Abstract
Purpose of Review
Blunt cerebrovascular injury (BCVI) can result in significant morbidity and mortality. Early screening, type of medical therapy, timing of medical therapy and the utilization of endovascular interventions are important factors in the management of BCVI.
Recent Findings
The development of screening criteria in the trauma patient population has improved diagnostic yield, allowing for earlier treatment and better outcomes. While different screening criteria schemas have been published, recent literature suggests a universal screening protocol may be justified. Medical therapy using anticoagulation or antiplatelet agents have been the mainstay of treatment. The use of endovascular intervention had support initially but has fallen out of favor and is not currently recommended for routine use.
Summary
BCVI management centers on broad screening of the trauma population, early diagnosis and management with anticoagulation or antiplatelet therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
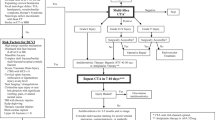
In modern reports, blunt cerebrovascular injury (BCVI) is diagnosed in 1–3% of patients who suffer from blunt trauma [1,2,3]. Subsequent stroke can result in debilitating morbidity and mortality. Patients with untreated BCVIs have a stroke risk of up to 40%, with variable morbidity based on the vessel injured and grade of injury [4]. Over the past several decades, regionalization of trauma care and advances in screening technology have increased recognition of BCVI, resulting in earlier treatment with medical therapy and decreased stroke rates.
Early screening was initially performed using diagnostic angiography. Today, computed tomography angiography (CTA) is the primary diagnostic modality with angiography reserved for select cases [5]. Systematic screening for BCVI has resulted in earlier diagnosis. Several screening guidelines have been proposed, all of which include patients considered high risk by injury pattern [6, 7]. Selective use of screening will underestimate the true incidence of BCVI and with the ever expanding “criteria” included in protocols, universal screening is becoming a more common alternative [8•, 9••].
The mainstay of BCVI treatment is medical therapy with anticoagulation or antiplatelet agents. No dedicated study has evaluated the use of pharmacologic agents compared to one another, however either therapy appears acceptable [10, 11••]. More importantly, it appears to be the early initiation of medical therapy that decreases stroke rate [2, 4]. Use of endovascular interventions was initially explored for higher grade injuries, but recent literature advises against routine use of stents with stroke rates remaining low with medical therapy alone [11••, 12, 13]. The aim of this review is to describe the evolution of diagnostic and management strategies in BCVI in the literature.
Diagnostic Modalities
BCVI was initially diagnosed after patients demonstrated neurologic sequelae following blunt trauma. Typically, the neurologic symptoms presented after a latent period and injury was confirmed using angiography. Fabian et al. identified 21 patients with blunt carotid artery dissections on angiography after they presented with a change in neurologic examination, or an initial neurologic deficit not explained by computed tomography (CT) imaging [14]. This prompted more liberal use of diagnostic angiography in blunt trauma patients. Over an 8-year period Cothren et al. screened 727 patients with diagnostic angiography, of which 244 were diagnosed with BCVI. The authors concluded comprehensive screening with diagnostic angiography prevented strokes and was cost-effective [15].
While angiography was the early diagnostic modality of choice, less invasive and less expensive modalities were explored as an alternative for screening. Berne et al. used 4-slice CTA to screen 486 patients and found 19 patients with BCVIs, which were subsequently confirmed with angiography. The authors stated there were no patients with a negative CTA that had a missed injury [16]. However, others found CTA inadequate compared to angiography with sensitivity for BCVI diagnosis ranging from 29 to 68% [17,18,19]. Magnetic resonance angiography (MRA) and duplex doppler ultrasound were also found to be insufficient with sensitivities of 50% to 75% and 38.5%, respectively [17, 18, 20].
CT imaging has improved with advancement in technology; however, angiography remains the gold standard in diagnosis. Despite superior sensitivity with angiography, CTA has emerged as the principal modality for screening likely due to its use in combination with liberal screening criteria, ease, cost, and non-invasive nature [11••, 21•]. At our institution, we screen patients with CTA and when BCVI is identified or imaging is equivocal, angiography is subsequently performed. While CTA is widely accepted, its diagnostic ability is still limited compared to angiography.
Screening Criteria Guidelines
The first reports of BCVI occurred after patients experienced neurologic sequelae followed by confirmatory imaging. Understanding the pathophysiology and common symptomatic presentation, led to the development of screening guidelines based on associated injuries, mechanism of injury, and symptoms to identify patients with potential BCVI.
Biffl et al. demonstrated patients with Glasgow coma score ≤ 6, petrous bone fracture, diffuse axonal injury, or Lefort II or III fracture, in the setting of high-risk mechanisms (hyperextension, hyperflexion, or direct blow) had a 41% risk of carotid injury. 39% of patients with cervical spine fractures were found to have vertebral artery injuries. Importantly, 20% of patients diagnosed with BCVI did not have any of those risk factors, which highlights the need for broad screening to prevent missed injuries [22]. Further investigation resulted in the more liberalized Memphis Criteria which included cervical spine fractures, Lefort II or III fractures, Horner’s syndrome, skull base fractures involving the foramen lacerum, neck soft tissue injury, or neurological abnormalities unexplained by CT head and yielded a 29% BCVI diagnosis when implemented as a screening protocol. After implementation of the screening criteria, 91% of BCVIs were detected prior to the development of neurologic sequelae [17]. Cervical spine fractures involving subluxation, extension into the foramen transversarium, and C1 to C3 fractures were injury patterns specifically associated with vertebral artery injuries [23].
The Denver Criteria incorporated signs and symptoms of BCVIs and risk factors associated with injury patterns and mechanism. Of 727 patients screened, 244 (34%) patients were diagnosed with BCVI [15]. When the Denver Criteria were incorporated into a screening protocol, a significant increase in BCVI diagnosis (0.52% vs 1.06%), yet a decreased rate of stroke was reported (40% vs 27%). Since the development of the Memphis and Denver screening criteria in the early 2000’s, the criteria have been refined and expanded [3, 4, 6, 24]. The American College of Surgeons Trauma Quality Improvement Program (ACS TQIP) Best Practices Guidelines in Imaging incorporates 15 signs and findings based on physical exam, mechanism, and injuries into a BCVI screening protocol [25].
Leichtle et al. implemented universal screening at their institution and found almost 20% of patients with BCVI would not have been diagnosed by using the expanded Denver criteria or the ACS TQIP Best Practices Guidelines in Imaging [25]. Most recently, Black et al. employed universal screening and concluded 25.3% to 52.7% of patients diagnosed with BCVI would not have been screened for BCVI using the Memphis, Denver, or expanded Denver criteria. 7.6% of patients who suffered from blunt trauma were diagnosed with BCVI [9••].
Early screening protocols were developed to identify high-risk patients without over utilizing resources [22]. Expansion of criteria and now universal screening have captured the true incidence of BCVI, decreasing the devastating morbidity and mortality from neurological sequelae. In our institution, we universally screen blunt trauma patients with CTA for BCVI with a protocol developed in conjunction with the radiology department to minimize contrast load.
Medical Therapy
BCVIs are difficult to operate on due to their inaccessible location, therefore treatment is with either systemic anticoagulation or antiplatelet therapy. Fabian et al. demonstrated heparin therapy to be independently associated with improvement in neurologic outcome and survival. Heparin therapy started before the onset of symptoms prevented neurologic deterioration [26]. Further studies found anticoagulation with heparin, antiplatelet therapy, or a combination of both demonstrated decreased incidence of stroke in patients with BCVIs [2, 4, 10].
In many studies, a combination of anticoagulation and antiplatelet therapy is used in treatment, making it difficult to determine the superiority of one therapy compared to another. Cothren et al. identified 282 asymptomatic BCVIs and initiated treatment with either heparin, aspirin, or aspirin and/or clopidogrel. Only one patient developed a stroke, injury healing rates were similar (39% vs 43% vs 46%) among treatment groups and injury progression rates were also similar (12% vs 10% vs 15%) among treatment groups [10]. Esposito et al. published a large multicenter trial evaluating factors associated with stroke formation. There were 777 BCVIs included in the study with a stroke rate of 8.9%. The authors found use of antiplatelet therapy, specifically aspirin, during the hospitalization was associated with lower stroke rates [27•].
At our institution, treatment with systemic heparin is initiated as soon as possible. Questionable findings or injuries identified on CTA are confirmed using angiography. Although we usually repeat imaging within 1 week after diagnosis to evaluate for resolution of injury, work from Wagenaar et al. showed early follow up imaging of high-grade injuries may not be warranted because high-grade injuries did not resolve [28]. This area requires further study. Long-term treatment is then transitioned to single or dual-antiplatelet therapy.
Timing of Medical Therapy
In early studies, BCVIs were diagnosed after neurologic symptoms presented in patients, and subsequent treatment with medical therapy was started reactively [14, 26]. The authors concluded that since patients improved with medical therapy, earlier diagnosis and treatment would improve outcomes. Management shifted to aggressive screening focusing on early identification and treatment of BCVIs during their “latent” period, optimizing outcomes. Several studies demonstrated decreased stroke rate when BCVIs were treated before patients developed neurologic sequalae [2, 4, 15].
A more challenging scenario is when to start treatment in patients who have concomitant traumatic brain injuries (TBI) and/or solid organ injuries (SOI). Management strategies in this patient population are controversial and not well-described. Shahan et al. evaluated 119 patients, with BCVI and either TBI or SOI or both, treated with anticoagulation or antiplatelet therapy. The mean time to therapeutic active partial thromboplastic time (aPTT) from diagnosis was 7 h. Medical therapy did not increase risk of worsening TBI or SOI [29].
However, a recent multi-institutional trial found treatment delay may not necessarily result in stroke. In the 16-center trial, 636 BCVIs were identified, and treatment was considered delayed if initiation was > 24 h. Median time to medical therapy was 62 h in the delayed group compared to 11 h in the non-delayed group. ISS was greater in the delayed group, but there was no overall difference in stroke rate between groups. The authors conclude necessary delays in treatment in patients with concomitant injuries may not result in increased stroke rate [30•].
Modern treatment of BCVI hinges on early diagnosis and initiation of medical therapy. At our institution we initiate medical therapy as soon as possible, including all patients with SOI, TBI and spine injuries (unless patients go directly to the operating room). Heparin infusion is initiated when patients with TBI demonstrate hemorrhage stability on CT imaging. This is usually achieved 6 h after their initial CT scan, however in cases when TBIs worsen on repeat imaging, time to heparin infusion can be delayed by 24 to 36 h. Patients are closely monitored in an intensive care unit setting.
Endovascular Interventions
Injuries with luminal narrowing and pseudoaneurysms were initially thought to benefit from therapeutic endovascular stenting. Early studies demonstrated feasibility and successful outcomes by decreasing the risk of embolism and rupture with stent placement [31, 32]. However, the role of endovascular stenting has evolved over that past few decades. After a significant increase in use, further studies reported risk of stent thrombosis and neurologic deficits after stent placement [33, 34]. Burlew et al. reported stent use had significantly decreased over the study period. The authors found antithrombotic treatment was effective in high grade BCVIs and routine stenting did not provide additional benefit [12]. Shahan et al. reported similar findings, with a decrease in stent use (34% vs 8.9%) over a 5-year period, yet no change in stroke rate [13]. Recently published practice management guidelines recommended against routine use of endovascular stenting [11••].
At our institution, the use of endovascular stents has dramatically decreased over the years. Occasional use in an enlarging carotid pseudoaneurysm or dissection with significant stenosis may be warranted. Since treatment with anticoagulation or antiplatelet therapy alone has demonstrated decreased risk of stroke and mortality, the role for endovascular stent placement is limited. Possible procedural complications, need for long-term antiplatelet therapy, and need for repeat imaging and/or procedures must be considered when determining the treatment plan for the individual patient.
Conclusion
Diagnosis and management of BCVI has evolved over the past several decades. CTA is a noninvasive diagnostic modality that has increased in use and is considered standard in screening. Its ease and cost make liberal screening possible which is critical to early diagnosis and treatment to prevent the devastating morbidity and mortality associated with stroke events. Universal screening results in BCVI diagnosis in a substantial number of patients who would not usually be identified using even the most aggressive screening criteria. CTA used in combination with universal screening helps overcome the diagnostic limitations of CTA. Medical therapy with anticoagulation or antiplatelet agents has been the mainstay of treatment and can safely be used in patients with TBI and SOI. The use of endovascular stents has decreased over time due to risk of complications and should only be considered in select cases.
Data Availability
Not applicable.
References
Papers of particular interest, published recently have been highlighted as • Of importance •• Of major importance
Kerwin AJ, Bynoe RP, Murray J, Hudson ER, Close TP, Gifford RR, Carson KW, Smith LP, Bell RM. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma. 2001;51(2):308–14.
Stein DM, Boswell S, Sliker CW, Lui FY, Scalea TM. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66:132.
Geddes AE, Burlew CC, Wagenaar AE, Biffl WL, Johnson JL, Pieracci FM, Campion EM, Moore EE. Expanded screening criteria for blunt cerebrovascular injury: a bigger impact than anticipated. Am J Surg. 2016;212(6):1167–74.
Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, Croce MA. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279–85 (discussion 285-6).
Paulus EM, Fabian TC, Savage SA, Zarzaur BL, Botta V, Dutton W, Croce MA. Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg. 2014;76(2):279–83 (discussion 284-5).
Emmett KP, Fabian TC, DiCocco JM, Zarzaur BL, Croce MA. Improving the screening criteria for blunt cerebrovascular injury: the appropriate role for computed tomography angiography. J Trauma. 2011;70(5):1058–63 (discussion 1063-5).
Burlew CC, Biffl WL, Moore EE, Barnett CC, Johnson JL, Bensard DD. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg. 2012;72(2):330–5 (discussion 336-7, quiz 539).
• Leichtle SW, Banerjee D, Schrader R, Torres B, Jayaraman S, Rodas E, Broering B, Aboutanos MB. Blunt cerebrovascular injury: the case for universal screening. J Trauma Acute Care Surg. 2020;89(5):880–6 (Without implementation of universal screening, 20% of BCVI would not be diagnosed).
•• Black JA, Abraham PJ, Abraham MN, Cox DB, Griffin RL, Holcomb JB, Hu PJ, Kerby JD, Liptrap EJ, Thaci B, Harrigan MR, Jansen JO. Universal screening for blunt cerebrovascular injury. J Trauma Acute Care Surg. 2021;90(2):224–31 (Universal screening identifies a significant number of patients with BCVI that would not have been screened with traditional criteria).
Cothren CC, Biffl WL, Moore EE, Kashuk JL, Johnson JL. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144(7):685–90.
•• Kim DY, Biffl W, Bokhari F, Brakenridge S, Chao E, Claridge JA, Fraser D, Jawa R, Kasotakis G, Kerwin A, Khan U, Kurek S, Plurad D, Robinson BRH, Stassen N, Tesoriero R, Yorkgitis B, Como JJ. Evaluation and management of blunt cerebrovascular injury: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;88(6):875–87 (A recent practice management guideline supporting use of systematic screening with CTA, treatment with antithrombotic therapy, and limited use of endovascular stents).
Burlew CC, Biffl WL, Moore EE, Pieracci FM, Beauchamp KM, Stovall R, Wagenaar AE, Jurkovich GJ. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg. 2014;218(5):1012–7.
Shahan CP, Sharpe JP, Stickley SM, Manley NR, Filiberto DM, Fabian TC, Croce MA, Magnotti LJ. The changing role of endovascular stenting for blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2018;84(2):308–11.
Fabian TC, George SM Jr, Croce MA, Mangiante EC, Voeller GR, Kudsk KA. Carotid artery trauma: management based on mechanism of injury. J Trauma. 1990;30(8):953–61 (discussion 961-3).
Cothren CC, Moore EE, Ray CE Jr, Ciesla DJ, Johnson JL, Moore JB, Burch JM. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg. 2005;190(6):845–9.
Berne JD, Norwood SH, McAuley CE, Villareal DH. Helical computed tomographic angiography: an excellent screening test for blunt cerebrovascular injury. J Trauma. 2004;57(1):11–7 (discussion 17-9).
Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, Qaisi WG, Felker RE, Timmons SD. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236(3):386–93 (discussion 393-5).
Biffl WL, Ray CE Jr, Moore EE, Mestek M, Johnson JL, Burch JM. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53(5):850–6.
Goodwin RB, Beery PR 2nd, Dorbish RJ, Betz JA, Hari JK, Opalek JM, Magee DJ, Hinze SS, Scileppi RM, Franz RW, Williams TD, Jenkins JJ 2nd, Suh KI. Computed tomographic angiography versus conventional angiography for the diagnosis of blunt cerebrovascular injury in trauma patients. J Trauma. 2009;67(5):1046–50.
Mutze S, Rademacher G, Matthes G, Hosten N, Stengel D. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology. 2005;237(3):884–92.
• Kik CC, Slooff WM, Moayeri N, de Jong PA, Muijs SPJ, Öner FC. Diagnostic accuracy of computed tomography angiography (CTA) for diagnosing blunt cerebrovascular injury in trauma patients: a systematic review and meta-analysis. Eur Radiol. 2022;32(4):2727–38 (CTA has limitations as a diagnostic modality compared to the gold standard of diagnostic angiography).
Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Elliott JP, Burch JM. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178(6):517–22.
Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE Jr, Johnson JL, Moore JB, Burch JM. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811–3.
Bruns BR, Tesoriero R, Kufera J, Sliker C, Laser A, Scalea TM, Stein DM. Blunt cerebrovascular injury screening guidelines: what are we willing to miss? J Trauma Acute Care Surg. 2014;76(3):691–5.
American College of Surgeons. ACS TQIP best practices guidelines in imaging. 2018. https://www.facs.org/-/media/files/quality-programs/trauma/tqip/imaging_guidelines.ashx. Accessed 22 Nov 2022.
Fabian TC, Patton JH Jr, Croce MA, Minard G, Kudsk KA, Pritchard FE. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223(5):513–22 (discussion 522-5).
• Esposito EC, Kufera JA, Wolff TW, Spalding MC, Simpson J, Dunn JA, Zier L, Burruss S, Kim P, Jacobson LE, Williams J, Nahmias J, Grigorian A, Harmon L, Gergen A, Chatoor M, Rattan R, Young AJ, Pascual JL, Murry J, Ong AW, Muller A, Sandhu RS, Appelbaum R, Bugaev N, Tatar A, Zreik K, Hustad L, Lieser MJ, Stein DM, Scalea TM, Lauerman MH. Factors associated with stroke formation in blunt cerebrovascular injury: an EAST multicenter study. J Trauma Acute Care Surg. 2022;92(2):347–54 (Protocolized management, treatment with antiplatelet therapy, and lower percentage of luminal stenosis are factors associated with lower stroke rates in patients with BCVI).
Wagenaar AE, Burlew CC, Biffl WL, Beauchamp KM, Pieracci FM, Stovall RT, Jurkovich GJ, Moore EE. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2014;77(4):540–5 (quiz 650).
Shahan CP, Magnotti LJ, McBeth PB, Weinberg JA, Croce MA, Fabian TC. Early antithrombotic therapy is safe and effective in patients with blunt cerebrovascular injury and solid organ injury or traumatic brain injury. J Trauma Acute Care Surg. 2016;81(1):173–7.
• Appelbaum RD, Esposito E, Spaulding MC, Simpson JP, Dunn J, Zier LB, Burruss S, Kim PP, Jacobson LE, Williams JM, Nahmias J, Grigorian A, Harmon L, Gergen AK, Chatoor M, Rattan R, Young AJ, Pascual JL, Murry J, Ong AW, Muller A, Sandhu RS, Bugaev N, Tatar A, Zreik K, Lieser MJ, Stein DM, Scalea TM, Lauerman MH. Does treatment delay for blunt cerebrovascular injury affect stroke rate?: An EAST multicenter study. Injury. 2022;53(11):3702–8. (Delayed medical therapy occurs in more severely injured patients but does not result in higher stroke rates).
Duke BJ, Ryu RK, Coldwell DM, Brega KE. Treatment of blunt injury to the carotid artery by using endovascular stents: an early experience. J Neurosurg. 1997;87(6):825–9.
Coldwell DM, Novak Z, Ryu RK, Brega KE, Biffl WL, Offner PJ, Franciose RJ, Burch JM, Moore EE. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000;48(3):470–2.
DuBose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65(6):1561–6.
Cothren CC, Moore EE, Ray CE Jr, Ciesla DJ, Johnson JL, Moore JB, Burch JM. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg. 2005;140(5):480–5.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DMF: writing manuscript, AJK: editing and revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial disclosures.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Filiberto, D.M., Kerwin, A.J. Blunt Cerebrovascular Injury. Curr Surg Rep 11, 81–85 (2023). https://doi.org/10.1007/s40137-023-00350-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40137-023-00350-3