Abstract
Purpose of Review
Review epidemiology, screening recommendations, management, and treatment of hepatitis C virus and strategies to expand diagnosis and effective linkage to care.
Recent Findings
Hepatitis C virus (HCV) infections have increased threefold from 2010 to 2016 and close to half of people infected remain unaware of their HCV status. Emergency departments and hospitals are points of care where health care is provided to people who often do not otherwise receive regular primary health care and recommended screening tests. Recent studies in emergency departments have shown that expanded testing can identify many people with chronic HCV infection who would otherwise remain undiagnosed.
Summary
Hepatitis C infection can impact the health of patients who are admitted to emergency departments and hospitals. Expanded screening can identify patients at risk of HCV-related liver disease, get them linked to care and treatment, and prevent progression of liver disease, HCV-related deaths, and transmission of infection. Actions that expand HCV screening in emergency departments are an important response to address the increasing number of HCV infections in the USA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: Why Is Hepatitis C a Concern in Emergency Departments and Hospitals?
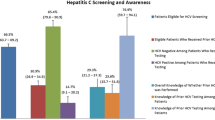
Hepatitis C virus (HCV) is the most common blood-borne infection in the USA with 2.4 million people currently estimated by the Centers for Disease Control and Prevention to be living with the infection [1], with as many as 71 million people infected worldwide according to the World Health Organization [2]. Only an estimated 56% of people with chronic HCV infection in the USA are aware of their status with awareness lowest among Hispanics, Asians, foreign-born individuals who live below the federal poverty level, and people who have a low level of education [3]. New infections are most common among people who inject drugs [4] who often are not getting regular health care and present at emergency departments after a drug overdose or with conditions exacerbated by drug use and poor health. For these reasons, emergency departments and hospitals are important settings to conduct screening for hepatitis C, followed by linkage to care.
HCV is an RNA virus that is transmitted parentally, usually through injection drug use and historically, though less frequently now, through poor infection control practices during health care procedures. Sexual transmission occurs rarely and among both men who have sex with men and heterosexual individuals; the likelihood of sexual transmission rises with the number of sex partners and when sex partners are coinfected with HIV [5, 6]. HCV can also be transmitted from infected mother to unborn child during pregnancy although the risk is low, ranging from 4.2 to 7.8% [7].
In 2016, the CDC received reports of 2967 cases of acute HCV infection. Since acute HCV infection can be mild or asymptomatic, each reported case is estimated to represent 12.3 actual cases, which suggests that the actual number of acute cases was 41,200. The CDC documented a threefold increase in new HCV infections between 2010 and 2016 and linked the increase to rising injection drug use, primarily among white adolescents and young adults who reside in nonurban settings [4]. HCV is considered to be a hardy virus; one study found that cell culture–derived HCV can remain infectious on surfaces up to 6 weeks, also suggesting that accidental contact with contaminated surfaces may be a source of health care–associated transmission [8].
Prevention of HCV remains a challenge, since currently there is no vaccine for HCV. Because of the increases in new HCV infections related to drug use behaviors, strategies such as comprehensive harm reduction, screening, linkage to care, and HCV treatment are critical to identify people at risk for HCV and decrease transmission of the virus. Treating people with HCV infection is essential not only to decrease HCV-related morbidity and mortality but also to prevent HCV transmission.
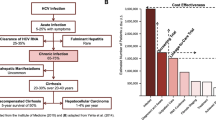
Acute infection progresses to chronic disease in 75 to 85% of cases. Little is known about the factors associated with spontaneous clearance of the virus, though immune response is determined in part by genetics, sex, and mode of acquisition. Chronic infection is usually asymptomatic, even in the presence of mild to severe liver disease. One recent analysis found that only 38.2% of people at risk for significant HCV-related liver fibrosis were aware of their infection [3]. Chronic HCV infection is a leading cause of liver disease–related death due to cirrhosis and hepatocellular carcinoma in the Western world. Persistent inflammation due to HCV infection causes cirrhosis within 30 years in 10 to 20% of those infected, with 1 to 5% of these patients at annual risk for hepatocellular carcinoma [9].
Emergency departments (EDs) and hospitals are often points of health care system entry for people who do not receive regular care. EDs and hospitals have been shown to be effective partners in expanding HCV screening and linkage to care that are key to identify people at risk for significant liver disease as well as individuals who are likely to transmit the infection to others.
Diagnosis of HCV
To confirm a diagnosis of HCV, serologic testing is required. Acute hepatitis C diagnosis requires evidence of seroconversion based on a previous negative test followed by a positive test or recent onset of acute hepatitis accompanied by a positive test. Most people do not present with symptoms of acute HCV, and most infections that are identified are chronic infections. There is no diagnostic test to determine acute versus chronic infection; newly diagnosed patients are considered to be chronically infected after 6 months of persistent infection [10].
Previous screening strategies for HCV were based on the identification of potentially stigmatizing risk factors such as a history of injection drug use or unprotected sex with multiple partners, which were seldom assessed by providers or disclosed by patients. Because this strategy failed to identify many patients who were chronically infected with HCV, the CDC and the U.S. Preventive Services Task Force (USPSTF) recommend expanded HCV serologic screening for all people considered to be at significant risk as well as for members of groups known to have high prevalence rates, such as people born between 1945 and 1965 [11, 12].
HCV serologic screening of patients at high risk for infection is a grade B USPSTF recommendation, qualifying this screening as a preventive service covered without copay under the Affordable Care Act (ACA). Because people with HCV infection are typically asymptomatic, expanding screening in emergency departments and hospitals to include asymptomatic people with an increased probability of having or developing HCV-related liver disease has been shown to be more effective in identifying people with HCV compared with diagnostic testing based on symptoms [13].
Who should be screened for HCV? People with the following risk factors should undergo HCV serologic screening at least once [11, 12]:
-
Birth date between 1945 and 1965, regardless of other risk factors
-
Receipt of clotting factor concentrates produced before 1987
-
Persistently abnormal alanine aminotransferase levels
-
History of long-term hemodialysis treatment
-
Receipt of blood, blood products, or an organ transplant prior to July 1992 or receipt of blood from a donor who later tested positive for HCV
Those in the following groups should undergo HCV serologic screening routinely:
-
People with HIV infection (all people with HIV should be screened for HCV at least once and those at high risk should be screened annually and after suspected exposure) [14]
-
Anyone who has ever used injection drugs (even once); annual screening is recommended for people who continue to use illicit drugs, as well as their sex partners. More frequent screening is recommended if recent exposure is suspected [15]
-
Health care, emergency medical, and public safety workers after needlestick injury or mucosal exposure to HCV-contaminated blood
-
Children born to mothers who test positive for HCV [11]
HCV screening is a two-step process. People recommended for screening should first be tested using an HCV antibody test. A positive antibody test indicates a history of exposure to HCV but cannot distinguish resolved from active infections. HCV antibodies can persist for years following a naturally resolved infection or successful antiviral therapy so further testing is required to determine whether infection is active. Quantitative RNA testing is the preferred test to confirm active HCV infection; this test can detect very low levels of virus in the blood and the results can be used as a baseline viral load prior to initiating treatment. Many laboratories now offer a reflex RNA test which can be ordered at the same time as the antibody test but is not completed if the antibody test is negative. Results of the antibody and the RNA tests should accompany all referrals to care upon discharge.
There are several genotypes of HCV, and identification of genotype is important in the selection of the treatment agents to be used. This test is typically done during the evaluation for treatment rather than during an acute emergency or hospital stay.
Evaluation of Liver Disease
Liver fibrosis evaluation in patients with chronic HCV is important because liver disease is often asymptomatic and the liver is responsible for critical functions such as filtering blood, detoxifying chemicals, and metabolizing many medications. Liver biopsy is no longer a primary tool in assessing liver disease. Liver disease severity can be measured using noninvasive methods including liver-directed physical exam; routine blood tests such as ALT, AST, albumin, bilirubin, INR, and CBC with platelet count; serum fibrosis marker panels; liver imaging such as ultrasound or CT scan; and transient elastography. As HCV-related disease evaluation and treatment has expanded, simple calculations derived from routine blood tests such as serum AST-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) offer lower cost and low threshold strategies to screen patients for liver disease. FIB-4 is a calculation ratio using age, AST, ALT, and platelet count values [16].
Clinical presentation of HCV is often unremarkable and nonspecific to the liver. Since most patients with chronic HCV infection are asymptomatic until they develop advanced liver disease, it is important to maintain a high level of clinical suspicion. Mild to moderate liver damage may produce vague symptoms, such as fatigue, over a period ranging from years to decades. HCV, however, may also have a number of extrahepatic manifestations, including cryoglobulinemia and a lymphoproliferative immune complex disorder that causes arthralgia, purpura, glomerular disease, peripheral neuropathy, central nervous system vasculitis, and reduced complement levels [17•].
HCV Treatment Advances
The goal of HCV therapy is to achieve sustained viral response (SVR), or viral clearance, which represents a cure for HCV [18]. HCV treatments result in a SVR, or cure, for more than 95% of patients who are treated with combination therapies for 8–12 weeks, leading to reduction of the sequelae of untreated chronic infection. These highly effective treatments are underutilized for a number of reasons. Notably, many patients are unaware of their HCV status, patients and many providers are unaware of new treatments, and the policies of insurance companies and other payers often restrict access to treatment because of the initial cost of the medications. The cost of these medications has been reduced greatly due to numerous treatment options resulting in market competition, use of group purchasing and subscription models of manufacturer negotiation, and partnering with covered entities to access 340B drug pricing.
Previously, interferon and ribavirin formed the backbone of HCV therapies. These were very difficult to tolerate, with many adverse effects and numerous contraindications. Since 2001—and until 2013—the standard of care for HCV was pegylated interferon (peginterferon) injected weekly paired with oral ribavirin. Both patients and providers were anxious about initiating the 48-week therapy regimen because of its considerable adverse effect profile and relatively low rate of SVR of 41% or less for HCV genotype 1, the most common genotype in the USA [19].
Since 2013, numerous very effective direct acting antivirals (DAAs) have been approved by the FDA, from 3 different classes of drugs (see Table 1). These 3 classes of drugs include protease NS3/4A inhibitors, protease NS5A inhibitors, and nucleotide/nucleoside and nonnucleoside polymerase NS5B inhibitors, which are given as combinations of at least 2 different classes of drugs. Currently, there are ten therapies available in the USA, which generally cure as many as 97% of patients with HCV. These are given as combinations of at least 2 different classes of DAAs, for 8–12 weeks, depending on the HCV genotype, the combination medication given, and whether this is initial therapy or treatment provided after an initial treatment failure [20]. See Table 1 for a list of approved DAAs, their composition, and indication.
Once SVR has been achieved and HCV is cured, liver fibrosis can significantly improve or reverse and many patients experience improvements in the extrahepatic manifestations of HCV. The demand for liver transplantation has decreased significantly since the use of DAAs to treat HCV infection [21]. Successful treatment also reduces the individual’s risk of developing liver cancer. However, the risk for liver cancer in people who have had chronic HCV remains elevated, especially among those with advanced fibrosis and they should be screened for liver cancer by ultrasound twice yearly [22].
Importance of HCV Screening in Emergency Departments
The emergency department is often the only site of ongoing health care for persons at significant risk of HCV infection, presenting a window of opportunity for identifying many with HCV who are completely unaware of their infection, and may have significant liver fibrosis. Despite recommendations for screening from CDC and USPSTF, implementation of HCV screening is incomplete and nearly half of infected individuals remain undiagnosed. Everyone with HCV infection should receive treatment to cure the infection and prevent long-term sequelae.
In many EDs, initial test results can be completed and given to the patient while they are still in the ED. Once diagnosed, referral for treatment of persons with HCV infection is essential. In 95–97% of cases, treatment will result in cure of HCV infection, documented by SVR. Many patients are unaware of the curative treatments for HCV or have never received a referral to be treated with one of the new, more-effective therapies. Treatment and cure of HCV infection not only result in decreased risk of morbidity and mortality from liver disease but also are essential to interrupt transmission of HCV in key populations often seen in ED, including those with opioid use disorder.
A recent modeling study found that one-time HCV testing for all individuals > 18 years old would be cost-effective, lead to improved clinical outcomes, and identify more people compared with current screening recommendations [23••].
The unique opportunities to address HCV presented in EDs were highlighted in a 2016 study at the Johns Hopkins ED. The researchers implemented universal HCV testing for everyone 17 years and older during 2 months in 2013. Of the nearly 5000 HCV tests performed, 13.8% were antibody positive and of those, 31% had undocumented HCV infections [24••].
Conclusions: Actions for Health System Administrators, Leadership, and ED Providers
-
Initiate a demonstration project in the ED to determine prevalence of HCV among the patient population > 18 years old. This will provide key information to inform future screening efforts.
-
Expand screening of HCV to all patients admitted for opioid use disorder. This will support the identification of people who have the highest rates of new infection so that they can receive counseling and referral to harm reduction services and linkage to care and treatment. Treating people at risk also reduces ongoing transmission of the virus and can support preventive public health efforts to eliminate HCV.
-
Identify community providers who treat HCV and ensure linkage to care and consideration for treatment. Most people who have recently acquired HCV do not have complex liver disease so a referral to a hepatologist is not necessary because of low toxicity of DAA medication.
-
Refer patients identified with significant liver disease to a specialist such as a hepatologist or gastroenterologist. People with chronic HCV and severe liver diseases are generally prioritized to receive treatment quickly and must have close and ongoing follow-up for management of liver disease. After cure of HCV infection, the risk of hepatocellular carcinoma decreases but individuals remain at increased risk and should be screened every 6 months.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, et al. Estimating prevalence of hepatitis C virus infections in the United States, 2013–2016. Hepatology. 2019;63(3):1020–31. https://doi.org/10.1002/hep.30297.
World Health Organization Global Hepatitis Report 2017. 2017. https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 27 Apr 2019.
Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: an update from the National Health and Nutrition Examination Survey. 2019. J Viral Hepat. https://doi.org/10.1111/jvh.13060.
Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States. Atlanta; 2018. 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm. Accessed 27 Apr 2019.
Workowski KA, et al. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110.
Fierer DS, Factor SH, Uriel AJ, Carriero DC, Dieterich DT, Mullen MP, et al. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men—New York City, 2005–2010. Morb Mortal Wkly Rep. 2011;60(28):945–50.
Koneru AK, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission – United States and Kentucky, 2011–2014. Morb Mortal Wkly Rep. 2016;65(28):705–10.
Paintsil E, Binka M, Patel A, Lindenback BD, Heimer R. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis. 2014;209(8):1205–11. https://doi.org/10.1093/infdis/jit648.
Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 Suppl):S58–68.
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. https://doi.org/10.1002/hep.22759.
Moyer VA, Preventive Services Task Force US. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349–57.
Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo GG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. Morb Mortal Wkly Rep. 2012;61(RR-4):1–32.
White DAE, Anderson ES, Pfeil SK, Trivedi TK, Alter HJ. Results of a rapid hepatitis C virus screening and diagnostic testing program in an urban emergency department. Ann Emerg Med. 2015;61(1):119–28.
AIDSInfo. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2018. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/26/hcv-hiv. Accessed 27 Apr 2019.
Belani H, Chorba T, Fletcher F, Hennessey K, Kroeger K, Lansky A, et al. Integrated prevention services for HIV infection viral hepatitis sexually transmitted diseases, and tuberculosis for persons who us drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. 2012. Morb Mortal Wkly Rep;61(5).
Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, Vijaydeva V, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57(2):240–6. https://doi.org/10.1093/cid/cit245.
• AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. 2009. http://www.hcvguidelines.org. Accessed 27 Apr 2019. Best reference for hepatitis C treatment.
Ward J. Hepatitis C virus: the 25-year journey from discovery to cure. Hepatology. 2018;60(5):1479–82. https://doi.org/10.1002/hep27377.
Rumi MG. Pegylated interferon [alpha]2b versus pegylated interferon [alpha]2a for chronic hepatitis C: the unreached goal of superiority. J Hepatol. 2009;15:1097–9. https://doi.org/10.1016/j.jhep.2009.09.013.
Younossi Z, Stepanova M, Manns MP, Reddy R, Gordon SC, Bourliere M. Patient-reported outcomes in chronic hepatitis C: the impact of placebo, active treatment, and sustained viral eradication. J Hepatol. 2018;68(1 Supp):S312–3. https://doi.org/10.1016/S0168-8278(18)30842-0.
Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16(8):1356–8. https://doi.org/10.1016/j.cgh.2017.11.045.
Jacobson IM, Lim JK, Fried MW. American Gastroenterological Association Institute clinical practice update-expert review: care of patients who have achieved a sustained virologic response after antiviral therapy for chronic hepatitis c infection. J Gastroenterol. 2017;152(6):1578–87. https://doi.org/10.1053/j.gastro.2017.03.018.
•• JA B, Tasillo A, Yazdi GE, Wang J, Vellozzi C, Hariri S, et al. Population-level outcomes and cost effectiveness of expanding the recommendation for age-based hepatitis C testing in the United States. Clin Infect Dis. 2018;67(4):549–56. https://doi.org/10.1093/cid/ciy098 Expanded HCV testing strategies increase case identification and number of people treated and cured, leading to improved clinical outcomes.
•• YH H, Rothman RE, Laeyendecker OB, Kelen GD, Avomu A, Patel EU, et al. Evaluation of the Center for Disease Control and Prevention recommendations for hepatitis C virus testing in an urban emergency department. Clin Infect Dis. 2016;62(9):1059–65. https://doi.org/10.1093/cid/ciw074 HCV testing of people born 1945–1965 in EDs would double identification of undocumented HCV infection, 1-time universal testing would further improve case identification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.The findings of this article are those of the authors.
They do not necessarily reflect the views of the U.S. Department of Health and Human Services.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infectious Disease
Rights and permissions
About this article
Cite this article
Dan, C., Kaplowitz, L. Update on Hepatitis C Screening and Management: Actions for Emergency Departments. Curr Emerg Hosp Med Rep 7, 53–58 (2019). https://doi.org/10.1007/s40138-019-00183-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-019-00183-4