Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic disorder that results in significant declines in respiratory function and a high mortality rate only a few years after diagnosis. Medical management of IPF has been attempted with various types of medications, such as immunosuppressants, anticoagulants, endothelin receptor antagonists, and anti-inflammatory drugs with less than conclusive results. However, with the approval of nintedanib and pirfenidone following the IMPULSIS-1, IMPULSIS-2, and ASCEND data, there is hope that medical therapy may be able to slow the progression of IPF and decrease the rate of acute exacerbations in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a type of chronic, fibrosing interstitial pneumonia of unknown cause that results in progressively worsening lung function over time [1]. In addition to chronic dyspnea, patients often present with cough, bibasilar inspiratory crackles, and finger clubbing. Incidence of IPF appears to be higher in older, male patients, but the exact incidence and prevalence of the disease is difficult to determine since a consensus definition was only recently established. The annual incidence in the United States has been estimated to range from 6.8–8.8 to 16.3–17.4 per 100,000 population, depending on the definition used, while in Europe the estimated incidence ranged from 0.22 to 7.4 per 100,000 population [1, 2]. The most significant risk factors appear to be cigarette smoking and environmental exposures, but others include exposure to microbes, GERD, diabetes, pulmonary hypertension, and various genetic factors [1, 3]. Modification or treatment of some of these risk factors, such as anti-acid therapy in patients with GERD, may help decrease progression of IPF and lead to longer survival [1, 4, 5].
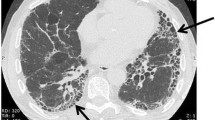
IPF is a diagnosis of exclusion and is recognized separately from other forms of interstitial pneumonia and from interstitial lung disease (ILD) secondary to other causes. A recently updated guideline provides criteria for diagnosing IPF using high-resolution computed tomography (HRCT) and/or surgical lung biopsy [1]. IPF is associated with histopathological or radiological pattern of usual interstitial pneumonia (UIP), characterized by reticular opacities and often traction bronchiectasis and minimal ground-glass opacifications [6]. In patients with biopsy-proven IPF, HRCT has been shown to have a positive predictive value of over 90 %, and is a crucial tool in the diagnostic process.
While the course of IPF varies widely among patients, symptoms typically occur long before IPF is officially recognized as the cause. On average patients survive approximately 3 years after the diagnosis is made, with only 20–30 % of patients alive 5 years after diagnosis [3, 6–9]. Mortality in patients with IPF can be due to both respiratory and non-respiratory causes, however over 75 % of deaths are secondary to a progression of IPF, acute exacerbation, ALI/ARDS, pneumonia, or other respiratory disorder [8]. IPF classically results in a slow, progressive decline in lung function (annual decrease in FVC of 0.13–0.21 L), and the majority of IPF-related deaths are due to a clinical process that has been ongoing for >4 weeks [6, 8]. However, there appears to be a cohort of patients that experience a more rapidly evolving course that results in a shorter survival. It is believed that these patients typically have various factors that have been linked to decreased survival, such as older age, smoking, low BMI, worsened symptoms, worsened radiographic findings, and the development of comorbid conditions [8].
Acute exacerbations have been reported in up to 20 % of patients with IPF, and are generally defined by a worsening of dyspnea within 1 month, hypoxemia, worsened infiltrates seen on radiography, and no other clear explanation [1, 10]. This sudden worsening of a chronic disease often leads to acute respiratory distress syndrome (ARDS), and is associated with increased mortality, both acutely and within 6 months [3, 11]. However, respiratory failure due to an acute worsening of IPF is difficult to diagnose, as the signs and symptoms are similar to other disorders, specifically infection, heart failure, pulmonary embolism, and drug-induced lung disease [11]. While it is important to exclude all of these as potential causes, evaluating for a possible infection is essential since the treatment for acute exacerbations of IPF may include significant immunosuppression. Treatment regimens involving corticosteroids, with or without cyclophosphamide, have been reported in small studies. While there is no consensus, doses of methylprednisolone up to 1000 mg/day for up to 3 days followed by 1–2 mg/ (kg day) have been used [1, 10, 12, 13]. However, due to a lack of convincing evidence, administration of corticosteroids for acute exacerbations of IPF is a weak recommendation by the most recent guidelines [1].
Therapies for IPF
While many options for the treatment of IPF have been studied, current consensus guidelines do not recommend the use of specific pharmacologic agents [1]. The majority of data reviewed is graded as low-quality evidence due to the observational nature and lack of placebo-controlled studies. Medications including corticosteroids, colchicine, cyclosporine, acetylcysteine, azathioprine (in combination with corticosteroids and acetylcysteine), interferon-γ 1b, bosentan, sildenafil, imatinib, anticoagulants, and etanercept have been studied but are not endorsed for the majority of patients with IPF [1, 6, 14–21]. While new evidence has led to the conclusion that some of these interventions, such as warfarin, may be harmful and should be avoided, many patients are treated with some combination of the above therapies [18, 22, 23].
In 2005, the IFIGENIA study compared high-dose acetylcysteine to placebo in patients receiving azathioprine and prednisone. While no difference in survival was seen, patients who received acetylcysteine had a slower deterioration in vital capacity and carbon monoxide diffusing capacity (DLCO) [24]. In 2012, the PANTHER-IPF study compared three groups of patients with mild to moderate IPF who received placebo, monotherapy with acetylcysteine, or combination therapy with acetylcysteine, azathioprine, and prednisone [25]. There was no significant difference between the acetylcysteine or combination groups in the change in FVC or in disease progression. Patients who received combination therapy had significantly higher all-cause mortality and all-cause hospitalizations, as well as a higher incidence of acute exacerbations, leading to the trial being stopped early. Data continued to be collected on the acetylcysteine and placebo groups until 60 weeks, and also found no difference in pulmonary metrics, such as change in FVC, or survival [26]. With these results, the benefits of classic regimens that patients have been managed with for decades have been called into question.
As discussed above, strong literature demonstrating efficacy of medications is lacking. Two promising agents, pirfenidone and nintedanib, completed phase 3 trials and recently received FDA approval for the slowing lung function decline in IPF and decreasing acute exacerbations. The details of the medications and their clinical trials are discussed here and are summarized in Table 1.
Pirfenidone
Pirfenidone works by inhibition of TGF-B stimulated collagen synthesis decreasing the extracellular matrix and in vivo blocks fibroblast proliferation [27–30]. Pirfenidone is initiated at 267 mg orally three times daily for 7 days, and titrated to 534 mg orally three times daily for 7 days, and then 801 mg orally three times daily thereafter. It is currently not recommended to exceed a maximum daily dose of 2403 mg per day of pirfenidone, and in the presence of moderate to strong CYP1A2 inhibitors, the daily dose should be reduced. Monitoring of liver function tests is recommended prior to initiation, monthly for the first 6 months of therapy, and every 3 months thereafter. To date, pirfenidone has been evaluated in several randomized trials. The first study conducted in Japanese patients randomized 275 patients in a 2:1:2 manner to 1800 mg/day of pirfenidone, 1200 mg/day of pirfenidone, or placebo [31]. The analysis found significant decreases in the rate of decline in FVC (p = 0.046) and increase in progression-free survival (p = 0.028) between the high-dose pirfenidone and placebo arms.
The CAPACITY trials (004 and 006) were two multinational double-blind RCTs conducted with patients from Europe, North America, and Australia comparing pirfenidone to placebo [32]. In the 004 study, patients were assigned in a 2:1:2 model to receive either 2403 mg/day of pirfenidone, 1197 mg/day of pirfenidone, or placebo. In the 006 study, patients were randomized 1:1 to receive either 2403 mg/day of pirfenidone or placebo. The primary endpoint was the change in percentage predicted FVC at week 72 of analysis. A significant decrease in decrease in FVC was only demonstrated in the 004 study. A decrease in decline in the 6 min walk test was observed in the 006 study, but not in the 004.
After the release of the CAPACITY trials, pirfenidone was approved for use in patients with mild-moderate IPF in Japan and Europe. The Food and Drug Administration however expressed concern about the CAPACITY 006 Study failing to show the same benefit as 004, and requested another RCT to be conducted before approval would be granted.
The ASCEND Trial randomized 555 patients to receive either pirfenidone 2403 mg/day or placebo [33••]. The investigators found the pirfenidone arm resulted in significant slowing of disease progression measured by changes in the 6 min walk test and mean decline in percent predicted or absolute FVC. Pirfenidone reduced the RR of death or disease progression by 43 % (HR 0.57, 95 % CI 0.43–0.77; p < 0.001). Analysis of mortality in the ASCEND trial did not detect a significant difference in mortality. The investigators did however prespecify a pooled analysis of all patients in the ASCEND and two CAPACITY studies. The pooled analysis demonstrated a decrease in all-cause mortality at 1 year by 48 % (p = 0.01) and death from IPF at 1 year by 68 % (p = 0.006) when compared to placebo. Following the publication of the ASCEND trial, the FDA approved pirfenidone for the treatment of IPF.
During the clinical trials of pirfenidone, the most common adverse effects were GI related: nausea, vomiting, abdominal discomfort, and diarrhea. Other adverse effects that occurred more commonly with pirfenidone than with placebo in RCTs and pooled analysis were rash, fatigue, photosensitivity, and LFT elevation. Adverse effects rarely resulted in discontinuation of therapy, though patients who experience elevations in liver function tests during pirfenidone therapy should be considered for a dose reduction or discontinuation of therapy.
With the demonstrated effects in clinical trials on slowing IPF progression with a possible mortality benefit in pooled analysis, pirfenidone has the potential to become a new standard therapy for IPF management.
Nintedanib
Nintedanib is an oral tyrosine kinase inhibitor thought to work on several receptors significant in the progression of IPF that also recently completed phase 3 evaluation for the treatment of IPF [34]. Nintedanib is dosed 150 mg orally twice daily. A reduced dose of 100 mg orally twice daily is available for patients who experience diarrhea on the higher dose. Interactions are expected with inhibitors of p-glycoprotein and CYP3A4 inhibitors. There also may be increased risk of bleeding with nintedanib in patients who are maintained on therapeutic anticoagulation. It is recommended that liver function tests are obtained prior to initiation of therapy, followed by monthly for 3 months, then every 3 months thereafter.
After promising phase II results [35], phase III evaluation of nintedanib took place in the INPULSIS-1 and INPULSIS-2 studies [36••]. The INPULSIS studies were two replicate double-blind RCTs of nintedanib 150 mg twice daily compared to placebo over the course of 52 weeks. The primary endpoint of both trials was the annual rate of decline in FVC. Between the two INPULSIS trials, 1066 patients were randomized in a 3:2 model to receive nintedanib or placebo. Significant decreases in the adjusted annual rate of change in FVC were observed in both IMPULSIS analyses (p < 0.001 for both) for nintedanib when compared to placebo. There was significant benefit in increasing time to first acute exacerbation in INPULSIS-2 that was not observed in INPULSIS-1. There was no detected improvement in mortality during the study period, though the analyses were not powered to a mortality endpoint.
After the results of the INPULSIS analyses were published, nintedanib received approval from the FDA and in Europe for the treatment of IPF.
Adverse Effects
There was no significant difference in the incidence of serious events between nintedanib and placebo (31.1 vs. 27.0 % in INPULSIS-1 and 29.8 and 32.9 % in INPULSIS-2). The most commonly experienced adverse effect with nintedanib in the INPULSIS studies was diarrhea (61.5 and 63.2 % of patients, respectively in INPULSIS-1 and INPULSIS-2). Diarrhea led to discontinuation of therapy in <5 % of patients in both analyses. Other adverse effects that were commonly observed with nintedanib therapy were nausea (22.7 vs. 5.9 % in INPULSIS-1, 26.1 vs. 7.3 % in INPULSIS-2), vomiting (12.9 vs. 2.0 % in INPULSIS-1, 10.3 vs. 3.2 % in INPULSIS-2), and elevation of liver function tests (4.9 vs. 0.5 % in INPULSIS-1, 5.2 vs. 0.9 % in INPULSIS-2). LFT abnormalities were reversible upon discontinuation of nintedanib.
Since the FDA approval of pirfenidone and nintedanib, there has been a wide range of reactions among the clinical community. Some providers report excitement at being able to provide hope to patients with an IPF diagnosis in ways that previously they have been unable to do so [37]. Other investigators are looking to see if additional benefit may be seen from combination therapy with pirfenidone and inhaled NAC when compared to pirfenidone alone [38]. The sense of hopefulness is not shared by all clinicians it seems, as many experts question the role of pirfenidone or nintedanib in all patients, or restricting use to patients who met the criteria of the clinical trials that evaluated their use [39], a practice that would remove many patients with severe IPF from qualifying for treatment.
Conclusions
Classic management strategies in patients with IPF have shown mixed results. The promising data with both pirfenidone and nintedanib demonstrating slowing of disease progression will likely lead to a paradigm shift in the chronic treatment of this disease state. Clinical familiarity with these new agents, particularly in the inpatient population, will evolve over the next several years of clinical practice.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ats/ers/jrs/alat statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21(126):355–61.
King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164(6):1025–32.
Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1(5):369–76.
Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(12):1390–4.
King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–61.
Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203.
Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40.
Tzouvelekis A, Bonella F, Spagnolo P. Update on therapeutic management of idiopathic pulmonary fibrosis. Ther Clin Risk Manag. 2015;11:359–70.
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–43.
Antoniou KM, Wells AU. Acute exacerbations of idiopathic pulmonary fibrosis. Respiration. 2013;86(4):265–74.
Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22(5):821–6.
Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128(5):3310–5.
Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–10.
King TE Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (inspire): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374(9685):222–8.
King TE Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, et al. Build-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81.
Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128(3):1475–82.
Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186(1):88–95.
Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125–33.
Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178(9):948–55.
Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8.
Alagha K, Secq V, Pahus L, Sofalvi T, Palot A, Bourdin A, et al. We should prohibit warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;191(8):958–60.
Peikert T, Daniels CE, Beebe TJ, Meyer KC, Ryu JH. Assessment of current practice in the diagnosis and therapy of idiopathic pulmonary fibrosis. Respir Med. 2008;102(9):1342–8.
Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–42.
Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and n-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77.
Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–101.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–7.
Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol. 1999;276(2 Pt 1):L311–8.
Misra HP, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem. 2000;204(1–2):119–26.
Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase Ii study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061–9.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–9.
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet. 2011;377(9779):1760–9.
•• King TE, Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. This study was a phase 3 international, multicenter study that evaluated the effects of pirfenidone vs. placebo for 52 weeks in patients with IPF. Patients who received perfenidone had significantly less decline in lung function. There also was improvement in exercise tolerance and progression-free survival.
Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–82.
Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87.
•• Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. The two IMPULSIS studies evaluated the safety and efficacy of nintedanib compared to placebo in patients with confirmed IPF. Both studies showed a beneficial effect of nintedanib on disease progression, and IMPULSIS-2 demonstrated a decrease in the rate of acute exacerbations.
Hunninghake GM. A new hope for idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2142–3.
Sakamoto S, Muramatsu Y, Satoh K, Ishida F, Kikuchi N, Sano G, et al. Effectiveness of combined therapy with pirfenidone and inhaled n-acetylcysteine for advanced idiopathic pulmonary fibrosis: a case-control study. Respirology. 2015;20(3):445–52.
Moodley Y, Corte T, Richeldi L, King TE Jr. Do all patients with idiopathic pulmonary fibrosis warrant a trial of therapeutic intervention? A pro-con perspective. Respirology. 2015;20(3):389–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on COPD.
Rights and permissions
About this article
Cite this article
DeGrado, J.R., Gilmore, J.F. Update on New Treatments for Idiopathic Pulmonary Fibrosis. Curr Emerg Hosp Med Rep 3, 134–138 (2015). https://doi.org/10.1007/s40138-015-0076-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-015-0076-8