Abstract
Purpose of Review
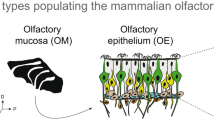
The olfactory epithelium is characterized as the main organ for the sense of smell in humans and vertebrates. The neuronal cells called olfactory receptor neurons (ORNs) play a key role in the olfactory epithelium by expressing the olfactory receptors (ORs) on their apical surface membrane, are specially differentiated.
Recent Findings
The model of odor identification and discrimination working in combination is well established; however, little is known about the action mechanisms of neuronal divergence for odor identification and discrimination. In this article, we present some basic theories on the transduction and analysis of odorant binding.
Summary
Resent research in the past decades has brought forth a better understanding of the science behind human olfaction. Still many more unanswered questions remain, leaving much to be discovered and unraveled in the field of olfaction science.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sense of smell is a chemo-sensoring system that enables the detection and discrimination of millions of different volatile molecules called odorants that provide critical information about the surrounding environment. In humans, odorants’ recognition is mediated by an extensive repertoire of olfactory receptors (O.R.s) encoded by 391 functional OR genes [1•]. The O.R.s are located across the plasma membranes of the ciliated dendrites of olfactory sensory neurons, which are localized in the olfactory epithelium. Each sensory neuron expresses a single allele of a single OR gene to ensure a distinct unique pattern of neuronal activation for every odorant [1•, 2•]. Mammalian O.R.s are classified into two classes according to the recognized type of odorant type: Class I, O.R.s bind primarily hydrophilic odorants, and Class II, O.R.s bind hydrophobic odorants. The odorant must cross a hydrophilic mucus, where the ciliated dendrites of olfactory neurons are immersed. The hydrophobic odorants need to be transported, which is believed to be the role of the small soluble proteins, the odorant-binding proteins (OBPs) [2•, 3].
In vertebrates, OBPs are expressed to a very high degree level in the nasal epithelia, where they bind and carry hydrophobic and volatile odorant molecules. Humans express two OBP genes, OBP2A and OBP2B, but their expression is not enhanced in the human olfactory epithelium [3]. In this article, we will present current evidence, knowledge, and theories on odorant binding to gain a deeper understanding of the binding and transport of odorants.
General Approaches to Olfactory Classification
Provided that an immediate association between percepts and a single or a few physical parameters has not yet been found in olfaction [4•], scientists have relied on more subjective attempts to classify odors [5•, 6]. Early odor classification systems were primarily based on the individual expertise of botanists, chemists, or perfumers and have mainly ruled out experimental confirmation [5•, 6, 7•]. The first empirical classifications were only published in the twentieth century and were based on 3 main approaches:
Features of the Sensory Organ
Several researchers have linked the qualities of smell to the function of olfactory receptors. Amoore [8, 9•, 10••, 11•] hypothesized an increased detection threshold for specific odors, together with an otherwise normal olfactory sensitivity, indicative of the malfunction of a particular receptor type. She selected subjects for specific types of anosmia and defined a classification system based on seven primary odors, each related to a different receptor type. Other studies have used empirical cross-matching approaches to study the relationship between odor classes and receptor types [12••, 13]. However, with the discovery of 320 types of odorant receptors in humans [14–16], the idea of a controllable primary odor system has been widely rejected, along with the attempt to establish olfactory classifications based on the physiological characteristics of the olfactory system.
Features of the Sensory Stimulus
The chemical structure of an odorous compound strongly determines its perceived quality. There have been attempts to establish reliable structure–odor relationships (SOR) by linking perceptual properties to molecular vibration [17–19], molecule shape [18, 19], character [16, 19–21], chain length [22], and other physicochemical parameters. However, all single measures have failed to predict odor sensations or systematically explain odor perception. Recent studies have revived early approaches [18, 19] and attempted to incorporate hundreds of physicochemical characteristics into a single measure [22, 23•, 24, 25].
Features of the Sensory Percept
Henning was the first to directly classify olfactory perceptions by classifying verbal descriptions of odors [26]. He presented 415 fragrances to N = 6 participants and asked them to express their perceptions. Based on a subjective summary of these verbal reports, Henning proposed 6 odor qualities. These were fragrant (flowery), ethereal (fruity), putrid (foul), spicy (aromatic), resinous (piny, balsamy), burned (burning, scorched).
Odorants and Their Role
An odorant is characterized as any molecule capable of eliciting a response from an olfactory neuron. Odorants are volatilized organic or inorganic molecules, mainly C-, H-, and O-atoms [23•, 27•]. Some odorants also contain N- and S-atoms, rarely metal ions or heteroatoms such as Cl- or Br-. Odorant size is at < 400 Da. If they were more significant, they would be either not volatile enough to be sensed or too big to fit into an olfactory receptor binding pocket. The latter conclusion stems from the observation that anosmia, the inability to sense an odor, is particularly pronounced at the upper size detection limit. Odorants are perceived at concentrations in the ppb to ppm range, although this number varies between odorants, individuals, and species [27•]. The detection of odorants occurs on the surface of olfactory neurons in the mucous epithelium of the nasal cavity of vertebrates and on the sensory antennae of invertebrates. Dogs are known to have 1000 times the sensitivity of humans. It is believed that the larger olfactory epithelial area and the increase in the number and type of olfactory receptors per cm2 are the main reasons for the increased sensitivity and selectivity [28, 29, 30•].
Theories in Odorant Binding
Two theories try to explain the odorant binding and the consequent transduction of olfactory signals, the shape and the vibration theory [31]. According to the first, a particular smell is due to a structural specificity between the Odorant and the operating room. According to vibrational theory, when an OR binds an odorous substance, tunneling of electrons through the binding site can occur if the vibrational mode is equal to the energy gap between the filled electron levels and empty. The electronic tunneling effect activates the G protein cascade [31].
The Transmission of Olfactory Stimuli
The structural theory is the most widely used theory to describe the sense of smell. Based on the traditional “lock-and-key” mechanism, it states that the aroused odor is explained solely by the structural compatibility between the “lock”/odor binding pouch from the operating room and the fragrance/button [32].
An odotope is a functional group/structural motif, and a fragrance is typically composed of several odotopes. Therefore, the perceived odor is believed to be a function of all odotopes in the molecule and their intramolecular arrangement. Another theory is the “vibration” theory. Vibration theory describes the character of odor as a result of the relative charge distribution within the total uncharged odor molecules, causing the O.R.s to function as inelastic electron tunnel spectroscopes. The elasticity or translational vibrations of filled or unfilled molecular orbitals explain the variability observed in apparently identical odorants [33••]. Olfactory cilia membranes present olfactory receptors (OR). ORs are members of the G-protein-coupled receptor (GPCR) family coupled to GTP-binding regulatory proteins (G-proteins). OR proteins are transmembrane receptor proteins with seven transmembrane domains with about 20–25 amino acids in each domain and a total length of about 350 amino acids.
The amino-acid sequences of various O.R.s are highly conserved in the G protein binding region while hypervariable in the ligand binding regions [31, 32, 33••]. OR proteins represent the most prominent family of genes in vertebrates. Genomic DNA analysis yielded estimates for the presumed OR gene family repertoire size of about 1000 in mammals, about 2–3% in the genome.
Currently available data indicate that each ORN expresses only one type of functional OR and that only one allele of a given OR is expressed [34–38]. The molecular mechanisms that regulate how each mature ORN selects a particular OR and how its expression pattern is maintained in the face of ongoing ORN replacement remain to be elucidated. In 2000, the vomeronasal receptor type 1, V1R, a GPCR and the receptor for pheromones in most animals, was found in the human olfactory mucosa [39, 40]. However, the vomeronasal organ and the terminal nerve, connecting the vomeronasal organ and the limbic system, are only rudimentary organs in humans, and the role of these receptors in humans is controversial.
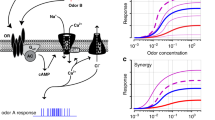
With the binding of an odorant, the OR at the ciliary membrane changes its structure to activate the G-protein of olfactory type (Golf) bound inside the ORN. The activation of Golf leads to the production of cAMP.
The cAMP opens cyclic nucleotide-gated (CNG) ion channels which allow an influx of calcium and sodium ions into the ORN, resulting in depolarization. The last leads to an action potential in the ORN, resulting in depolarization and an action potential, which carries information centrally through the neuron [30•, 31].
The inositol triphosphate (IP3)-linked pathway is another known mechanism of ORN stimulation in mammals, including humans [30•, 31]. This pathway uses IP3 as a secondary messenger instead of cAMP. Another type of G-protein can activate the membrane-bound enzyme phospholipase C. Together with diacylglycerol, IP3 is converted from a lipid phosphatidylinositol 4,5-bisphosphate in the plasma membrane by phospholipase C. The influx of calcium ions into the ORN through CNG channels or IP3-gated channels triggers an action potential with the amplification of voltage-gated calcium channels, which leads to the further influx of calcium ions and the opening of calcium-dependent chloride channels to enhance the efflux of chloride ions [30•, 31]. Calcium and cAMP activate ion channels and various protein kinases, including protein kinase A and calcium/calmodulin-dependent kinase II, which are involved in the termination of the odorant signal [30•, 31].
After depolarization, protein kinase acts as a phosphorylase to inactivate the ion channels and other cascade components, resulting in the adaptation of the ORN to odorant stimulus. Intracellular calcium ions are exchanged through sodium/calcium exchangers located at the ciliary layer of the ORN [30•]. 2,4,6-trichloroanisole can suppress olfactory signal transduction by suppressing CNG channels, thus reducing smell [30•, 31].
The Odorant Binding Proteins (OBPs)-Structure, Role, and Importance
The first OBP was discovered in 1981 [32]. A sex pheromone from the silk moth Anthernae Polyphemus was labeled and incubated with an exact number of antennae of this species. The pheromone bound a small protein of approximately 15 kD detected in the antenna antennae of male moths but not female moths. This protein will be referred to herein by the more general term odorant-binding protein (OBP). Mammals also have proteins called Obps, first identified in cows' nasal mucosa [31, 32, 33••]. Previous studies have suggested that vertebrate odorant binding odor-binding proteins (OBPs) are pheromone carriers, either in certain biological glands or secretory body fluids like urine, saliva, seminal fluid, and semen [34]. They may be involved in perceiving volatile transient chemical cues in the olfactory epithelium [35]. Indeed, it has been well established that these proteins respond to airborne stimuli from pheromones in olfactory systems, and the trigger can elicit adaptive behavioral responses. It may stimulate downstream physiological processes, suggesting a functional role in olfaction-evoked odor-related behaviors. In the biological system, the function of lipocalins, like vertebrate OBPs is OBPs, lies at the interface between the external environment and membrane olfactory membrane receptors (O.R.s). Certain biophysical studies revealed that OBPs are considerably stable in the pH range from 4.0 to 7.5 [38] and are substantially resistant to proteolysis and solvent denaturation compared to O.R.s. The presence of ligands inside ligands in the binding pocket of OBP further increases its thermal stability. It seems to be a promising attractive feature for designing the development of suitable sensors for biotechnological applications. It also includes a variety of transducers, gas sensors, quartz crystal microbalance microbalances (QCM), surface acoustic wave devices, and organic field effect field-effect transistors [39]. It has also been proposed suggested that OBPs expressed in both nasal mucus and saliva of buffalo may play an essential role in odor olfactory perception and sexual communication [40–42, 43••].
Since 2006, another class of odor receptors has been identified, called trace amine-associated receptors (TAARs), which exist for volatile amines commonly found in animal urine [40]. However, the role of TAAR in the human olfactory mucosa is still unknown. An OR displays affinity not for only a kind of specific molecule but a range of odor molecules, and conversely, a single odorant molecule may bind to several different olfactory receptors with varying affinities [41, 42, 44, 45•, 46, 47••, 48].
The molecular mechanisms that regulate how each mature ORN selects a particular OR and how its expression pattern is maintained in the face of ongoing ORN replacement have yet to be explained. In 2000, the vomeronasal type 1 receptor, V1R, a GPCR and the receptor for pheromones in most animals, was found in the human olfactory mucosa [49•, 50].
However, the role of TAAR in the human olfactory mucosa is still unknown. An OR displays affinity not only for a kind for one type of specific molecule but for a range of odor molecules. Conversely, a single odorant molecule may bind to various olfactory odorant receptors with varying affinities [49•, 50, 51•]. To reach their membrane receptors embedded in the membrane of the olfactory neurons, airborne odorants, commonly usually hydrophobic molecules, must be transported through the aqueous watery nasal mucus. The odorant-binding proteins (OBPs) [51•, 52] are abundant low-molecular-weight soluble, highly soluble, low molecular weight (about 20 kDa) proteins (around 20 kDa) secreted by the olfactory epithelium in the vertebrate nasal mucus [52]. These proteins reversibly bind odorants with dissociation constants in the micromolar range. Although their functions are still unclear, OBPs are also suspected to be involved in the odorant deactivation of odorants [51•]. Vertebrate OBPs belong to the lipocalin superfamily [52]. However, members of this superfamily display share low sequence similarity (usually lower (typically less than 20% amino acid identity), all share a conserved folding pattern, an 8-stranded a-barrel flanked by an R-helix at the C-terminal end C-terminus of the polypeptide chain. The a-barrel defines a central apolar cavity, called the Calix, whose sole task is to bind and transport hydrophobic odorant odor molecules [53].
Various proteins have been detected in human nasal mucus, including a protein with an N-terminal identical to tear lipocalin [52, 53]. However, contrary to all other vertebrates so far studied, the presence of OBP-like proteins in the olfactory mucus has not yet been observed in humans. Recently, two putative human OBP genes (hOBPIIa and hOBPIIb) localized on chromosome 9q34 have been described [38, 54, 55]. Alternatively, spliced mRNAs have been observed for both genes generating genes, producing proteins with different C-termini.
The human olfactory epithelium, containing sensory neurons, is situated on the cribriform plate and extends a short distance down to the septum, the superior turbinate, and possibly the superior upper part of the middle turbinate [38]. It covers a surface area of about 100–400 about 100–400 mm2 in the uppermost top part of the nasal cavity, named the so-called olfactory cleft. A recent study demonstrated, by demonstrating through biopsy and electrophysiological recordings, recordings that this anterior part portion contains olfactory neurons that generate elicit odor-induced electrical responses in the majority of subjects [38, 42, 54, 55]. The presence of hOBPIIaR only in the olfactory cleft is partly in disagreement with partially contradicts the localization of mRNAs, which were mRNAs detected in acinar cells from the middle meatus and turbinates, but not searched sought in the olfactory cleft [34, 55–57]. Previous attempts to find OBPs in the human nasal cavity [28, 30•, 31, 32, 33••] have failed, probably because these approaches were based on analysis of washings of the whole nasal cavity in which lavages where proteolysis could occur [58].
The small area of the olfactory cleft and its inaccessible location make it difficult for the sampling to collect mucus from the olfactory cleft mucus [58, 59••, 60].
Conclusion
The past few decades have brought a better understanding of the science behind human olfaction. Some research findings have been supported by the mainstream and most scientific community, while others have been controversial. In addition, many more issues and other problems remain unresolved and unsolved, leaving much to be discovered and unraveled in the field of olfaction olfactory science. Scientists and physicians should concentrate on obtaining knowledge on generating insights from basic olfaction olfactory science research in preclinical settings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Persuy, M.A., et al. Mammalian olfactory receptors: molecular mechanisms of odorant detection, 3D-modeling, and structure-activity relationships. Prog Mol Biol Transl Sci. 2015;130:1–36. • Findings of this study suggest the different molecular mechanisms of odorant detection.
Clyne PJ, et al. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22(2):327–38.
• Olender T, et al. The human olfactory transcriptome. BMC Genomics. 2016;17(1): p. 619. The article gives a thorough insight on the olfactory transcriptome.
• Friedrich RW, Korsching SI. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci. 1998;18(23):9977–88. It is important because the odorant representations are explained on a neurophysiological basis.
• Chastrette M, Elmouaffek A, Sauvegrain P. A multidimensional statistical study of similarities between 74 notes used in perfumery. Chem Senses. 1988;13(2):295–305. The authors try to explain how different odors can be perceived and “analysed” in CNS.
Chastrette M, Laumer JY, Sauvegrain P. Analysis of a system of description of odors by means of four different multivariate statistical methods. Chem. Sense. 1991;6(1):81–93.
• Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4(4):266–75. Although a little bit peculiar as a title, it gives an insight about the “olfactory” memory.
Amoore JE. Stereochemical Theory of Olfaction. Nature. 1963;199:912–3.
• Amoore JE. Stereochemical and vibrational theories of odor. Nature. 1971;233(5317):270–1. The article provides evidence on stereochemical and vibrational theories. An “all-time classic” article!.
•• Amoore JE. Specific anosmia and the concept of primary odors. Chem Sense. 1977;2(3):267–281. Anosmia is explained on an extent basis.
• Amoore JE, Palmieri G, Wanke E. Molecular shape and odor: pattern analysis of PAPA. Nature. 1967;216(5120):1084–7. The article provides useful information about molecular shape and odor perception.
•• Cain WS, Johnson Jr F. Lability of odor pleasantness: influence of mere exposure. Perception. 1978;7(4):459–65. The article analyses the influence of the molecular shape of odor and its influence on the receptors.
Cain WS, Polak EH. Olfactory adaptation as an aspect of odor similarity. Chem Senses. 1992;17(5):481–91.
de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30(2):537–52.
Glusman G, et al. The complete human olfactory subgenome. Genome Res. 2001;11(5):685–702.
Malnic B, et al. Combinatorial receptor codes for odors. Cell. 1999;96(5):713–23.
Dyson GM. The scientific basis of odour. J Chem Technol Biotechnol. 1938;57:647–51.
Schiffman S, Robinson DE, Erickson RP. Multidimensional scaling of odorants: examination of psychological and physiochemical dimensions. Chem Sense. 1977;2(3):375–390. Of importance.
Wright R, Serenius R. Odour and molecular vibration. II. Raman spectra of substances with the nitrobenzene odour. J Appl Chem. 1954;4(11):615–621.
Blum MS, Doolittle RE, Beroza M. Alarm pheromones: Utilization in evaluation of olfactory theories. J Insect Physiol. 1971;17(12):2351–61.
Uchida N, et al. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3(10):1035–43.
Doving KB. An electrophysiological study of odour similarities of homologous substances. J Physiol. 1966;186(1):97–109.
• Jacob S, Hayreh DJ, McClintock MK. Context-dependent effects of steroid chemosignals on human physiology and mood. Physiol Behav. 2001;4(1–2):15–27. Findings give an explanation between physiology of olfaction and psychology.
Piferi RL, et al. An alternative approach for achieving cardiovascular baseline: viewing an aquatic video. Int J Psychophysiol. 2000;37(2):207–17.
Savic I, et al. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron. 2001;31(4):661–8. Of importance.
Henning H. Der geruch. 1916, Leipzig (Germany): Johann Ambrosius Barth. 1916.
• Kaeppler K, Mueller F. Odor classification: a review of factors influencing perception-based odor arrangements. Chem Sense. 2013;38(3):189–209. A review, which provides useful information on factors influencing perception-based odor arrangements.
Dalton P, et al. The use of semantic differential scaling to define the multi-dimensional representation of odors. J Sens Stud. 2008;23(4):485–97.
Moran DT, et al. The fine structure of the olfactory mucosa in man. J Neurocytol. 1982;11(5):721–46.
• Noe J, et al. Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int. 1997;30(6):523–31. Although the article is based on experimental data, the authors provide important clues on the function of olfactory neurons.
Park S-W. Understanding the human sensory conduction of smell. hmr. 2014;34(3):100–106.
Silva C, et al. Odorant binding proteins: a biotechnological tool for odor control. Appl Microbiol Biotechnol. 2014;98(8):3629–38.
•• Pelosi P, Zhu J, Knoll W. Odorant-binding proteins as sensing elements for odour monitoring. Sensors. 2018;18(10):3248. The availability of many structures solved both as apo-proteins and in complexes with some ligands, it is feasible to design mutants.
Whitson KB, Whitson SR. Human odorant binding protein 2a has two affinity states and is capable of binding some uremic toxins. Biochem Analytic Biochem. 2014;1–7.
Goncalves F, et al. Two Engineered OBPs with opposite temperature-dependent affinities towards 1-aminoanthracene. Sci Rep. 2018;8(1):14844.
Zhao H, et al. Functional expression of a mammalian odorant receptor. Science. 1998;279(5348):237–42.
Freitag J, et al. Olfactory receptors in aquatic and terrestrial vertebrates. J Comp Physiol A. 1998;183(5):635–50.
Mombaerts P. Odorant receptor genes in humans. Curr Opin Genet Dev. 1999;9(3):315–20.
Chess A, et al. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78(5):823–34.
Rodriguez I, et al. A putative pheromone receptor gene expressed in human olfactory mucosa. Nat Genet. 2000;26(1):18–9.
Cunningham M, Crady CA. Identification of olfactory dimensions by semantic differential technique. Psychon Sci. 1971;23(6):387–8.
Ko HJ, Park TH. Enhancement of odorant detection sensitivity by the expression of odorant-binding protein. Biosens Bioelectron. 2008;23(7):1017–23.
•• Sun JS, Xiao S, Carlson JR. The diverse small proteins called odorant-binding proteins. Open Biol. 2018;8(12): 180208. The article provides useful information about the structure and physiological characteristics of odorants.
Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293(5828):161–3.
• Briand L, et al. Evidence of an odorant-binding protein in the human olfactory mucus: location, structural characterization, and odorant-binding properties. Biochemistry. 2002;41(23):7241–52. According to the article the relatively limited specificity of hOBP(IIa)(alpha) suggests that other human OBPs are expected to take into account the large diversity of odorant molecules.
Cavaggioni A, Mucignat-Caretta C. Major urinary proteins, alpha(2U)-globulins and aphrodisin. Biochim Biophys Acta. 2000;1482(1–2):218–28.
•• Archunan G. Odorant Binding Proteins: a key player in the sense of smell. Bioinformation. 2018;14(1): 36–37. The authors discuss about the role of OBPs and their importance in industrial, pest management and therapeutic developments.
Zarzo M. The sense of smell: molecular basis of odorant recognition. Biol Rev Camb Philos Soc. 2007;82(3):455–79.
• Hess B. Lincs P. A parallel linear constraint solver for molecular simulation. J Chem Theory Comput. 2008;4(1):116–22. In this paper the parallel linear constraint solver (P-LINCS) is presented. The last allows the constraining of all bonds in macromolecules.
Schiefner A, et al. Crystal structure of the human odorant binding protein. OBPIIa Proteins. 2015;83(6):1180–4.
• Tegoni M, et al. Mammalian odorant binding proteins. Biochim Biophys Acta. 2000;1482(1–2):229–40. The three-dimensional structure of these proteins presents antiparallel β-sheets and a short α-helical segment close to the C terminus. This is an important element for understanding the mechanisms of olfaction.
Briand L, et al. Odorant and pheromone binding by aphrodisin, a hamster aphrodisiac protein. FEBS Lett. 2000;476(3):179–85.
Dravnieks A. Odor quality: semantically generated multidimensional profiles are stable. Science. 1982;218(4574):799–801.
Bianchet, M.A., et al., The three-dimensional structure of bovine odorant binding protein and its mechanism of odor recognition. Nat Struct Biol. 1996;3(11):934–9. Of importance.
Bignetti E, et al. Purification and characterisation of an odorant-binding protein from cow nasal tissue. Eur J Biochem. 1985;149(2):227–31.
Haberly LB. Olfactory cortex., in The synaptic organization of the brain, G.M. Shepherd, Editor. Oxford University Press: New York. 1998;377–416.
Morales B, Bacigalupo J. Chemical reception in vertebrate olfaction: evidence for multiple transduction pathways. Biol Res. 1996;29(3):333–41.
Giessel AJ, Datta SR. Olfactory maps, circuits and computations. Curr Opin Neurobiol. 2014;24(1):120–32.
•• Kaur R, et al. IP3-gated channels and their occurrence relative to CNG channels in the soma and dendritic knob of rat olfactory receptor neurons. J Membr Biol. 2001;181(2): p. 91–105. The data of the article suggest that the cAMP-pathway dominates the IP3 pathway in mammalian olfactory transduction.
Nef P, et al. Spatial pattern of receptor expression in the olfactory epithelium. Proc Natl Acad Sci USA. 1992;89(19):8948–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on RHINOLOGY: Taste and Smell Disorders
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malliou, F., Pavlidis, P. Current Theories in Odorant Binding. Curr Otorhinolaryngol Rep 10, 405–410 (2022). https://doi.org/10.1007/s40136-022-00437-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-022-00437-y