Abstract
Delirium is highly prevalent among elderly post-operative patients with no pharmacological intervention approved by the Food and Drug Administration for prevention or treatment. We conducted a systematic evidence review to critically appraise literature related to the pharmacotherapy of post-operative delirium. Ten studies fulfilled our inclusion criteria with two interventions for delirium treatment and eight interventions for delirium prevention in post-operative patients. The quality of evidence of delirium treatment studies was poor, whereas the quality of evidence in delirium prevention studies ranges from moderate to high. Delirium treatment studies find similar delirium duration and length-of-stay outcomes between haloperidol and either morphine or ondansetron. Risperidone was found to reduce the conversion of sub-syndromal delirium to delirium in one study compared to placebo. Haloperidol, olanzapine, and ketamine were each found to reduce delirium incidence, whereas rivastigmine had no impact on delirium incidence or duration. Lighter anesthesia as monitored by bi-spectral index led to a decreased delirium incidence. Considering results from studies conducted prior to the dates of this review, the current evidence suggests that certain pharmacologic classes and lighter sedation using BIS monitoring may prevent post-operative delirium, although a conclusive recommendation for clinical practice must await further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delirium is a form of acute brain failure characterized by altered consciousness with a reduced ability to focus, sustain, or shift attention that develops quickly and tends to fluctuate over the course of the day [1]. Delirium is common among post-operative elderly patients with up to 70 % of patients suffering an episode of delirium after surgery [2–4]. Multiple risk factors have been implicated in the development of post-operative delirium including advanced age, pre-existing cognitive impairment and limited functional status, severity of illness, chronic comorbidities, and use of multiple pharmacological agents [5, 6]. The presence of delirium increases the rate of post-operative complications, delays functional recovery, predisposes to cognitive decline, increases length of hospital stay and in-hospital mortality, and results in staggering health care costs in billions of dollars [7–11].
Although no pharmacologic intervention has been approved by the Food and Drug Administration (FDA) in the treatment or prevention of delirium in any population, a number of studies have tested the impact of a number of medication classes [6, 12]. In addition, national and international societies have developed guidelines for practicing clinicians to prevent and treat delirium, such as the recently published Society of Critical Care Medicine guidelines on pain, agitation, and delirium among critically ill patients [13], and the American Geriatrics Society clinical practice guidelines for post-operative delirium that are currently in development. In addition, delirium-focused organizations like the American Delirium Society and the European Delirium Association have called for an international coalition to improve the understanding of delirium pathogenesis and impact of delirium research [14].
A number of clinical trials have been published focusing on pharmacological management of post-operative delirium. We conducted this systematic review to identify recent advances in the pharmacotherapy of post-operative delirium, and to provide clinicians with an updated summary to guide clinical decision making. This review builds upon our prior work [6, 12] with an exclusive focus on pharmacologic prevention and treatment of post-operative delirium.
Data Sources
A PubMed, OVID Medline, and Google Docs search were performed to identify clinical trials concerned with preventing or treating post-operative delirium. The following search terms were used: delirium, altered mental status, confusion, or agitation; surgery, surgical, anesthesia, procedure, operative, or sedation; and haloperidol, antipsychotic, benzodiazepine, lorazepam, clonazepam, alprazolam, midazolam, dexmedetomidine, quetiapine, propofol, gabapentin, or ketamine. The search terms were limited to English language and human subjects and to articles published between 2008 and July of 2014.
Methods
We included all available controlled clinical trials that used a pharmacologic intervention for prevention or treatment of delirium in the peri-operative setting. The treatment strategies could include either placebo or active control comparisons. Studies that enrolled patients under the age of 18 or not specific to surgical populations were excluded. Titles and abstracts were screened by three reviewers (DG, BK, and NC). Potentially relevant studies were reviewed in full to determine their applicability. After the initial data set of articles was comprised, their bibliographies and references were reviewed for any additional articles that may have also fit the search criteria. The results were used in conjunction with the surgical-related papers produced from this group’s previous systematic delirium treatment review comprised articles from January 1966 to October 2008 [14].
Independent assessment of each article was done by the three co-authors (DG, BK, and NC) to ensure the quality using the JADAD scale [15]. This scale assesses parameters critical to the scientific credibility of a clinical trial, allocating a score to each study between 0 and 5, with higher scores indicating a higher quality in the conduct and/or reporting of the trial [15]. The studies included were heterogeneous in regard to their interventions, control, clinical setting, and population, and therefore were not appropriate to merge into a pooled meta-analysis. In prior systematic reviews, the authors have used a similar method to critically assess articles [1, 14, 16].
We categorized studies as treatment studies if the pharmacologic intervention (or control/usual care) was given to subjects only after delirium occurred, and prevention studies as those in which the pharmacologic intervention was given to subjects before delirium occurred. Studies may have evaluated delirium incidence as well as duration regardless of the treatment or prevention intervention.
Results
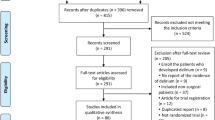
Our search yielded 854 potential studies, out of which 10 fulfilled the inclusion criteria (Fig. 1). Figure 1 describes results of our search strategy [17, 18•, 19–25, 26•]. Of these 10 studies, two focused on the treatment of post-operative delirium with the use of haloperidol, [17] morphine, [17] or ondansetron. [19] Six studies evaluated the role of haloperidol, [18•] dexmedetomidine, [21] ketamine, [23], risperidone, [24] rivastigmine, [25] and olanzapine [22] to prevent delirium. Two additional studies evaluated the depth of sedation versus normal anesthesia care, using bi-spectral index monitoring (BIS) monitoring, in the prevention of post-operative delirium [20, 26•]. Tables 1 and 2 provide a summary of the articles included in our review, and are described in further detail below.
Treatment of Post-operative Delirium
The literature search produced two trials that evaluated pharmacologic management approaches to prevention of delirium in the post-operative setting [17, 19]. Both studies compared the use of non-conventional treatments against haloperidol [17, 19], and both studies used haloperidol as the standard of care for a pharmacologic rescue medication when delirium was not controlled. The two trials enrolled cardiac surgery patients predominately in the 6th decade of life or older, with majority being males. Exclusion criteria were similar in both studies and were comprised neurologic or psychiatric disorders that may complicate delirium identification.
The trial by Atalan and colleagues [17] compared morphine as an alternative to haloperidol in the treatment of hyperactive delirium. Fifty-three post-cardiac surgery patients diagnosed with hyperactive delirium using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) [27] and Richmond Agitation Sedation Scale [28] were randomized to intramuscular haloperidol versus intramuscular morphine. No difference in duration of delirium was reported (haloperidol group: 33.92 ± 16.70 vs. morphine group: 31.56 ± 16.60 h; p = 0.607). The haloperidol group required more rescue medication and had no differences in pain scores between the two groups. RASS scores were lowered more quickly in the morphine group than the haloperidol group, though no measure of delirium severity was reported. The authors concluded that the morphine worked faster than the haloperidol and controlled the hyperactive delirium with similar effectiveness to the haloperidol.
One trial compared the use of serotonin 5HT-3 antagonist ondansetron with haloperidol for treating post-cardiac surgery delirious patients [19]. Delirium detection was based on a four-point rating system ranging from 0-normal to 4-totally disoriented, aggressive. This system has not been validated and therefore limits interpretation of results. The baseline mean rating for this scale was 3.1 in both groups. Groups were either given 8 mg intravenous (IV) ondansetron or 5 mg IV haloperidol. Each patient group was then reassessed 10 min after intervention medication which was given using the rating tool. No difference in the four-point delirium detection scale was found between the two groups.
Prevention of Post-operative Delirium
Eight studies met our search criteria that focused on the prevention of post-operative delirium in adults [18•, 20–25, 26•]. Studies primarily focused on older adults. Study populations included cardiac surgery in four [21, 23–25], orthopedic surgery in two, [20, 22] and non-cardiac surgeries in two trials [18•, 26•]. The interventions were predominantly pharmacologic treatment to attenuate risk of delirium, but two of the studies looked specifically at the depth of anesthesia as a risk factor for developing post-operative delirium. All studies excluded patients with known cognitive dysfunction or psychiatric disorders. Four of the studies used the Mini-Mental Status Exam (MMSE) [29] to determine baseline cognitive dysfunction prior to the procedure [20, 24, 25, 26•]. A fifth did specify study patients underwent unspecified neuropsychiatric battery 1 week prior to surgery and excluded subjects with severe neurologic damage (details not provided) [23]. A range of assessment tools were used to determine the development of delirium, and the patients were monitored for delirium incidence from 24 h to 6 days. None of the studies identified patient baseline cognitive status; therefore, a comparative stratification of cognitive decline could not be used as an endpoint.
Two studies focused on reducing sedation depth, in contrast to giving additional medications, using BIS-guided anesthesia. [20, 26•] The larger of the two by Chan [26•] randomized 921 patients to receive either BIS-guided anesthesia versus usual care in elderly patients undergoing non-cardiac surgery. A BIS value of 40–60 was targeted in the control group, and delirium was assessed using the confusion assessment method (CAM) [30]. BIS guidance reduced propofol delivery by 21 % and volatile anesthetics by 30 %. Fewer patients in the BIS group developed delirium versus routine care (15.1 vs. 24.1 %; p 0.01). The authors concluded that targeting higher BIS values will prevent 83 patients from delirium from every 1,000 elderly patients undergoing major surgery. The trial by Sieber and colleagues [20] randomized 114 elderly patients (≥65 years) undergoing hip fracture repair under spinal anesthesia to either receive light (BIS ≥80) sedation or deep (BIS ~50) sedation using propofol. They assessed patients with the CAM [30] on the second post-operative day for delirium and showed a 50 % reduction in the development of post-operative delirium in the elderly when BIS-guided propofol is used with a target of 80 or higher (19 vs. 40 %; p 0.02). Delirium duration was shorter in the light sedation group than in the deep sedation group (0.5 ± 1.5 vs. 1.4 ± 4 days; p 0.01). Significantly higher amounts of propofol were utilized in the deep sedation arm (10.2 mg/kg ±5.6) compared to the light sedation group (2.5 mg/kg ±2.7) (p: < 0.001), whereas midazolam dose was lower (1.26 ± 6.36 vs. 5.53 ± 12.42; p 0.02).
Hakim et al. [24] conducted a randomized, double-blinded, parallel-arm trial comparing risperidone versus placebo for treatment of sub-syndromal delirium in patients undergoing on-pump cardiac surgery. Hakim and colleagues [24] used validated screening tools to exclude patients with cognitive impairment or depression, the MMSE [29] and the 15-item Geriatric Depression Scale [31]. One hundred patients were randomized to receive either 0.5 mg risperidone or placebo orally every 12 h once they showed signs of sub-syndromal delirium using the Intensive Care Delirium Screening Checklist. [32] Results showed a statistically significant reduction in delirium incidence after treatment of sub-syndromal delirium with risperidone compared to placebo (13.7 vs. 34 %; p 0.031). No differences were observed in length of ICU and length of hospital stay.
Wang et al. [18•] conducted a randomized, double-blind placebo-controlled trial using low-dose haloperidol (0.5 mg IV bolus followed by continuous infusion of 0.1 mg/h for 12 h) as a prophylactic measure to prevent the incidence of delirium in 457 elderly patients (≥65 years) undergoing non-cardiac surgery. The primary end point was measured by the CAM-ICU within the first 7 days after surgery. Patients in the haloperidol arm had an incident delirium rate of 15.3 % compared to 23.2 % in the control group (p = 0.031).
Shehabi et al. [21] evaluated the use of post-operative dexmedetomidine versus morphine infusion in a randomized, double-blinded fashion in 299 elderly patients (≥60 years) undergoing cardiac surgery. The infusions were started within 1 h after arrival to the intensive care unit following the surgical procedure. Study medicines were titrated based on a predetermined protocol to maintain target sedation. Additional morphine and propofol were used as open-label interventions to achieve additional sedation or analgesia if required in both groups. Delirium development in the first five post-operative days was assessed using the CAM-ICU scale. Median dexmedetomidine dose was 0.49 µg/kg/h and that of morphine was 49 µg/kg/h. The mean total dose of propofol infusion was significantly lower in the dexmedetomidine group than in the morphine group (30.3 ± 4.7 vs. 35.3 ± 5.2 mg/h; p < 0.001). Supplemental morphine use was similar in both groups. Incident delirium rates were 8.6 % in the dexmedetomidine and 15 % in the morphine group (p = 0.08); and delirium duration was 2 days in the dexmedetomidine arm versus 5 days in the morphine arm (p = 0.03).
One study intervened during induction of anesthesia with a hypothesis that ketamine can blunt the systemic inflammatory response syndrome and can reduce post-operative delirium. This trial by Hudetz et al. [23] administered a single bolus of ketamine (0.5 mg/kg IV) versus placebo during the general anesthetic induction of 58 male patients undergoing cardiopulmonary bypass surgery. After surgical completion and transfer to the intensive care unit three psychologists each monitored the patients for delirium using the Intensive Care Delirium Screening Checklist (ICDSC) [31] for up to a maximum of 5 days. The incidence of delirium was 3 % in the ketamine group compared to 31 % in placebo (p = 0.01).
The randomized, double-blind placebo-controlled trial by Larsen et al. [22] evaluated the efficacy of olanzapine 5 mg orally just before and after elective knee or hip replacement surgery in preventing delirium among 495 elderly (≥65 years) patients. Comprehensive evaluation tools for delirium including DSM-III-R, MMSE, [27], CAM [32], and Delirium Rating Scale-Revised (DRS-R-98) [33] were utilized. Patients were followed until post-operative day 8. Olanzapine prophylaxis produced significantly lower rates of post-operative delirium than placebo (14.3 vs. 40.2 %; p < 0.001). However, among delirious patients, duration of delirium was longer (2.2 ± 1.3 vs. 1.6 ± 0.7 days; p = 0.02) and severity of delirium was higher (expressed by maximum DRS-R-98 score on first day of delirium, 16.44 ± 3.7 vs. 14.5 ± 2.7; p = 0.02) in the olanzapine group compared to placebo.
Gamberini et al. conducted a randomized, double-blind placebo-controlled trial of rivastigmine, a cholinesterase inhibitor for prevention of post-operative delirium among cardiac surgery patients [25]. One hundred and twenty patients aged ≥65 years were randomized to receive either rivastigmine at a dose of 1.5 mg orally every 8 h, starting the evening prior to surgery and continuing until the sixth post-operative day or placebo. No difference in delirium incidence was observed in the rivastigmine (30 %) and the placebo groups (32 %) (p = 0.8).
Discussion
This review provides an update to prior work summarizing published literature on the role of pharmacologic interventions for the prevention and treatment of delirium, with a specific focus on post-operative delirium. Our results must be considered within the context of previously published studies in delirium literature, which have historically lacked high-quality randomized, placebo-controlled studies from any population [6, 12]. Existing literature is further complicated by heterogeneous treatment approaches and populations that prevent meta-analyses and derivation of summative recommendations. Therefore, further work that improves scientific quality should be conducted to determine if pharmacotherapy for delirium treatment can be considered.
The studies included in our review suggest that reducing peri-operative sedation levels using methods such as BIS monitoring may offer the highest likelihood of delirium prevention, although the optimal sedation target level and pharmacologic approach to sedation warrants further study. Related work by Pandharipande and colleagues in a mixed medical and surgical population further highlight the level of sedation as a rational target for interventions to prevent post-operative delirium [34]. With respect to pharmacotherapy for the prevention of delirium, our results suggest that certain classes, particularly first- and second-generation antipsychotics, may reduce incident delirium and duration; however, given results of prior work not included in our review, the heterogeneity in results, population, agents and doses studied, further work is necessary before recommendations can be made regarding the role of antipsychotic medications in both prevention and treatment of delirium.
As highlighted above, notable studies contributing to the post-operative delirium body of literature were not included in our review because they were published prior to 2008. Therefore, any summative recommendations made as a result of this review should also consider findings from other relevant work, including pilot work by Leung and colleagues using gabapentin to reduce post-operative pain as well as incident delirium [35]. Our review included only one study that employed a narcotic analgesic as an intervention arm [21]. Our search criteria also restricted the inclusion of notable delirium prevention studies published by Kalisvaart, Liptzin, and Sampson who used antipsychotic and pro-cholinergic agents for delirium prevention and each found no reduction of delirium incidence when compared to placebo [36–38]. Considering prior work by Liptzin and Sampson as well as results from our review, we can conclude that pro-cholinergic interventions (such as citicoline and the acetylcholinesterase inhibitors) should not be considered for delirium prevention or to reduce delirium duration [37, 38].
Our review is limited by the inclusion of studies that were published and did not include studies that may have been conducted and not carried forward to publication. This review was focused on studies that exclusively enrolled surgical populations, therefore did not include medically ill populations, and therefore does not extrapolate results to this population. Other notable limitations in comparing the results of the included studies are the requirement of mechanical ventilation following the surgical procedure, the type and duration of surgical procedure, and the drug, dose, and duration of medications used for pain and sedation during and after the surgical procedure.
Our findings are consistent with current guidelines for the management of pain, agitation, and delirium [13] among adult patients in the ICU (mixed population) with respect to the choice of sedative in mechanically ventilated populations and use of BIS monitors for procedural sedation. Our findings are also consistent with the PAD guidelines that refrain from a recommendation on the use of pharmacotherapy for the prevention or treatment of delirium among critically ill adults. Studies included in this review suggest further work describing the role of pharmacotherapy for delirium prevention, and treatment is necessary before a recommendation for or against such interventions can be made.
Conclusion
The ability of the current evidence for prevention and treatment of post-operative delirium to influence clinical practice is currently limited by inconsistency in intervention design and methodological challenges. Therefore, clinicians are encouraged to limit use of pharmacologic interventions in delirium to those with severe agitation and behavioral features that interrupt the delivery of care or put other patients or care providers at risk of harm. The existing evidence does suggest that pharmacologic interventions may have a role in future delirium prevention and treatment strategies; however, the developments of high-quality clinical trials evaluating all risks and benefits are necessary before such interventions should be implemented in the clinical environment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Inoyue SK. Delirium in older persons. N Eng J Med. 2006;354:1157–65.
Fong HK, Sands LPP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review. Anesth Analg. 2006;102:1255–66.
Newman MF. Longitudinal assessment of neurocognitive function coronary artery bypass surgery. N Eng J Med. 2001;344:395–402.
Sadler PD. Incidence, degree and duration of post-cardiotomy delirium. Heart Lung. 1981;10:1084–92.
Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2005;54(10):1578–89.
Khan BA, Zawahiri M, Campbell NL, et al. Delirium in hospitalized patients: implications of current evidence on clinical practice and future avenues for research—a systematic evidence review. J Hosp Med. 2012;7(7):580–9.
Scazynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Eng J Med. 2012;367(1):30–9.
Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62.
Khan BA, Lasiter S, Boustani MA (2014). Critical care recovery center. Making the case for an innovative collaborative care model for ICU survivors. Am J Nurs (In press).
Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16.
Leslie DL, Marcanatonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32.
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306.
Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59:S237–40.
Campbell NL, Bostani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med. 2009;24(7):848–53.
Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Campbell NL, et al. Medication adherence in older adults with cognitive impairment: a systematic evidence-based review. Am J Geriatr Pharmacother. 2012;10(3):165–77.
Atalan N, et al. Morphine is a reasonable alternative to haloperidol in the treatment of postoperative hyperactive-type delirium after cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(5):933–8.
• Wang W, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40(3):731–9. This study by Wang present a rigorously designed randomized controlled trial of a medication intervention to reduce the incidence of delirium compared to placebo. The existing delirium prevention literature lacks high-quality medication versus placebo clinical trials. Among a population at high risk of delirium due to age and stress of surgical procedures, this study shows a unique dosing protocol of low-dose haloperidol reduced the incidence of delirium compared to those given placebo.
Tagarakis GI, et al. Ondasetron versus haloperidol for the treatment of postcardiotomy delirium: a prospective, randomized, double-blinded study. J Cardiothorac Surg. 2012;7:25.
Sieber FE, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair.[Erratum appears in Mayo Clin Proc. 85(4):400 Note: dosage error in article text]. Mayo Clinic Proc. 2010 Apr;85(1):18–26.
Shehabi Y, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology. 2012;111(5):1075–84.
Larsen KA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409–18.
Hudetz JA, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23(5):651–7.
Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987–97.
Gamberini M, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery—a randomized controlled trial. Crit Care Med. 2009;37(5):1762–8.
• Chan MT, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. This study suggests that BIS-guided anesthesia during surgical procedures may have both short- and long-term benefits in cognitive function. Short-term benefits include a reduced incidence of post-operative delirium. The study also suggests a BIS-guided approach to anesthesia during surgical procedures may reduce post-operative cognitive decline 3 months after a procedure.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
D’Ath P, et al. Screening, detection and management of depression in elderly primary care attenders. I: the acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract. 1994;11(3):260–6.
Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). J Am Med Assoc. 2001;286:2703–10.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, et al. The Richmond Agitation-Sedation Scale. Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44.
Bergeron N, Dubois MJ, Dumont M, et al. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–64.
Inouye SK, VanDyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method, a new method for detection of delirium. Ann Intern Med. 1990;113:941–8.
Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–42.
Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine versus lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–53.
Leung JM, Sands LP, Rico M, Petersen KL, Rowbotham MC, Dahl JB, Ames C, Chou D, Weinstein P. Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients. Neurology. 2006;67(7):1251–3.
Kalisvaart KJ, de Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, Eikelenboom P. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658–66.
Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post surgical delirium. Am J Geriatr Psychiatry. 2005;13:1100–6.
Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343–9.
Acknowledgments
Dr. Khan’s work on the project was supported by a grant from the National Institute on Aging (NIA K23-AG043476) and Indiana University Health Values Fund Award (VFR-398). Dr. Campbell’s work on the project was supported by a grant from the National Institute on Aging (NIA K23-AG044440).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Perioperative Delirium.
Rights and permissions
About this article
Cite this article
Khan, B.A., Gutteridge, D. & Campbell, N.L. Update on Pharmacotherapy for Prevention and Treatment of Post-operative Delirium: A Systematic Evidence Review. Curr Anesthesiol Rep 5, 57–64 (2015). https://doi.org/10.1007/s40140-014-0090-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-014-0090-5