Abstract
Management options of vestibular schwannomas continue to evolve as surgical, radiation, and radiologic imaging techniques and technology change and improve over time. Currently, three options for these tumors exist and include primary surgical extirpation, stereotactic radiation, and observation with serial imaging. Skull base surgeons are charged with providing the patient with a comprehensive discussion of each option based on the inherit risks and benefits of each option to craft a management plan based on numerous factors that include age, tumor location, and size, hearing level at presentation and any medical comorbidities that may influence functional outcomes and quality of life. The purpose of this article was to review the surgical and non-surgical approaches to tumor management highlighting the risks and benefits and ongoing controversies between modalities regarding optimal functional outcomes. A particular emphasis is placed on hearing preservation and facial nerve function after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vestibular schwannomas (VS, aka, acoustic neuromas) are benign neoplasms that arise from Schwann cells. They comprise more than 80 % of all tumors within the cerebellopontine angle (CPA). Recent advances in skull base surgical technique and radio-surgical options as well as sophisticated imaging modalities have yielded improved cranial nerve function, quality of life, and decreased morbidity and mortality. Despite the advancements, considerable controversy persists between surgical and non-surgical approaches in terms of optimal VS treatment and outcome. Cranial base surgeons are faced with selecting options including microsurgical extirpation, stereotactic radiosurgery, and watchful waiting/observation as primary treatment modalities. Factors including audiometric status, tumor location, and size as well as medical co-morbidities and patient age are important selection criteria that influence the therapeutic modality and the risk of cranial base/intra-cranial surgery, functional preservation, and long-term quality of life. The purpose of this review article was to compare current surgical and non-surgical (radiosurgery and observation) approaches with a particular emphasis on indications and therapeutic outcome.
Surgical Options
Surgical Approaches
Three surgical approaches [middle cranial fossa (MCF), retro-sigmoid/sub-occipital (RS), or translabyrinthine (TL)] to the lateral cranial base/temporal bone are employed for VS extirpation. Each approach is individually selected based on criteria that include tumor size, location, pre-operative hearing status, patient preference, and individual medical co-morbidities. The MCF approach is typically reserved for small tumors confined to the medial aspects of the internal auditory canal (IAC) with optimal characteristics for hearing preservation [1]. The retrosigmoid (RS) approach is used for large CPA tumors with or without considerable brainstem compression or for all tumors except those isolated to the lateral IAC. The TL approach is hearing ablative as it provides a direct route through the inner ear to the IAC and CPA without brain retraction. This approach provides ideal access and visualization of the lateral IAC for complete tumor extirpation and early facial nerve (FN) identification [2].
Middle Cranial Fossa Approach (MCF)
Indications/Technique
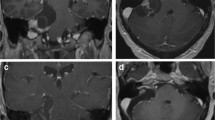
With availability of fine-cut MRI (VIII nerve or IAC protocol), identification of small (<1.5 cm) tumors in patients with normal or near-normal hearing has increased. In younger, healthy patients with serviceable hearing and a medially positioned tumor within the IAC, a MCF approach is indicated (Fig. 1). Demonte and Gidley [3] outlined the ideal patients for a MCF approach and VS resection with hearing preservation to include those with tumors with 1 cm or less extension into the CPA located in the medial end of the IAC with a hearing loss no greater than 40 dB on pure tone average (PTA) testing and a word recognition score (WRS) of at least 80 % (AAO-HNS Class A and upper Class B hearing). They also identified those younger than 65 years since dural elevation becomes more difficult in older patients.
Tumors suitable for hearing preservation via a middle cranial fossa (MCF) approach. Note the small tumor in the medial aspect of the right internal auditory canal (IAC) on this coronal T1-weighted MRI. Tumors isolated in this area of the IAC are well suited for a MCF approach as there is no extension to the delicate cochlear modiolar fibers in the lateral IAC. If pre-operative hearing levels are meaningful in a younger patient who desires a hearing preservation surgery, a MCF approach is indicated
Complications/Outcomes
Any intracranial surgery that involves brain retraction has potential surgical risks that include epidural hematoma, brain edema, pneumocephalus, CSF leak, meningitis, seizure, and wound complications/infections. Specific to MCF surgery are temporal lobe injury (if dominant lobe could lead to transient aphasia/dysphasia), CSF leak, hearing loss, and FN injury. With more improved surgical technique leading to shorter operative and brain retraction time, many complications secondary to MCF surgery are largely limited to CSF leak, FN injury, and hearing loss.
Hearing Preservation
Hearing preservation is defined as maintenance of post-operative hearing of at least 50 dB PTA and 50 % WRS (50/50 rule) and encompasses classes A and B of the American Academy of Otolaryngology-Head and Neck Surgery system. It is generally acknowledged that for those undergoing MCF surgery hearing preservation rates are better in tumors arising medially in the IAC [4] with an adequate CSF cap lateral to the tumor buffering the cochlear modiolus (Fig. 2) and if it arises from the superior vestibular nerve (SVN) rather than the inferior vestibular nerve (IVN). Given the proximity of the IVN to the cochlear nerve (CN), tumor adhesion and mechanical damage to the CN during surgery are more likely. Opening of the IAC from above with resultant direct visualization of tumors in the superior compartment also make SVN tumors more amenable to resection. Pre-operative identification of the nerve of origin is therefore an important prognostic consideration and is often possible with small tumors and MRI.
Differences in MRI imaging modality help to determine surgical candidacy and surgical approach. Various weighted (T1 vs T2) MRI images may be helpful in determining the extent of tumor infiltration into various structures. This figure shows an axial T2-weighted image through the IAC demonstrating the right space occupying lesion consistent with a vestibular schwannoma from the same tumor seen on T1-weighted images (Fig. 1). Note the utility of seeing the CSF cap lateral to the tumor on T2 imaging that represents an incomplete extension into the delicate cochlear modiolar fibers that suggest space for surgical removal with the potential to spare delicate auditory structures
Using AAO criteria, Brackmann [5] reported that 59 % of patients following MCF surgery for VS had serviceable hearing with 50 % maintaining pre-operative hearing levels. Satar [6] reported 62 % preservation of hearing following MCF approach, while others have reported approximately 70 % of patients retaining serviceable hearing 5–10 years after VS resection [7, 8•, 9]. When hearing preservation rates are compared across surgical approach, Staecker [10] reported a 57 % success rate following MCF versus 47 % in comparable tumors removed via RS, while Noudel [11] reported 62 % for MCF and 58 % for RS. These rates are widely reported and range from 37 to 77 % with MCF generally being superior with hearing results that will typically remain stable over time [12].
Facial Nerve Function
One main disadvantage of the MCF approach is the proximity of the FN within the superior fallopian canal and manipulation of the nerve during tumor extirpation. Tumor size is therefore deemed to be the most important prognostic factor of post-operative FN outcome. At 1-year following MCF resection of tumors 1.5 cm or smaller, Arriaga [13] reported that 96 % of patients retained normal or near-normal FN function (House-Brackmann grades I or II) with a more recent report of 94.5 % retaining HB I–II following MCF resection of tumors smaller than 1 cm [14]. Other groups have reported similar findings with smaller IAC tumors [6].
Retrosigmoid Approach (RS)
Indications/Technique
Of the noted surgical approaches to the CPA/IAC, the RS or sub-occipital approach is the most commonly utilized and provides wide visualization of the CPA [15]. As such, it can be employed for small to medium-sized tumors that have minimal extension within the IAC (Fig. 3) with the goal of hearing preservation (same AAO selection criteria as outlined for MCF approach above) and for large tumors, to alleviate brainstem and neurovascular compression. It is ideally utilized as a hearing sparing technique and provides ideal exposure of the brainstem and cranial nerves IV through XII.
Larger tumors with minimal IAC extension are ideal for a retrosigmoid surgical approach. This T1-weighted axial image shows the large right gadolinium-enhancing lesion consistent with vestibular schwannoma largely confined to the cerebellopontine angle. Note the minimal extension of the tumor within the IAC. Based on size and minimal IAC extension, a retrosigmoid approach could be utilized to remove the lesion while potentially sparing any residual hearing and protecting facial nerve function
Complications/Outcomes
Like the MCF approach, the RS also involves brain retraction and may therefore be fraught with similar surgical complications including brain (brainstem and cerbellar) edema, hematoma, dural venous sinus congestion, vascular and/or lower cranial nerve injury, chronic headache from intradural drilling, CSF leak, FN injury, and hearing loss. Given the posterior approach for larger tumors, the FN is often found on the anterior surface of the tumor and is therefore not at immediate risk of injury as it is in the MCF approach (Fig. 3). However, the cisternal segment of the FN lacks a true epineurium and is therefore prone to splaying with mass effect from larger tumors, which may place the nerve at greater risk for stretch or transection injury often leading to subtotal tumor resection.
Hearing Preservation
The main disadvantages of the RS approach include limited exposure of the lateral IAC and the potential for postoperative headache and occipital pain syndromes. Hilman [8•] reported improved hearing preservation rates for small tumors with the MCF approach (59.3 % of 88 patients) versus RS (38.5 % of 50 patients), similar to results published over 10 years ago [10, 16, 17]. Two recent publications, however, report no significant difference between hearing preservation between MCF and RS approaches for small tumors [12, 18•].
Facial Nerve Function
Injury to the FN may occur during any component of VS resection and is often secondary to drilling of the bony IAC or following stretch or transection injury during tumor resection. Extirpation of large tumors places the FN at particular risk as the nerve becomes thinned as it stretches over a large tumor capsule where visualization is compromised (Fig. 3). The FN is most vulnerable at the interface between the cisternal and meatal segments. An extensive review of the literature reports favorable outcomes for FN function following RS approach for VS resection. Sughrue [19] reported HB I–II in 90 % of cases for tumors <2 cm that dropped to 67 % when the tumor size exceeded 2 cm. These numbers compare favorably to the authors MCF and TL approaches, where 85 and 81 % of patients had HB I–II following surgery, respectively. Cardoso [20] reported similar outcomes in tumors less than 3 cm where HB I–II was achieved in 90 % of cases, while Zhao [21] reported only 50 % rates of HB I–II 1-year following RS approach for large (>4 cm) tumors. The less favorable FN outcomes with larger tumors highlight the susceptibility of the cisternal segment of the nerve where a clear epineurium is absent allowing for splaying of nerve bundles over the tumor capsule making them particularly vulnerable to stretch or transection during tumor resection.
Translabyrinthine Approach (TL)
Indications/Technique
The TL approach is often utilized for larger CPA/IAC tumors when pre-operative hearing is poor or in cases where the chance of sparing hearing during surgery would be unlikely (Fig. 4). As with the MCF and RS approaches, there are advantages and disadvantages. One main advantage to the TL approach is that it provides the most direct route to the CPA and exposes the IAC in its entirety. This is accomplished without brain retraction, and the FN can be easily located and unmolested due to the vast exposure of the IAC and translocation if required. The TL approach therefore provides ideal tumor exposure and a safe and reliable tissue plane between the tumor and the FN. In addition to VS, the TL approach may be used for other lesions of the CPA including meningiomas, epidermoid cysts, glomus tumors, lipomas, metastatic lesions, and choroid plexus papillomas. The main disadvantages of the TL approach lie in the obliteration of residual hearing and balance function for the ipsilateral ear and the need for a fat graft repair to prevent CSF leaks post-operatively.
Tumors filling the entire IAC with poor pre-operative hearing levels are ideal for a translabyrinthine approach. This axial T1-weighted MRI shows a compact vestibular schwannoma within the entire IAC with compression up to the cochlear modiolus making this tumor not a candidate for a hearing preservation approach. Given the bulk of the tumor being isolated to the IAC with minimal CPA extension, the hearing ablative, translabyrinthine approach is most ideal as it will allow for complete tumor extirpation, facial nerve preservation, and no brain retraction
Complications/Outcomes
As with the MCF and RS techniques, surgical complications also exist for the TL approach. Since the TL approach obliterates the inner ear, residual hearing and balance function are sacrificed entirely and are therefore expected outcomes rather than surgical complications. Due to the lack of brain retraction required for this approach, complications are largely limited to FN injury, bleeding, CSF leak, meningitis, and wound complications/infections.
Facial Nerve Function
Given the wide exposure of the IAC and complete visualization of the FN, most published reports have shown favorable preservation rates for FN outcome following TL surgery. One variable that predicts FN success is tumor size. A review of 512 patients one-year following TL surgery where approximately 95 % of the tumor was removed, HB I–II was seen in greater than 80 % of patients with tumors less than 2.5 cm, with HB III–IV seen approximately 15 % of the time. In cases where the tumor exceeds 3.5 cm, the rates of HB I–II fell to 53 % with near complete FN paralysis seen at 17 %. These data are consistent with other reports where larger tumors (>3 cm) resulted in HB I–III 1-year following resection [22], while others have shown HB I–II in 78 % of patients with similar-sized tumors 6 months after surgery [23]. For the largest tumors (>4 cm) where a combined trans-otic or trans-cochlear approach is required for tumor access with anterior apical extension, FN function was found to be intact in nearly 77 % of cases with HB I–III being retained in 63 % of cases 1-year following surgery [2].
Non-Surgical Options
Observation
Management of VS has recently seen a transition from complete tumor extirpation to optimization of functional outcomes for patients. Associated with improved ability to diagnose smaller tumors, there is an increasing trend to observe tumors versus surgical resection or radiation [24–26]. The change in treatment paradigm is largely based on the benign and slow growing nature of VS, the relative risks to hearing, balance, and FN with treatment intervention. Of 552 tumors, 17 % of IAC lesions demonstrated growth (defined as progression to an extrametal tumor) and 29 % of extrametal tumors demonstrated growth (>2 mm) over a follow-up period of 3.6 years (range 1–15 years), while <20 % of patients ultimately required treatment [25]. Thus, observation as a management option can be appealing in the elderly and in those with small tumors at presentation. It is also a viable option in those with serviceable hearing at presentation, but whose tumor is not compatible with hearing preservation surgery, either based on size or anatomical location (Fig. 5). In patients who presented with excellent (100 %) WRS, 69 % maintained hearing over 10 years later. Alternatively, in those with WRS <100 % at presentation, only 38 % maintained serviceable hearing over time [27•]. When considering observation for VS management, it is critical to impart that hearing will likely continue to unpredictably deteriorate in a manner not proportionate to tumor growth [28–30]. Thus, observation may not be the best management option in those with small tumors who consider long-term hearing preservation a priority. Patients should also understand that the extent of tumor growth allowed before proceeding to treatment could impact risk stratification in terms of FN preservation and other complications associated with treatment.
Tumor characteristics and intact hearing often warrant observation. This T1-weighted axial MRI in a younger patient reveals a gadolinium-enhancing lesion within the right IAC that is compacted into the cochlear modiolus (arrow). In this patient, the tumor has not grown significantly over several months with intact, meaningful hearing in place. This tumor based on the location is not removable without sacrificing hearing; therefore, repeat MRI every 6–12 months is warranted to observe for any changes that may warrant surgical removal, while ongoing hearing evaluations are conducted to allow the patient to enjoy intact function for as long as possible
As observation is increasingly chosen for VS management, accurate measurement of tumor size and growth becomes an issue. Most studies currently report size based on maximal tumor diameter, diameter of the tumor parallel to the petrous apex, or as orthogonal measurements. Each of these methods has limitations as VS typically have an IAC and cisternal component and are often irregular in shape (Fig. 6). There is an increasing argument for measuring and reporting tumor size based on total tumor volume [31]. Proposed methods include the Cavalieri, the maximal diameter, the orthogonal (XYZ), and the maximum slice area methods.
The size classification of VS is challenging as there typically is an IAC and a CPA component. This T1-weighted coronal MRI shows a left gadolinium-enhancing lesion that fills the IAC with extension medial to the porous acousticus into the CPA. The multi-location makes a common classification on size challenging when measuring the tumor for changes over time on serial MRI
Regardless of measurement strategy regular surveillance imaging is critical. The exact timing of surveillance imaging varies, and it is generally agreed that imaging should be repeated more frequently (e.g., every 6 months) initially, and once a 1–2 year period of no growth is documented, imaging may be repeated at longer intervals (e.g., 1–2 year intervals).
Stereotactic Radiation
Radiation of VS has gained popularity [24, 32], largely from reported success in arresting tumor growth without long-term sequelae and improved quality of life [29, 33–35]. Stereotactic radiation may be delivered in a single dose, termed fractionated stereotactic radiosurgery or in fractions, termed stereotactic radiotherapy. Tumor control rates are similar between both modalities, and there are conflicting reports as to whether one strategy is superior in terms of hearing preservation [33, 36–40]. Initially, VS were treated with higher doses (16–20 Gy) of radiation, and most tumors were found to decrease in size. However, higher rates of hearing loss, FN, and trigeminal nerve neuropathies led to decreased doses for single (12–14 Gy) or hypofractionated (18–21 Gy) treatment paradigms [41].
Indications for Stereotactic Radiation Treatment
Stereotactic radiation (STR) may be used for actively growing small to medium VS or to treat residual or recurrent tumors following micro-surgical resection or for elderly or poor surgical candidates. Reports of successful treatment of larger tumors (>2.5–3 cm) exist [42, 43], yet are noted to have greater risks as compared to smaller tumors [44]. Peritumoral edema, hydrocephalus, and radiotoxicity to the cochlea and adjacent cranial nerves are a known concern. While radiation of tumors in the NF2 population is appealing [41], STR in this population may be associated with lower tumor control rates and higher rates of malignant transformation [45].
Outcomes
The goal of STR of VS is arrest of tumor growth while minimizing risk to hearing, FN, trigeminal nerve, and adjacent brainstem. Evaluating success after STR is a challenge due to changes in technology, treatment protocols (radiation dose), and the relatively short duration of follow-up reported and variability in how treatment success is defined. Current studies have a mean follow-up of less than 5 years [31, 36, 46–50], and long-term outcomes are often based on actuarial estimates of tumor control and hearing preservation rates [41–43, 46, 47]. There is also significant variability in how successful tumor control or hearing preservation is defined. For example, some studies define successful tumor control as no growth on follow-up imaging [35, 47, 50, 51], while others report a lack of symptomatic growth or progression that required further treatment [31, 42, 49]. To illustrate, Vivas [31] reported tumor growth rates and percentage of patients that progressed to further intervention. They reported 17 % of tumors demonstrated growth (>2 mm) and 26 % of tumors demonstrated >20 % volume increase 1-year following treatment (tumor control rate of 80–83 %). Only 5 % required further intervention, and therefore a tumor control rate of 95 % could also be reported [31].
Despite limitations, STR is an effective means of tumor control with reported rates at 71–99 % [29, 31, 33, 35, 47, 50–53]. The variability in control rates reflects a lack of unified parameters whereby in general improved tumor control rates are defined as no growth on follow-up imaging [35]. Lower tumor control rates have been associated with larger tumors, inadequate radiation dose, and cystic tumors [43, 52, 54; Fig. 7]. However, many studies reporting control rates fail to clarify whether the tumor demonstrated growth prior to STR [42, 47, 49, 51–53]. Therefore, in many studies, it is unclear if the lack of tumor growth is a result of STR or whether the tumor naturally was not growing.
Large cystic tumors may be less responsive to stereo radiosurgery. This axial T1-weighted MRI shows a large left CPA tumor with mild IAC extension. Of note are the large size and the cystic nature as evidenced by the heterogeneous gadolinium signal throughout the body of the tumor. Lesion of this size and cystic nature are not particularly responsive to radiation and that known fact makes surgical extirpation more relevant
Hearing is successfully preserved in up to 93 % of VS patients after STR but uniformly degenerates over time to rates as low as 23 % 10 years later [31, 33, 41, 46, 48–53, 55•, 56]. There are no absolute predictors of long-term hearing preservation, yet many agree that level of radiation dose to the cochlea may portend a poor prognosis. It is impossible to avoid all radiation exposure; however, higher doses to the cochlea are associated with poorer hearing outcomes [46–48]. Advanced age, treatment dose >13 Gy, transient tumor volume expansion (>20 %) following treatment, any hearing loss at initial presentation, and larger tumors are all associated with poorer hearing outcomes [31, 36, 43, 47, 51, 52, 55•, 56]. When evaluating outcomes of STR, pre-treatment tumor growth rates, duration of follow-up after treatment, and hearing levels must all be considered [33, 37, 42, 43, 46, 47, 52, 55•, 56, 57].
Complications
Despite being a non-invasive procedure, complications from STR may still occur. Most complications are related to direct or indirect effects of radiation over time. Unsuccessful tumor control or recurrent growth, while uncommon, does occur. Persistent, sequential tumor growth, not to be confused with pseudo-progression, following radiation treatment may require either additional radiation treatment or microsurgical excision. Surgical excision following STR is more challenging due to changes in tumor texture and vascularity and increased adherence/scarring to the FN resulting in sub-total tumor extirpation to optimize FN outcomes [58].
The FN and trigeminal nerve (up to 3 %) [29, 33, 41, 52] are most susceptible to injury with STR. The incidence of injury has decreased secondary to lower and more accurate radiation dosing. Nonetheless, facial paresis continues to occur (up to 4 %) [29, 41, 50], is typically temporary and may improve with steroids, yet can be permanent (1.6 %) [33, 41] and is more likely when treating larger tumors [43] or with use of higher radiation doses (13 Gy) [52]. Post-radiation dysfunction of the nervus intermedius, manifested as disturbances in taste, salivation, or lacrimation (22–45 %), has been documented despite normal facial nerve motor function [59].
The incidence of vestibulopathy is unpredictable following STR. Chung and colleagues reported balance disturbance in 25 % of those following STR of large VS [42]. In 175 patients following STR, 28.6 % of patients reported improvement in vestibular symptoms and 14.3 % reported further impairment [57].
Hydrocephalus may occur following STR and often occurs within the first 1–2 years following treatment. Obstructive hydrocephalus may result from edema and typically resolves over time and can be treated successfully with oral steroids. Non-obstructive hydrocephalus may be secondary to radiation-induced tumor necrosis and release of tumor proteins into CSF that impairs resorption by arachnoid granulations [54]. Hydrocephalus requiring a ventricular shunt occurs in up to 7 % of cases [40, 52, 53, 59] and at higher rates with larger VS [42, 43, 60, 61].
Pseudo-progression or peritumoral edema occurs in up to 30 % of patients following radiation [47, 50, 54, 61, 62] and is symptomatic in 21 % [61, 63]. The incidence of edema correlates with tumor size, and larger (>3 cm) tumors have a 50 % rate of pseudo-progression [61]. As a result of edema, tumors commonly are larger 1–2 years following treatment and should not be mistaken for unsuccessful tumor control [61]. However, there is an associated risk of hydrocephalus and loss of serviceable hearing even with transient edema [61]. Some tumors (9 %) will be persistently enlarged and do not demonstrate sequential growth and therefore are not considered treatment failures [41, 62]. Mild cases of edema are often successfully treated with oral steroids.
Serious complications following STR include radiation-induced cerebrovascular injury, brainstem necrosis, or tumorigenesis. Radiation-related cerebrovascular injury, including stroke, intra-tumoral hemorrhage, or subarachnoid hemorrhage, may occur immediately or years after treatment [52, 54]. The incidence is unknown and may be related to endothelial injury and changes in blood–brain barrier and acceleration of atherosclerosis [63]. While rates of radiation-induced malignant transformation are very rare, there are at least 33 cases of malignant tumors following STR for VS [42, 45, 52, 64–69] with nearly half of these cases occurring in NF2 patients [45, 67, 69]. The most commonly reported form of malignancy has been peripheral nerve sheath tumors; however, sarcomas, malignant meningiomas, and glioblastomas have been reported. The mean latency to detection of these malignancies is just over 5 years following STR but may present as late as 19 years after treatment [45, 66]. The prognosis is uniformly poor [42, 45, 52, 64–69]. There have additionally been rare reports of brainstem radionecrosis [40] or fatalities as a result of direct sequelae of radiation treatment, such as post-radiation hydrocephalus [31, 52].
Conclusions
Vestibular schwannomas are benign slowing growing lesions and have numerous management options. Surgical extirpation, stereotactic radiosurgery, and observation are the commonly employed options with choices made based on tumor location and size, medical comorbidities, and the potential for hearing preservation. Each modality has risks and benefits that must be weighed carefully when deciding the optimal management course. There continues to be controversy when comparing each in terms of functional outcome and optimal quality of life. Each surgical option should be thoroughly discussed if relevant along with expectations and when discussing radiation therapy as a potential treatment modality for VS patients should understand that arrest of tumor growth, rather than eradication, is the goal of treatment. As such, STR is only indicated in growing tumors. The patient must be informed of and prepared to undergo post-treatment surveillance imaging over a period of many years following STR to ensure no further tumor growth or evidence of malignant transformation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Quesnel A, McKenna M. Current strategies in management of intracanicular vestibular schwannoma. Curr Opin Otolaryngol Head Neck Surg. 2011;19:335–40.
Arriaga M, Lin J. Translabyrinthine approach: indications, techniques, and results. Otolaryngol Clin N Am. 2012;45:399–415.
DeMonte F, Gidley P. Hearing preservation for vestibular schwannoma: experience with the middle fossa appraoch. Neurosurg Focus. 2012;33:1–6.
Wang AC, Chinn SB, Than KD, Arts HA, Telian SA, El-Kashlan HK, Thompson BG. Durability of hearing preservation after microsurgical treatment of vestibular schwannoma using the middle cranial fossa approach. J Neurosurg. 2013;119:131–8.
Brackmann DE, Owens RM, Friedman RA, et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol. 2000;21:417–24.
Satar B, Jackler RK, Oghalai J, et al. Risk-benefit analysis of using the middle fossa approach for acoustic neuromas with >10 mm cerebellopontine angle component. Laryngoscope. 2002;112:1500–6.
Friedman RA, Kesser B, Brackmann DE, Fisher LM, Slattery WH, Hitselberger WE. Long-term hearing preservation after middle fossa removal of vestibular schwannoma. Otolaryngol Head Neck Surg. 2003;129:660–5.
• Hillman T, Chen DA, Arriaga MA, Quigley M: Facial nerve function and hearing preservation acoustic tumor surgery: does the approach matter? Otolaryngol Head Neck Surg. 2010;142:115–9. This large, single institution study provides a comparison of the middle fossa and retrosigmoid approaches for hearing preservation with small tumors.
Woodson EA, Dempewolf RD, Gubbels SP, Porter AT, Oleson JJ, Hansen MR, et al. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol. 2010;31:1144–52.
Staecker H, Nadol JB Jr, Ojeman R, et al. Hearing preservation in acoustic neuroma surgery: middle fossa versus retrosigmoid approach. Am J Otol. 2000;21:399–404.
Noudel R, Gomis P, Duntze J, et al. Hearing preservation and facial nerve function after microsurgery for intracanalicular vestibular schwannomas: comparison of middle fossa and retrosigmoid approaches. Acta Neurochir (Wien). 2009;151:935–44.
Sameshima T, Fukushima T, McElveen JT Jr, et al. Critical assessment of operative approaches for hearing preservation in small acoustic neuroma surgery: retrosigmoid vs middle fossa approach. Neurosurgery. 2010;67:640–4.
Arriaga M, Laxford W, Berliner K. Facial nerve function following middle fossa and translabyrinthine acoustic tumor surgery: a comparison. Am J Otol. 1994;15:620–4.
Fayad JN, Brackmann DE. Treatment of small acoustic tumors (vestibular schwannomas). Neurosurg Q. 2005;15:127–37.
Elhammady MS, Telischi FF, Morcos JJ. Retrosigmoid approach: indications, techniques and results. Otolaryngol Clin N Am. 2012;45:375–97.
Hecht CS, Honrubia VF, Wiet RJ, Sims HS. Hearing preservation after acoustic neuroma resection with tumor size used as a clinical prognosticator. Laryngoscope. 1997;107:1122–6.
Irving RM, Jackler RK, Pitts LH. Hearing preservation in patients undergoing vestibular schwannoma surgery: comparison of middle fossa and retrosigmoid approaches. J Neurosurg. 1998;88:840–5.
• Phillips DJ, Kobylarz EJ, De Peralta ET, et al. Predictive factors of hearing preservation after surgical resection of small vestibular schwannomas. Otol Neurotol. 2010; 31:1463–8. In this retrospective evaluation of patients with small tumors, the authors did not find that preoperative hearing or filling of the entire auditory canal were statistically significant predictors of postoperative hearing preservation.
Sughrue ME, Yang I, Rutkowski MJ, et al. Preservation of facial nerve function after resection of vestibular schwannoma. Br J Neurosurg. 2010;24:666–761.
Cardoso AC, Fernandes YB, Ramina R, et al. Acoustic neuroma (vestibular schwannoma): surgical results on 240 patients operated on dorsal decubitus position. Arq Neuropsiquiatr. 2007;65:605–9.
Zhao X, Wang Z, Ji Y, et al. Long-term facial nerve function evaluation following surgery for large acoustic neuromas via retrosigmoid transmeatal approach. Acta Neurochir (Wien). 2010;152:1647–52.
Jacob A, Robinson LL, Bortman JS, et al. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope. 2007;117:2087–92.
Anderson DE, Leonetti J, Wind JJ, et al. Resection of large vestibular schwannomas: facial nerve preservation in the context of surgical approach and patient- assessed outcome. J Neurosurg. 2005;102:643–9.
Patel J, Vasan R, van Loveren H, Downes K, Agazzie S. The changing face of acoustic neuroma management in the USA: analysis of the 1998 and 2008 patient surveys from the acoustic neuroma association. Br J Neurosurg. 2014;28:20–4.
Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27:547–52.
Theodosopoulos PV, Pensak ML. Contemporary management of acoustic neuromas. Laryngoscope. 2011;121:1133–7.
• Stangerup SE, Thomsen J, Tos M, Caye-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol. 2010;31:271–5. All patients in Denmark with VS diagnosed since 1976 are referred to a single national referral center and entered prospectively in a national database, thereby eliminating referral or retrospective review biases. This is the largest study looking at long term hearing outcomes in those with vestibular schwannomas who are observed, and in those who presented with a word recognition score of 100 % in the affected ear, the hearing preservation rate was 69 % at 10 years. The hearing preservation rate fell drastically to 38 % at 10 years in those with good word recognition scores less than 100 % at presentation.
Kondziolka D. Hearing after gamma knife surgery. J Neurosurg. 2012;117:874–5.
Maniakas A, Saliba I. Conservative management versus stereotactic radiation for vestibular schwannomas: a meta analysis of patients with more than 5 years’ follow-up. Otol Neurotol. 2012;33:230–8.
Pennings RJ, Morris DP, Clarke L, Allen S, Walling S, Bance ML. Natural history of hearing deterioration in intracanalicular vestibular schwannoma. Neurosurgery. 2011;68:68–77.
Vivas EX, Wegner R, Conley G, Torok J, Heron DE, Kabolizadeh P, Burton S, Ozhasoglu C, Quinn A, Hirsch BE. Treatment outcomes in patients treated with cyberknife radiosurgery for vestibular schwannoma. Otol Neurotol. 2014;35:162–70.
Lau T, Olivera R, Miller T, Downes K, Danner C, van Loveren HR, Agazzi S. Paradoxical trends in the management of vestibular schwannoma in the United States: Clinical article. J Neurosurg. 2012;117:514–9.
Combs SE, Welzel T, Kessel K, Habermehl D, Rieken S, Schramm O, Debus J. Hearing preservation after radiotherapy for vestibular schwannoma is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcomes. Radiother Oncol. 2013;106:175–80.
Park SS, Grills IS, Bojrab D, Pieper D, Kartush J, Martin A, Perez E, Hahn Y, Ye H, Martinez A, Chen P. Longitudinal assessment of quality of life and audiometric test outcomes in vestibular schwannoma patients treated with gamma knife surgery. Otol Neurotol. 2011;32:676–9.
Varughese JK, Wentzel-Larsen T, Pedersen PH, Mahesparan R, Lund-Johansen M. Gamma knife treatment of growing vestibular schwannoma in Norway: a prospective study. Int J Radiat Oncol Biol Phys. 2012;84:161–6.
Fong BM, Pezeshkian P, Nagasawa DT, De Salles A, Gopen Q, Yang I. Hearing preservation after LINAC radiosurgery and LINAC radiotherapy for vestibular schwannoma. J Clin Neurosci. 2012;19:1065–70.
Litre F, Rousseaux P, Jovenin N, Bazin A, Peruzzi P, Wdowczyk D, Colin P. Fractionated stereotactic radiotherapy for acoustic neuromas: a prospective monocenter study of about 158 cases. Radiother Oncol. 2013;106:169–74.
Puataweepong P, Dhanachai M, Dangprasert S, Narkwong L, Sitathanee C, Sawangsilpa T, Janwityanujit T, Yongvithisatid P. Linac-based sterotactic radiosurgery and fractionated stereotactic radiotherapy for vestibular schwannomas: comparative observations of 139 patients treated at a single institution. J Radiat Res. 2014;55:351–8.
Rasmussen R, Claesson M, Stangerup SE, Roed H, Christensen IJ, Caye-Thomasen P, Juhier M. Fractionated stereotactic radiotherapy of vestibular schwannomas accelerates hearing loss. Int J Radiat Oncol Biol Phys. 2012;83:e607–11.
Woolf DK, Williams M, Goh CL, Henderson DR, Menashy RV, Simpson N, Mastroianni B, Collis CH. Fractionated stereotactic radiotherapy for acoustic neuromas: long-term outcomes. Clin Oncol. 2013;25:734–8.
Link MJ, Driscoll CL, Foote RL, Pollock BE. Radiation therapy and radiosurgery for vestibular schwannomas: indications, techniques, and results. Otolaryngol Clin North Am. 2012;45:353–66.
Chung WY, Pan DH, Lee CC, Wu HM, Liu KD, Yen YS, Guo WY, Shiau CY, Shih YH. Large vestibular schwannomas treated by gamma knife surgery: long-term outcomes. J Neurosurg. 2010;113:112–21.
Milligan BD, Pollock BE, Foote RL, Link MJ. Long-term tumor control and cranial nerve outcomes following gamma knife surgery for larger-volume vestibular schwannomas. J Neurosurg. 2012;116:598–604.
Ganz JC. Complications of gamma knife neurosurgery and their appropriate management. Acta Neurochir Suppl. 2013;116:137–46.
Husseini ST, Piccirillo E, Sanna M. On, malignant transformation of acoustic neuroma/vestibular schwannoma 10 years after gamma knife stereotactic radiosurgery. Skull Base. 2011;21:135–8.
Baschnagel AM, Chen PY, Bojrab D, Pieper D, Kartush J, Didyuk O, Naumann IC, Maitz A, Grills IS. Hearing preservation in patients with vestibular schwannoma treated with gamma knife surgery. J Neurosurg. 2013;118:571–8.
Hasegawa T, Kida Y, Kato T, Lizuka H, Yamamoto T. Factors associated with hearing preservation after gamma knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg. 2011;115:1078–86.
Hayden Gephart MG, Hansasuta A, Balise RR, Choi C, Sakamoto GT, Venteicher AS, Soltys SG, Gibbs IC, Harsh GR, Adler JR, Chang SD. Cochlea radiation dose correlates with hearing loss after stereotactic radiosurgery for vestibular schwannoma. World Neurosurg. 2013;80:359–63.
Karam SD, Tai A, Strohl A, Steehler MK, Rashid A, Gagnon G, Harter KW, Jay AK, Collins SP, Kim JH, Jean W. Frameless fractionated stereotactic radiosurgery for vestibular schwannomas: a single-institution experience. Front Oncol. 2013;17:121.
Sager O, Beyzadeoglu M, Dincoglan F, Demirai S, Uysal B, Gamsiz H, Oysul K, Dirican B, Sirin S. Management of vestibular schwannomas with linear accelerator-based stereotactic radiosurgery: a single center experience. Tumori. 2013;99:617–22.
Tsai JT, Lin JW, Lin CW, Chen YH, Ma HI, Jen YM, Chen YH, Ju DT. Clinical evaluation of cyberknife in the treatment of vestibular schwannomas. Biomed Res Int. 2013. doi:10.1155/2013/297093
Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: a 10-year follow-up. J Neurosurg. 2013;119:10–6.
Roos DE, Potter AE, Brophy BP. Stereotactic radiosurgery for acoustic neuromas: what happens long term? Int J Radiat Oncol Biol Phys. 2012;82:1352–5.
Roche PH, Noudel R, Regis J. Management of radiation/radiosurgical complications and failures. Otolaryngol Clin North Am. 2012;45:367–74.
• Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CL, Link MJ. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013;118:579–87. Evaluated serviceable hearing preservation rates over time, with a median follow up of 9.3 years (range 5–14 years), and used the contra-lateral, non-radiated ear to control for age related hearing loss over time. Found an 80 % hearing preservation rate at 1 year, which decreased to 23 % at 10 years.
Kim YH, Kim DG, Han JH, Chung HT, Kim IK, Song SW, Park JH, Kim JW, Kim YH, Park CK, Kim CY, Paek SH, Jung HW. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Bil Phys. 2013;85:61–7.
Badakhshi H, Graf R, Bohmer D, Synowitz M, Wiener E, Budach V. Results for local control and functional outcome after linac-based image-guided stereotactic radiosurgery in 190 patients with vestibular schwannoma. J Radiat Res. 2014;55:288–92.
Friedman RA, Berliner KI, Bassim M, Ursick J, Slattery WH, Schwartz MS, Brackmann DE. A paradigm shift in salvage surgery for radiated vestibular schwannoma. Otol Neurotol. 2011;32:1322–8.
Park SH, Lee KY, Hwang SK. Nervus intermediate dysfunction following gamma knife surgery for vestibular schwannoma. J Neurosurg. 2013;118:566–70.
Lee SH, Seol HJ, Kong DS, Nam DH, Park K, Kim JH, Lee JL. Risk factors and tumor response associated with hydrocephalus after gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir. 2012;154:1679–84.
Aoyama H, Onodera S, Takeichi N, Onimaru R, Terasaka S, Sawamura Y, Shirato H. Symptomatic outcomes in relation to tumor expansion after fractionated stereotactic radiation therapy for vestibular schwannomas: single-institutional long-term experience. Int J Radiat Oncol Biol Phys. 2013;85:329–34.
Hayhurst C, Zadeh G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro Oncol. 2012;14:87–92.
Ujifuku K, Matsuo T, Toyoda K, Baba S, Okunaga T, Hayashi Y, Kamada K, Morikawa M, Suyama K, Nagata I, Hayashi N. Repeated delayed onset cerebellar radiation injuries after linear accelerator-based stereotactic radiosurgery for vestibular schwannoma: case report. Neurol Med Chir. 2012;52:933–6.
Akamatsu Y, Murakami K, Watanabe M, Jokura H, Tominaga T. Malignant peripheral nerve sheath tumor arising from benign vestibular schwannoma treated by gamma knife radiosurgery after two previous surgeries: a case report with surgical and pathological observations. World Neurosurg. 2010;73:751–4.
Lee SH, Rhee BA, Choi SK, Koh JS, Lim YJ. Cerebellopontine angle tumors causing hemifacial spasm: types, incidence, and mechanism in nine reported cases and literature review. Acta Neurochir. 2010;152:1901–8.
Markou K, Elmer S, Perret C, Huchet A, Goudakos J, Liguoro D, Franco-Vidal V, Maire JP, Darrouzet V. Unique case of malignant transformation of a vestibular schwannoma after fractionated radiotherapy. Am J Otolaryngol. 2012;33:168–73.
Puataweepong P, Janwityanujit T, Larbcharoensub N, Dhanachai M. Radiation-induced peripheral malignant nerve sheath tumor arising from vestibular schwannoma after linac-based stereotactic radiation therapy: a case report and review of literatures. Case Rep Med. 2012;2012:648191.
Schmitt WR, Carlson ML, Giannini C, Driscoll CL, Link MJ. Radiation-induced sarcoma in a large vestibular schwannoma following stereotactic radiosurgery: case report. Neurosurgery. 2011;68:840–6.
Yang T, Rockhill J, Born DE, Sekhar LN. A case of high-grade undifferentiated sarcoma after surgical resection and stereotactic radiosurgery of a vestibular schwannoma. Skull Base. 2010;20:179–83.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Ear Surgery.
Rights and permissions
About this article
Cite this article
Basura, G.J., Budenz, C. & Arts, H.A. Vestibular Schwannomas: Surgical and Nonsurgical Management. Curr Surg Rep 3, 5 (2015). https://doi.org/10.1007/s40137-015-0082-5
Published:
DOI: https://doi.org/10.1007/s40137-015-0082-5