
Central vs. Peripheral Location of RA
All RA techniques that are used in adults are also used in children, although the epidemiology of pediatric surgical conditions makes the distribution of RA techniques different [3••, 4••, 22]. The reduced frequency of serious adverse events with PNBs compared with neuraxial blocks has increased the use of PNBs in direct proportion to increasing age [3••, 4••, 22]. Overall, PNBs now constitute the majority, 50–66%, of RA. Neuraxial techniques, however, continue to be more prevalent in neonates and infants < 6 months where they represent 50% (ADARPEF)–81.4% (PRAN) of RA. The vast majority of these are caudal and subarachnoid blocks. The most common PNBs are penile and trunk blocks (ilioinguinal-iliohypogastrc, rectus sheath, and transversus abdominus plane {TAP}). The common use of penile blocks may place this group at greater risk for LAST [3••, 4••, 13••, 14]. Continuous catheters in neonates and infants are less common and represent only 5.9% (ADARPEF)–15.5% of all RA in this group. In contrast, for patients 10–12 years and older, neuraxial blocks represent only 18.5% (ADARPEF)–21% (PRAN) of blocks with the majority being thoracic and lumbar continuous epidural catheters. In older patients, the most common PNBs are, by far, blocks of the lower extremity. Catheters are used more frequently in older children and represent about 24% of RA [3••, 4••].
Adjuvants and Additives
Although most blocks in younger children are single-shot caudal epidurals, the brief duration of analgesia (4–8 h) is a limitation [23]. The addition of adjuvant medications to LA for caudals improves the quality of analgesia and prolongs analgesia [8]. The most common and best studied adjuvant is clonidine, a centrally acting alpha-agonist, whose efficacy and safety are established in the pediatric literature [8, 24]. Meta-analysis of addition of clonidine, 1–2 mcg/kg, to LA in caudals demonstrates an average prolongation of analgesia of 4 h with no difference in complications and side effects. Somnolence is reported with larger doses of clonidine.
A meta-analysis of 21 clinical trials that compared dexmedetomidine, an alpha-agonist with 8-times greater affinity for receptors than clonidine, as an additive (1 mcg/kg) to LA demonstrated a 2.5–3 fold greater duration of analgesia [25•]. Side effects include an increased emergence time and PACU somnolence, but decreased emergence delirium in children who received dexmedetomidine. Hemodynamic effects were not observed with doses limited to 1 mcg/kg. Dexmedetomidine has not undergone rigorous testing on nerves and is not FDA approved for perineural use but animal testing of the effects of dexmedetomidine on the rat sciatic nerve failed to elicit histologic changes to nerves at 24 h and 14 days [26]. The addition of dexmedetomidine to bupivacaine results in less perineural inflammation at 24 h compared with bupivacaine and saline control groups. In rabbits, 10 mcg/kg (10× clinical doses) epidural dexmedetomidine revealed demyelination. The significance of this study remains debated [27].
Ketamine causes spinal cord toxicity in clinically relevant doses in animal models and is not recommended for perineural use in children [28]. Due to limited toxicity data of all adjuvants in children, limiting doses to the minimum doses needed for efficacy are recommended.
Either perineural or intravenous dexamethasone increased the duration of sensory block in upper extremity blocks in adults compared with placebo by about 6 h, without side effects [29]. Perineural dexamethasone prolonged the duration of block by 3 h compared with intravenous dexamethasone. Postoperative opioid consumption in the dexamethasone groups was significantly less than in the placebo group. There is insufficient evidence to evaluate the effectiveness of dexamethasone in lower extremity blocks and in all blocks in children.
Ultrasound
The embrace of ultrasound guidance for RA has dramatically changed the practice of pediatric RA by accelerating the development of new RA techniques and improving the effectiveness of the blocks [30•, 31]. Ultrasound guidance for PNBs in children has increased from approximately 30% to over 90% in 10 years [4•]. Ultrasound imaging allows real-time visualization of key landmarks, the target structure (a nerve or fascial plane), organs and vessels relative to an advancing needle, and spread of LA. Ultrasound guidance may be more important in children than adults as their smaller size makes critical structures closer to target structures. In pediatric RA, ultrasound guidance generally decreases block performance time when compared to nerve stimulation technique, results in increased efficacy including greater success rates, improved block quality, reduces doses of LA, and greater duration of block [30•, 32, 33]. The adult literature documents a decreased incidence of LAST with ultrasound guidance [34], although pediatric-specific data documenting changed incidence of infrequent complications as a result of ultrasound guidance is lacking.
Ultrasound is still infrequently used for placement of neuraxial blocks but evidence suggests opportunities for increased success rate and decreased complications [4••, 35,36,37,38,39]. Many new ultrasound-guided techniques of RA previously performed in children using landmark or nerve stimulation techniques are appearing [32, 33, 37, 40,41,42,43,44,45,46,47]. Ultrasound guidance has enabled the creation of a new and expanding group of PNBs, especially those that target nerves within fascial planes. These blocks include the fascia iliaca (FI), transversus abdominus plane (TAP), quadratus lumborum (QLB), and erector spinae plane (ESP) blocks. There are sparse data on the use of most of these newer blocks in children.
Novel PNB Techniques
The FI block deposits LA under the investing fascia of the iliacus muscle at the level of the anterior superior iliac spine (ASIS) and covers the femoral, lateral femoral cutaneous and, less reliably, the obturator nerves. This block is effective for surgeries that involve the hip, anterolateral thigh, and distal femur [40]. This block was traditionally performed via landmarks and, in spite of demonstrated efficacy [47, 48], was under-used until the introduction of ultrasound guidance. Advantages include a more peripheral location compared to epidual or lumbar plexus blocks, thus imparting additional safety and removing the need (and time) to turn the patient lateral. Studies document efficacy and evidence of dermatomal coverage of the targeted nerves [40, 47, 48]. FI blocks with the needle tip directed above the inguinal ligament likely have greater success rates than those performed below the ligament although direct comparative studies in either adults or children have not been forthcoming [47, 49].
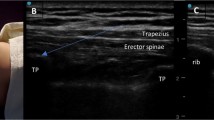
The TAP block is an abdominal wall block with conflicting efficacy reported in the pediatric literature [50,51,52,53]. This block is classically performed using ultrasound guidance with the transducer positioned in the midaxillary line with LA being deposited between the internal oblique and transversus abdominis muscles (Fig. 1). The dermatomal coverage in children is undocumented but dermatomal coverage in adults is shown to be unreliable which likely explains the conflicting effectiveness [54••]. The quadratus lumborum (QLB) muscle block, also referred to as a posterior TAP block, is an alternative abdominal wall block that targets the thoracoabdominal nerves in a more proximal location compared with the TAP (Fig. 1) [46, 55, 56]. More reliable dermatomal coverage has been demonstrated in both adults and children with predictable coverage from L1/T12 up to T9/T8 [46, 56•]. In randomized controlled trials, better efficacy and greater duration of analgesia are reported for the QLB when compared with a midaxillary TAP block [56•, 57, 58]. In addition to providing abdominal wall analgesia, case reports document analgesic efficacy for surgeries of the hip [59]. Different techniques for the QLB are described with LA being deposited at either the lateral-posterior border (lateral; QL1), the medial-posterior border (medial; QL2), or the anterior border (QL3; transmuscular) of the quadratus lumborum muscle [60]. The clinical differences between these variations remain unclear.
The erector spinae plane (ESP) block, a fascial plane block with deposition of LA at the anterior aspect of the ES muscle adjacent to the 5th transverse process, has been used for thoracoabdominal surgeries [61, 62]. Visceral coverage is unclear but dermatomal coverage to the midline has been demonstrated [61]. There are no randomized controlled trials in adults or children, although a review containing 242 case reports, including 23 children, reported reduced opioid consumption in 76.0% of patients [61]. These data must be interpreted cautiously as 90.9% of patients also received IV or PO multimodal medications and there were no comparison groups. Compared with an epidural, the location remote from the spinal cord has the advantages of decreased likelihood of direct cord trauma, epidural bleed, and deep infection. It is also theoretically safer than more proximal PNBs such as intercostal or paravertebral when presented with a mild coagulopathy or anticoagulants. No hemodynamic instability is reported and there is one major reported complication, a pneumothorax [63•]. There is insufficient evidence to support routine use of ESP as an alternative to more conventional RA.
Pudendal nerve blocks impede sensation to the perineum and are widely used in adult patients with sparse pediatric studies [45, 64, 65]. Studies indicate that the pudendal nerve block is an effective alternative to caudal analgesia in children with better postoperative pain management. There is, however, new interest in this block due to the association between caudal epidurals and the increased incidence of fistulous complications in hypospadias surgery [9••, 66]. Both ultrasound and nerve stimulation guided techniques are described with ultrasound guidance offering the potential advantage of visualizing vessels and the rectum.
Contraindications
Absolute contraindications to RA are similar to those for adults and include absolute allergy to LA agents, (guardian) refusal, systemic infection, or infection at the insertion site. True allergic reactions are rare and require further identification of the LA class as there is no cross reactivity between amides and esters. The frequency of allergic reactions with ester LA is greater than with amides.
Relative contraindications to RA are more controversial. Placement of RA in the presence of coagulopathies depends on the degree of anticoagulation and the location of the nerve relative to the neuroaxis and ability to compress [67, 68•]. For neuraxial blocks (epidurals and spinals), a coagulopathy is an absolute contraindication. The evidence to direct decisions on deep plexus (lumbar plexus) and paraneuraxial (paravertebral) blocks in the anticoagulated patient is less clear, although guidelines suggest practitioners adhere to the ASRA guidelines for these blocks. For more peripheral PNBs, there is little evidence to support making any recommendations. Therefore, the experience of the proceduralist and the risk-to-benefit ratio must be considered. Treated bacteremia is not an absolute contraindication to RA but, when present, the risk and benefits need to be carefully considered.
RA in Neonates and Young Infants: Special Consideration
It is often challenging to provide safe and effective perioperative care to increasingly complex neonates and younger infants. While newer anesthetic medications and better monitoring are available in modern operating rooms, young infants, especially preterm infants, continue to be sensitive to the myocardial and respiratory depressant effects of both inhaled and intravenous anesthetics used for GA. Prematurity, age < 12 months, and postoperative status are the strongest predictors for critical events [69•, 70, 71, 72]. Exposure to GA also raises concerns about the potential effects of GA on neurodevelopment [73,74,75, 76••]. There are reports of accelerated neuroapoptosis in developing brain tissue in primate and rodent models after exposure to GA with long-term behavioral and intellectual sequelae. The relevance of these data on developing human brains remains unclear and widely debated [77, 78]. RA as a sole anesthetic and substitute for GA may, thus, be desirable in younger and more fragile patients in whom it is well tolerated and effective [79,80,81,82,83]. The GAS study compared GA with spinal anesthesia (SA) in infants undergoing inguinal herniorrhaphy. Although there was no evidence of developmental delay or neurotoxicity from either anesthetic technique, better hemodynamic stability was apparent in the SA group. Infants in the GA group exhibited significant hypotension with MAPs < 45 and/or MAP < 35 in 87% and 49% respectively compared with the RA group with 41% and 16% respectively [76••]. The incidence of early postoperative apneas was decreased when RA was used compared with GA but late apneas were similar [83]. PNBs are rarely used as a substitute for GA in neonates and infants but provide excellent analgesia. Techniques for use in infants and neonates have been described [42,43,44,45]. Thus, RA may confer both theoretical and practical benefits in young infants.
The dose of LA in infants requires special consideration. Amide LA are highly protein bound and thus prone to accumulation in neonates and young infants due to decreased plasma proteins and immature hepatic enzymes [84,85,86]. The expression of various cytochrome P450 enzymes differs as a function of age and development but, in general, the enzymes are all immature at birth and have an activity of roughly 0–10% of adult values, increase to 30–40% by 1 month and approach adult levels at about 1 year making clearance and half-life (t1/2) of LA very dependent on age. Initial bolus doses are not significantly different between different ages of infants and children and drug accumulation occurs with continuous infusions or repeated bolus administration. The literature supports a maximum recommended infusion dose of bupivacaine, ropivacaine, and levobupivacaine of 0.2 mg/kg/h in neonates and infants < 3 months of age, 0.3 mg/kg/h for < 6 months, and 0.4 mg/kg/h if > 6 months [87, 88•]. When these age-related dosing recommendations are followed, plasma concentrations are well below the threshold for systemic toxicity for less than 48-h infusions [8, 87]. (Children with chronic disease and significant co-morbidities may have hypoalbuminemia and delayed metabolism which may increase drug accumulation. In such cases, a cautious approach to dosing should be used.)
2-Chloroprocaine is an aminoester class LA that is not protein bound, is metabolized by serum plasma-esterase, and characterized by a short half-life [85]. For these reasons, it is popular in infants for LA infusions. In neonates and infants, continuous epidural and paravertebral infusions of 2-chloroprocaine report widespread efficacy without complications [88•, 89, 90]. Infusion rates of 1.5% 2-chloroprocaine at 0.45–0.7 mL/kg/h (6–10 mg/kg/h) are documented with safety. There are 2 isolated reports of toxicity in infants associated with 2-chloroprocaine (a seizure and a cardiac arrhythmia) and each event was short-lived with no sequelae [91, 92].
Ambulatory Catheters
Pain should also be adequately addressed after hospital discharge as inadequate pain relief and adverse drug effects are the two most common reasons for prolonged length of stay and hospital readmission [93]. Single-shot PNBs and ambulatory RA catheter programs are available for pediatric patients to allow the benefit of RA at home with demonstrated safety, decreases in unanticipated hospital admissions, and cost savings [6•, 94]. Although the role of ambulatory catheters to decrease exposure to opioids in the outpatient setting is undocumented, there is no reason to suspect that catheters will not decrease opioid demand. This may be particularly important in adolescents in whom opioid abuse may be as great as 5.9% in patients first exposed in the perioperative setting [95••, 96••].
Controversies in Pediatric RA
Caution should be exercised when administering RA to patients with preexisting neuropathies. “Although PNBs may theoretically increase the risk of new or progressive postoperative neurologic complications, existing data can neither confirm nor refute this theory in clinical practice. A careful risk-to-benefit assessment of regional anesthesia to alternatives is warranted” [97]. When placing a PNB in a patient with a preexisting neuropathy, ultrasound use is suggested for nerve localization to minimize the number of needle passes and help to maintain a safe distance from the nerve. Smaller volumes and lower concentrations of LA medications are advised for both bolus and infusion administration.
Safe and effective pain management with RA in pediatric patients after surgery of the upper and lower extremities can represent a challenge of balancing adequate pain control with the risk of masking ischemic conditions, most notoriously, acute compartment syndrome (ACS). Pediatric series demonstrate the safe use of RA in patients at risk for ACS and there is no evidence that RA, especially PNBs, mask the ischemic pain of ACS [98••, 99]. The use of RA in patients at risk for ACS is, however, debated with many surgeons still taking a very conservative approach of avoidance. ACS occurs when pressure is increased in a closed muscle compartment and occurs most often after trauma with or without long bone fractures in the lower leg and forearm [100••]. Knowledge of presenting signs and symptoms of and a low index of suspicion for diagnosis of ACS is imperative for best patient care and outcomes. Pediatric patients with ACS present with the “3 As,” agitation, anxiety, and (increasing) analgesic requirement in contrast to adults who present with the “6 Ps,” pain, paresthesia, pallor, paralysis, pulselessness, and poikilothermia [100••]. While most patients present with escalating pain as a major component of their evolving compartment syndrome, painless compartment syndrome can occur and practitioners need to be vigilant to this possibility [101•]. Vascular injuries with occlusion and overly tight bandaging and casting can also cause ischemia and warrant the same vigilance and concerns as ACS.
To speed diagnosis, at-risk patients should be identified in advance and guidelines established for the Institution should be followed. When a perineural catheter is used in a patient at risk for ACS and ischemia, the LA bolus and infusion must use the lowest dose and concentration of LA that provides relief and prevents motor blockade [100••, 102]. PNBs have an advantage over epidurals as a selective PNB will spare blocking of non-operative aspects of the extremity and, thus, pain occurring outside the operative and blocked area should facilitate the diagnosis of ACS. ACS must be ruled out in any child with notable escalation in pain from baseline rather than a presumption of block failure. (This rule also applies when RA is not used and opioid administration is escalating.) Education of all involved caregivers is essential for timely recognition and treatment. Guidelines should include rapid escalation of information to more experienced physicians as delay of treatment beyond 4–8 h likely will result in permanent injury [100••, 102]. An example of guidelines is presented in Table 1.
Conclusions
Increasing amounts of pediatric-specific data and experience with pediatric RA is directing analgesic choices. Current data demonstrate a very low occurrence of serious complications, which is comparable with adult data and validate the use of GA for block placement in children. There is a shift away from neuraxial blocks in favor of PNBs as the use of ultrasound guidance has made new blocks possible and old blocks better and safer. Adjuvant medications and ambulatory catheter programs allow for increased RA duration and are factors in cost-effective, low-risk hospital discharge. Neonates and infants have more RA options. A revised LAST checklist reinforces new guidelines including less delay to ILE administration. Progress in pediatric RA should continue with large well-designed studies to precisely define the risks and benefits, and appropriate applications of novel ideas and technologies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Bosenberg A. Benefits of regional anesthesia in children. Paediatr Anaesth. 2012;28(8):684–5 Overview of basic pediatric regional anesthesia from a beloved expert.
• American Society of Anesthesiologists Task Force. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–73 Outlines increasingly important concepts of multi-modal therapy from the highest sources.
•• Ecoffey C, Lacroix F, Giaufré E, Orliaguet G, Courrèges P. Epidemiology and morbidity of regional anesthesia in children: a follow-up one-year prospective survey of the French-Language Society of Paediatric Anaesthesiologists (ADARPEF). Paediatr Anaesth. 2010;20(12):1061–9 Landmark data collection study in pediatric regional anesthesia for safety analysis.
•• Walker BJ. Complications in pediatric regional anesthesia. An analysis of more than 1,000.000 blocks from the Pediatric Regional Anaesthesia Network. Anesthesiology. 2018;129(4):721–32 Most recent landmark data collection study in pediatric regional anesthesia for safety analysis with over 100,000 children.
• Vecchione T, Zurakowski D, Boretsky K. Thoracic paravertebral nerve blocks in pediatric patients: safety and clinical experience. Anesth Analg. 2016;123(6):1588–90 In-depth analysis of safety and applications of paravertebral nerve blocks.
• Gurnaney H, Kraemer FW, Maxwell L, Muhly WT, Schleelein L, Ganesh A. Ambulatory continuous peripheral nerve blocks in children and adolescents: a longitudinal 8-year single center study. Anesth Analg. 2014;118(3):621–7 Single-center study looking at a stable, ongoing ambulatory catheter program.
Long JB, Joselyn AS, Bhalla T, Tobias JD, De Oliveira GS, Suresh S. The use of neuraxial catheters for postoperative analgesia in neonates: a multicenter safety analysis from the Pediatric Regional Anesthesia Network. Anesth Analg. 2016;122(6):1965–70.
Suresh S, Ecoffey C, Bosenberg A, Lonnqvist PA, De Oliveira GS, De Leon Casasola O, et al. The European Society of Regional Anaesthesia and Pain Therapy/American Society of Regional Anesthesia and Pain Medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43(2):211–6.
•• Taicher BM, Routh JC, Eck JB, Ross SS, Wiener JS, Ross AK. The association between caudal anesthesia and increased risk of postoperative surgical complications in boys undergoing hypospadias repair. Paediatr Anaesth. 2017;27(7):688–94 Study reveals a surprising association between caudal analgesia and urologic complications after hypospadias repair.
•• Taenzer AH, Walker BJ, Bosenberg AT, Martin L, Suresh S, Polaner DM, et al. Asleep versus awake: does it matter?: Pediatric regional block complications by patient state: a report from the Pediatric Regional Anesthesia Network. Reg Anesth Pain Med. 2014;39(4):279–83 Study specifically addresses the question of safety of performing RA with patients asleep.
Gurnaney H, Muhly WT, Kraemer FW, Cucchiaro G, Ganesh A. Safety of pediatric continuous interscalene block catheters placed under general anesthesia: a single center’s experience. Acta Anaesthesiol Scand. 2015;59(3):377–83.
Taenzer A, Walker B, Bosenberg A, Krane E, Martin L, Polaner D, et al. Interscalene brachial plexus blocks under general anesthesia in children: is this safe practice?: A report from the Pediatric Regional Anesthesia Network (PRAN). Reg Anesth Pain Med. 2014;39(6):502–5.
•• Gitman M, Barrington MJ. Local anesthetic systemic toxicity: a review of recent case reports and registries. Reg Anesth Pain Med. 2018;43(2):124–30 First study to identify risk factors for LAST in infants and children.
Yu RN, Houck CS, Casta A, Blum RH. Institutional policy changes to prevent cardiac toxicity associated with bupivacaine penile blockade in infants. A A Case Rep. 2016;7(3):71–5.
Groban L, Deal DD, Vernon JC, James RL, Butterworth J. Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine, and ropivacaine in anesthetized dogs. Anesth Analg. 2001;92(1):37–43.
Stewart J, Kellett N, Castro D. The central nervous system and cardiovascular effects of levobupivacaine and ropivacaine in healthy volunteers. Anesth Analg. 2003;97(2):412–6.
•• Neal JM, Barrington MJ, Fettiplace MR, Gitman M, Memtsoudis SG, Mörwald EE, et al. The third American Society of Regional Anesthesia and Pain Medicine practice advisory on local anesthetic systemic toxicity: executive summary 2017. Reg Anesth Pain Med. 2018;43(2):113–23 Very recent update on the understanding of LAST with revisions to guidelines for treatment treatment.
Hiller DB, Di Gregorio G, Ripper R, Kelly K, Massad M, Edelman L, et al. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009;111(3):498–505.
Presley JD, Chyka PA. Intravenous lipid emulsion to reverse acute drug toxicity in pediatric patients. Ann Pharmacother. 2013;47(5):735–43.
•• Fettiplace MR, Weinberg G. The mechanisms underlying lipid resuscitation therapy. Reg Anesth Pain Med. 2018;43(2):138–49 Outlines current understanding of the mechanism of ILE for the treatment of LAST.
•• Neal JM, Woodward CM, Harrison TK. The American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2017 version. Reg Anesth Pain Med. 2018;43(2):150–3 An excellent checklist for treatment of LAST.
Rochette A, Dadure C, Raux O, Troncin R, Mailhé P, Capdevila X. A review of pediatric regional anesthesia practice during a 17-year period in a single institution. Paediatr Anaesth. 2007;17(9):874–80.
Tsui BCH, Berde CB. Caudal analgesia and anesthesia techniques in children. Curr Opin Anaesthesiol. 2005;18:283–8.
Schnabel A, Poepping DM, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of clonidine as additive for caudal regional anesthesia: a quantitative systematic review of randomized controlled trials. Paediatr Anaesth. 2011;21:1219–30.
• Trifa M, Tumin D, Tobias JD. Dexmedetomidine as an adjunct for caudal anesthesia and analgesia in children. Minerva Anestesiol. 2018;84(7):836–47 Nice meta-analysis of studies on perineural administration of dexmedatomidine, the newest addition to the LA adjuvants.
Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. 55.
Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25:403–9.
Vranken JH, Troost D, De Haan P, Pennings FA, Van Der Vegt MH, Dijkgraaf MGW, et al. Severe toxic damage to the rabbit spinal cord after intrathecal administration of preservative-free S(+)-ketamine. Anesthesiology. 2006;105:813–8.
Pehora C, Pearson AME, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017, Issue 11. Art. No: CD011770.
• Lam DKM, Corry GN, Tsui BCH. Evidence for the use of ultrasound imaging in pediatric regional anesthesia: a systematic review. Reg Anesth Pain Med. 2016;41(2):229–41 Identifies the benefits of adding ultrasound guidance to RA.
Bosenberg AT. Innovative peripheral nerve blocks facilitated by ultrasound guidance. Paediatr Anaesth. 2018;28(8):684–5.
Oberndorfer U, Marhofer P, Bösenberg A, Willschke H, Felfernig M, Weintraud M, et al. Ultrasonographic guidance for sciatic and femoral nerve blocks in children. Br J Anaesth. 2007;98:797–801.
Dolan J, Lucie P, Geary T, Smith M, Kenny GNC. The rectus sheath block: accuracy of local anesthetic placement by trainee anesthesiologists using loss of resistance or ultrasound guidance. Reg Anesth Pain Med. 2009;34(3):247–50.
Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med. 2013;38(4):289–99.
Tsui BCH, Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology. 2010;112(3):719–28.
Lundblad M, Lönnqvist PA, Eksborg S, Marhofer P. Segmental distribution of high-volume caudal anesthesia in neonates, infants, and toddlers as assessed by ultrasonography. Paediatr Anaesth. 2011;21(2):121–7.
Keplinger M, Marhofer P, Klug W, Reiter B, Stimpfl T, Kettner SC, et al. Feasibility and pharmacokinetics of caudal blockade in children and adolescents with 30–50 kg of body weight. Paediatr Anaesth. 26(11):1053–9.
Sinskey JL, Vecchione TM, Ekstrom BG, Boretsky K. Benefits of ultrasound imaging for placement of caudal epidural blockade in 3 pediatric patients. A A Pract. 2018;10(11):307–9.
Kelleher S, Boretsky K, Alrayashi W. Images in anesthesiology: use of ultrasound to facilitate neonatal spinal anesthesia. Anesthesiology. 2017;126(3):561.
Miller BR. Ultrasound-guided fascia iliaca compartment block in pediatric patients using a long-axis, in-plane needle technique: a report of three cases. Paediatr Anaesth. 2011;21(12):1261–4.
Kelleher S, Boretsky K. Use of ultrasound to facilitate placement of spinal anesthetic in a 1.5 kg infant. Anesthesiology. 2017;126(3):561.
Boretsky K, Visoiu M, Bigeleisen P. Ultrasound-guided approach to the paravertebral space for catheter insertion in infants and children. Paediatr Anaesth. 2013;23(12):1193–8.
Fredrickson MJ, Seal P. Ultrasound-guided transversus abdominis plane block for neonatal abdominal surgery. Anaesth Intensive Care. 2009;37(3):469–72.
Tognù A, Cauli V, De Simone N, Aurini L, Manfrini M, Bonarelli S. In-plane ultrasound-guided lumbar plexus block using catheter-over-needle technique in a 14-month-old baby. Reg Anesth Pain Med. 2016;41(4):538–41.
Gaudet-Ferrand I, De La Arena P, Bringuier S, Raux O, Hertz L, Kalfa N, et al. Ultrasound-guided pudendal nerve block in children: a new technique of ultrasound-guided transperineal approach. Paediatr Anaesth. 2018;28(1):53–8.
Hernandez MA, Vecchione T, Boretsky K. Dermatomal spread following posterior transversus abdominis plane block in pediatric patients: our initial experience. Paediatr Anaesth. 2017;27(3):300–4.
Eastburn E, Hernandez MA, Boretsky K. Technical success of the ultrasound-guided supra-inguinal fascia iliaca compartment block in older children and adolescents for hip arthroscopy. Paediatr Anaesth. 2017;27(11):1120–4.
Neubrand TL, Roswell K, Deakyne S, Kocher K, Wathen J. Fascia iliaca compartment nerve block versus systemic pain control for acute femur fractures in the pediatric emergency department. Pediatr Emerg Care. 2014;30(7):469–73.
Shariat AN, Hadzic A, Xu D, Shastri U, Kwofie K, Gandhi K, et al. Fascia iliaca block for analgesia after hip arthroplasty: a randomized double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2013;38(3):201–5.
Sandeman DJ, Bennett M, Dilley AV, Perczuk A, Lim S, Kelly KJ. Ultrasound-guided transversus abdominis plane blocks for laparoscopic appendicectomy in children: a prospective randomized trial. Br J Anaesth. 2011;106(6):882–6.
Carney J, Finnerty O, Rauf J, Curley G, McDonnell JG, Laffey JG. Ipsilateral transversus abdominis plane block provides effective analgesia after appendectomy in children: a randomized controlled trial. Anesth Analg. 111(4):998–1003.
Lapmahapaisan S, Tantemsapya N, Aroonpruksakul N, Maisat W, Suraseranivongse S. Efficacy of surgical transversus abdominis plane block for postoperative pain relief following abdominal surgery in pediatric patients. Paediatr Anaesth. 2015;25(6):614–20.
Lorenzo AJ, Lynch J, Matava C, El-Beheiry H, Hayes J. Ultrasound guided transversus abdominis plane vs surgeon administered intraoperative regional field infiltration with bupivacaine for early postoperative pain control in children undergoing open pyeloplasty. J Urol. 2014;192(1):207–13.
•• Støving K, Rothe C, Rosenstock CV, Aasvang EK, Lundstrøm LH, Lange KHW. Cutaneous sensory block area, muscle-relaxing effect, and block duration of the transversus abdominis plane block: a randomized, blinded, and placebo-controlled study in healthy volunteers. Reg Anesth Pain Med. 2015;40(4):355–62 Key study showing the lack of predictable dermatomal coverage with TAP blocks paving the way for QL blocks.
Blanco R, McDonnell JG. Optimal point of injection: the quadratus lumborum type I and II blocks. http://www.respond2articles.com/ANA/ forums/post/1550.aspx. 2013. Accessed 17 Jan 2019.
• Murouchi T, Iwasaki S, Yamakage M. Quadratus lumborum block: analgesic effects and chronological ropivacaine concentrations after laparoscopic surgery. Reg Anesth Pain Med. 2016;41:146–50 Demonstrates analgesic duration of 24+ hours for QL block.
Öksüz G, Bilal B, Gürkan Y, Urfalioǧlu A, Arslan M, Gişi G, et al. Quadratus lumborum block versus transversus abdominis plane block in children undergoing low abdominal surgery: a randomized controlled trial. Reg Anesth Pain Med. 2017;42(5):674–9.
Blanco R, Ansari T, Riad W, Shetty N. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain after cesarean delivery: a randomized controlled trial. Reg Anesth Pain Med. 2016;41(6):757–62.
La Colla L, Ben-David B, Merman R. Quadratus lumborum block as an alternative to lumbar plexus block for hip surgery: a report of 2 cases. A A Case Rep. 2017;8(1):4–6.
El-Boghdadly K, Elsharkawy H, Short A, Chin KJ. Quadratus lumborum block nomenclature and anatomical considerations. Reg Anesth Pain Med. 2016;41(4):548–9.
Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53(September 2018):29–34. https://doi.org/10.1016/j.jclinane.2018.09.036.
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2018;47:47–9.
• Ueshima H. Pneumothorax after the erector spinae plane block. J Clin Anesth. 2018;48:12 First major complication for ESP block.
Naja ZM, Ziade FM, Kamel R, El-Kayali S, Daoud N, El-Rajab MA. The effectiveness of pudendal nerve block versus caudal block anesthesia for hypospadias in children. Anesth Analg. 2013;117:1401–7.
Kendigelen P, Tutuncu AC, Emre S, Altindas F, Kaya G. Pudendal versus caudal block in children undergoing hypospadias surgery a randomized controlled trial. Reg Anesth Pain Med. 2016;41:610–5.
Saavedra-Belaunde JA, Soto-Aviles O, Jorge J, Escudero K, Vazquez-Cruz M, Perez-Brayfield M. Can regional anesthesia have an effect on surgical outcomes in patients undergoing distal hypospadia surgery? J Pediatr Urol. 2017;13(1):45e1–4.
Chelly JE, Clark LD, Gebhard RE, Raw RM, Atchabahian A. Consensus of the orthopedic anesthesia, pain, and rehabilitation society on the use of peripheral nerve blocks in patients receiving thromboprophylaxis. J Clin Anesth. 2014;26(1):69–74.
• Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK. Executive summary: regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anesth Pain Med. 2018;35(1):102–5 Nice collection of all the data on bleeding complications associated with RA.
• Hohn A, Trieschmann U, Franklin J, Machatschek J-N, Kaufmann J, Herff H, et al. Incidence of peri-operative paediatric cardiac arrest: influence of a specialised paediatric anaesthesia team. Eur J Anaesthesiol. 2018;36(1):55–63 Study demonstrates continued increased risk of perioperative complications associated with age < 12 months.
Westerkamp AC, De Geus AF, Molenbuur B, Meyer P, Wietasch JKG, Struys MMRF, et al. Comparing peri-operative complications of paediatric and adult anaesthesia. Eur J Anaesthesiol. 2018;35(4):280–8.
Morton NS, Errera A. APA national audit of pediatric opioid infusions. Paediatr Anaesth. 2010;20(2):119–25.
Chidambaran V, Olbrecht V, Hossain M, Sadhasivam S, Rose J, Meyer MJ. Risk predictors of opioid-induced critical respiratory events in children: naloxone use as a quality measure of opioid safety. Pain Med (United States). 2014;15(12):2139–49.
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–4.
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–82.
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796–804.
•• Davidson AJ, Disma N, De Graaff JC, Withington DE, Dorris L, Bell G, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239–50 Study showing no increased risk of neurotoxicity in infants exposed to a single short GA compared to SA but more hypotensive episodes.
Soriano SG, Vutskits L, Jevtovic-Todorovic V, Hemmings HC. Thinking, fast and slow: highlights from the 2016 BJA seminar on anaesthetic neurotoxicity and neuroplasticity. Br J Anaesth. 2017;119(3):443–7.
Crosby G, Davis PJ. General anesthesia in infancy is associated with learning disabilities - or not. Anesth Analg. 2013;117(6):1270–2.
Jones LJ, Craven PD, Lakkundi A, Foster JP, Badawi N. Regional (spinal, epidural, caudal) versus general anaesthesia in preterm infants undergoing inguinal herniorrhaphy in early infancy. Cochrane Database Syst Rev. 2015;6:CD003669.
Williams RK, Adams DC, Aladjem EV, Kreutz JM, Sartorelli KH, Vane DW, et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont infant spinal registry. Anesth Analg. 2006;102:67–71.
Puncuh F, Lampugnani E, Kokki H. Use of spinal anaesthesia in paediatric patients: a single centre experience with 1132 cases. Pediatr Anesth. 2004;14:564–7.
Mueller CM, Sinclair TJ, Stevens M, Esquivel M, Gordon N. Regional block via continuous caudal infusion as sole anesthetic for inguinal hernia repair in conscious neonates. Pediatr Surg Int. 2017;33(3):341–5.
Davidson AJ, Morton NS, Arnup SJ, De Graaff JC, Disma N, Withington DE, et al. Apnea after awake regional and general anesthesia in infants: the general anesthesia compared to spinal anesthesia study-comparing apnea and neurodevelopmental outcomes, a randomized controlled trial. Anesthesiology. 2015;123(1):38–54.
Lerman J, Strong HA, LeDez KM, Swartz J, Rieder MJ, Burrows FA. Effects of age on the serum concentration of α1-acid glycoprotein and the binding of lidocaine in pediatric patients. Clin Pharmacol Ther. 1989;46(2):219–25.
Mazoit JX. Local anesthetics and their adjuncts. Paediatr Anaesth. 2012;22(1):31–8.
Gunter JB. Benefit and risks of local anesthetics in infants and children. Pediatr Drugs. 2002;4(10):649–72.
Tsui BCH, Boretsky K, Berde C. Maximum recommended dosage of ropivacaine and bupivacaine for pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43(8):895–6.
• Veneziano GTJ. Chloroprocaine for epidural anesthesia in infants and children. Pediatr Anesthesiol. 2017;27:581–90 Excellent summary of chloroprocaine use in infants and young children for epidurals.
Muhly WT, Gurnaney HG, Kraemer FW, Ganesh A, Maxwell LG. A retrospective comparison of ropivacaine and 2-chloroprocaine continuous thoracic epidural analgesia for management of postthoracotomy pain in infants. Paediatr Anaesth. 2015;25(11):1162–7.
Boretsky KR, Bardain S, Jennings R, Zurakowski D, Dodson B, Waisel D. Survival and neurodevelopmental outcomes of preterms resuscitated with different oxygen fractions. Pediatrics. 2015;25(11):1151–7.
Cladis FP, Litman RS. Transient cardiovascular toxicity with unintentional intravascular injection of 3% 2-chloroprocaine in a 2-month-old infant. Anesthesiology. 2004;100(1):181–3.
Hernandez MA, Boretsky K, Polaner D. Chloroprocaine: local anesthetic systemic toxicity in a 9-month infant with paravertebral catheter. Paediatr Anaesth. 2016;26(6):665–6.
Joshi G, Beck D, Emerson R, Halaszynski T, Jahr J, Lipman A, … Sinatra R. Defining new directions for more effective management of surgical pain in the United States: highlights of the inaugural Surgical Pain Congress™. Am Surg. n.d.;80(3):219-228.
Visoiu M, Joy LN, Grudziak JS, Chelly JE. The effectiveness of ambulatory continuous peripheral nerve blocks for postoperative pain management in children and adolescents. Paediatr Anaesth. 2014;24:1141–8.
•• Harbaugh CM, Lee JS, Hu HM, McCabe SE, Voepel-Lewis T, Englesbe MJ, et al. Persistent opioid use among pediatric patients after surgery. Pediatrics. 2018;141(1):e20172439. https://doi.org/10.1542/peds.2017-2439Horrifyingly high incidence of persistent opioid use after first exposure in the perioperative period.
•• Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017;152(6):e170504. Published online April 12, 2017. Corrected on January 9, 2019. Reinforces the results of the Harbough study. Again, a horrifyingly high incidence of persistent opioid use after first exposure in the perioperative period.
Neal JM, Barrington MJ, Brull R Hadzic A, Hebl JR, Horlocker TT, et al. The second ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine. Executive Summary 2015 Reg Anesth Pain Med. 2015;40(5):401–30.
•• Walker BJ, Noonan KJ, Bosenberg AT. Evolving compartment syndrome not masked by a continuous peripheral nerve block: evidence-based case management. Reg Anesth Pain Med. 2012;37(4):393–7 Evidence of timely diagnosis of ACS with continuous PNBs in place.
Ivani G, Suresh S, Ecoffey C, Bosenberg A, Lonnqvist PA, Krane E, et al. The European Society of Regional Anaesthesia and Pain Therapy and the American Society of Regional Anesthesia and Pain Medicine joint committee practice advisory on controversial topics in pediatric regional anesthesia. Reg Anesth Pain Med. 2015;40(5):526–32.
•• Livingston KS, Glotzbecker MP, Shore BJ. Pediatric acute compartment syndrome. J Am Acad Orthop Surg. 2017;25(5):358–64 Excellent state-of-the-art review of pediatric acute compartment syndrome.
• Badhe S, Baiju D, Elliot R, Rowles J, Calthorpe D. The “silent” compartment syndrome. Injury. 2009;40(2):220–2 Evidence that compartment syndrome can ocassionally present without a significant pain component.
Boretsky K, Cody S. Chapter 25: pediatrics-orthopedic surgery. In: Anderson, Wilson, Rosenblatt, editors. Decision-making in orthopedic and regional anesthesiology: a case-based approach; 2015. p. 74–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Karen R. Boretsky declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Anesthesia
Rights and permissions
About this article
Cite this article
Boretsky, K.R. Pediatric Regional Anesthesia Advances. Curr Anesthesiol Rep 9, 100–109 (2019). https://doi.org/10.1007/s40140-019-00318-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-019-00318-z