Abstract
Purpose of Review
Children with unilateral deafness may experience challenges with language development, educational progress, and social interaction. Rehabilitation with a cochlear implant (CI) may minimize these impacts. This review examines the characteristics of children with unilateral deafness presenting for candidacy assessment.
Recent Findings
Forty-nine children with unilateral deafness were assessed. Many (15/49) did not meet candidacy criteria due to cochlear nerve aplasia/hypoplasia (12/49), while 17/49 elected not to pursue CI. The most common etiologies in those 17/49 (35%) who met candidacy and consented to CI were congenital cytomegalovirus (cCMV) (41%) and trauma (26%).
Summary
Many children with unilateral deafness who present for assessment do not go on to receive an implant due to anatomic contraindications or their desire for non-intervention. This review highlights the high prevalence of cCMV amongst children with unilateral deafness presenting for CI where the potential for progression to bilateral hearing loss may influence decision for implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment of unilateral hearing loss in children without delay is warranted to protect from development of an “aural preference syndrome” in which hearing is biased to one ear and spatial hearing is compromised [1]. The present study examines clinical features of unilateral deafness which came to light when evaluating the type of hearing device most appropriate for use in a cohort of children with restricted duration (< 4 years) of unilateral severe to profound deafness.
Rationale for Treatment of Unilateral Sensorineural Hearing Loss in Children
Without access to binaural cues, children with unilateral sensorineural hearing loss (or single-sided deafness) do not develop sound localization [2]. Listening in noise becomes more difficult [2,3,4], as spatial cues are not available to help distinguish one sound from another. Some animal data [5] show a re-weighting of cues towards pinna spectral cues for localization with temporary conductive hearing loss (HL). However, despite the fact that children with unilateral deafness have access to pinna cues in one hemifield, these are not helpful for many sounds as they depend on high frequencies [6] and are used more for elevation, not sufficient for horizontal localization.
The effects of unilateral deafness extend beyond spatial hearing to language development, with significantly poorer spoken language than normal [7, 8], and slower rates of educational progress have been reported [9,10,11]. Social interaction and daily activities, such as safely riding a bike or crossing the street, can also be negatively impacted by unilateral hearing loss [9,10,11,12], suggesting deficits to spatial awareness because of impaired sound localization. Balance skills can also be reduced in children with unilateral hearing loss [13], and this along with the absence of binaural hearing may impact how these children navigate their environments. The impact of unilateral hearing loss in children has typically been minimized by clinicians despite clear evidence of impairments in children with a unilateral hearing loss relative to peers with normal hearing [7, 14]. Unilateral hearing loss is also associated with abnormal changes to resting/default networks and networks important for cognitively demanding tasks, executive functions, attention, working memory, performance monitoring, etc. [15,16,17,18]. The integrity of these networks correlates with educational outcomes [15].
Possible Treatments for Unilateral Sensorineural Hearing Loss in Children
Treatment options have been at times limited and/or inconsistent for children with single-sided deafness. The trivialization of the impact of unilateral hearing loss in the face of evidence may be a reflection of these therapeutic limitations. While cochlear implants (CI) are now considered standard of care for bilateral severe to profound sensorineural hearing loss, largely because conventional hearing aids typically do not provide sufficient audibility even with high gain amplification of sound, they have only recently been considered for individuals with unilateral deafness. Thus, until recently, treatments for unilateral listeners were limited to routing signal(s) from the deaf ear to the contralateral hearing ear either through conventional CROS aids or through bone anchored technologies. Our growing familiarity and use of cochlear implants in the setting of bilateral hearing loss suggests that these devices might be useful to treat unilateral deafness. Over the last several years, a number of studies revealed benefit of cochlear implant surgery in adult patients with deafness in one ear. Initially, cochlear implants were used in an attempt to treat intractable tinnitus in such adults. Van de Heyning et al. [19] reported complete tinnitus cessation in a group of 3 of 22 adults with single-sided deafness after 6 months of CI use. Additional hearing benefit was reported in other similar cohorts of adults [19, 20] with significant improvement in speech perception in noise, sound localization ability, and subjective hearing performance [4, 19,20,21,22,23,24,25,26].
Given the positive results of cochlear implantation in adults with acquired unilateral deafness, it is possible that children with single-sided deafness could also benefit from cochlear implantation. Delay to implantation is likely to be a significant contributor of benefit as shown by persistent aural preference in children not only who were bilaterally deaf in early life but also who received stimulation from a unilateral cochlear implant for several years before bilateral implantation [27]. Early reports of cochlear implantation in children with unilateral deafness appear to be consistent with this. Improved speech recognition in noise and sound localization were reported for three children who received a cochlear implant after acquiring deafness in one ear post-lingually [28] and of four children in another study [29]. Outcomes were best in the two children with limited duration of deafness, one child with congenital single-sided deafness implanted at 17 months of age and one child with acquired post-lingual loss in one ear post-meningitis with a short duration of deprivation at 5 weeks. In comparison, outcomes were poorer in the two children with longer periods of early deprivation, congenital single-sided deafness implanted at 4.5 and 6.8 years of age. Similarly, Arndt et al. [4] presented results of 12 months follow-up of a cohort of 13 children, demonstrating no evidence of binaural benefits for two children with congenital unilateral sensorineural hearing loss implanted at 4.3 and 13.8 years of ages, whereas a younger child (1.8 years) showed some clinical evidence of benefits comprising of substantial improvements in speech comprehension in noise and sound localization. Arndt suggested that CI intervention should take place before the age of 4 years in the setting of congenital deafness [4], but larger cohorts of children within this range are needed to better define the short and long-term benefits of cochlear implantation in children with unilateral sensorineural hearing loss [4, 7,8,9,10,11,12, 14, 30, 31].
Treatment of Unilateral Hearing Loss in Children Will Depend on Etiology
Several factors could affect implantation in children with unilateral deafness. A major contributor could be the etiologies associated with unilateral rather than bilateral deafness. While both can be caused by a variety of lesions of the inner ear and central nervous system [31], cochlear nerve deficiency (CND), a rare form of bilateral deafness, is a common cause of unilateral deafness in children. In a series of magnetic resonance imaging (MRI) scans from 128 children with unilateral hearing loss, Clemmens and colleagues found cochlear nerve deficiency in over 25%; the prevalence rose to 48% in children with severe to profound single-sided deafness [30]. This means that cochlear implantation is not a treatment option for many children with unilateral deafness. Other recognized etiologies for congenital unilateral sensorineural hearing loss are also important to consider, including congenital cytomegalovirus (CMV) infections, cochleovestibular anomalies and malformations (i.e., enlarged vestibular aqueduct (EVA)), labyrinthitis, meningitis, premature birth, trauma (cranio-cerebral injury), and auditory neuropathy [4, 30]. Importantly, this group of etiologies can be further subdivided into those in which the prognosis of the hearing loss is expected to progress and those more likely to remain stable. Likewise, in certain etiologies, the contralateral normal hearing ear may also be at risk of eventual hearing loss for example, as is the case for unilateral hearing loss due to congenital CMV [32]. In the present study, we present clinical characteristics of children with unilateral deafness presenting to our cochlear implant program for candidacy assessment for inclusion in a clinical trial examining the benefit of unilateral implantation.
Methods
Children with unilateral deafness were recruited under a study approved by the Hospital for Sick Children research ethics board (REB no. 10000002954). This portion of the study consisted of a retrospective review of the charts of children presenting to the clinic with severe to profound unilateral deafness. All families met with members of the cochlear implant team as part of the regular candidacy assessment. Implant candidacy was determined by the team using a Graded Profile Analysis [33].
Hearing evaluation consisted of ear-specific pure tone audiometric testing or auditory brainstem responses with impedance measures.
When performed, imaging included non-enhanced magnetic resonance imaging (MRI) of the brain and internal auditory canal and/or low dose, non-enhanced high resolution computed tomography (CT) of the temporal bones. MRI was performed on 3 T magnet utilizing a steady-state coherent axial images and direct multi-planar T2-weighted images in order to visualize labyrinth and nerves. Routine brain MRI was performed at the same time. CT utilized low-dose (70 mAs) thin sections (0.625 mm) limited to the petrous bones.
All available imaging was reviewed with a staff neuro-radiologist (SIB). Specific attention was paid to cochleovestibular anatomy, size of the internal auditory canal (IAC), as well as size and symmetry of the cochlear nerve canal on CT and cochlear nerve on MRI. MRI of the brain was also assessed for any abnormalities, particularly any confluent white matter changes, temporal lobe cysts, or cortical dysplasias that may be suggestive of congenital exposure to CMV [34]. CT imaging was assessed for brain calcifications that may also suggest congenital CMV exposure; however, CT imaging was typically limited to the petrous bones and, therefore, would miss any supratentorial calcifications. Likewise, MRI was blind to ossicular or window abnormalities.
For children with available neonatal dried blood spots, polymerase chain reaction (PCR) testing for CMV DNA was performed. In these instances, the possibility of congenital CMV as the underlying etiology could only be assessed by the presence or absence of the characteristic central nervous system findings seen on MRI brain as outlined above [34] in cases where imaging had been performed.
All families were offered a comprehensive 80-gene next-generation sequencing (NGS) panel, including genes associated with both syndromic and non-syndromic forms of hearing loss.
Results
Our program assessed 49 children (35 boys and 14 girls) with unilateral deafness (33 had left-sided deafness and 16 had right-sided deafness) between July 2013 and February 2017. The mean age at presentation to our clinic was 3.6 years (SD 4.5), but this represented two main age groups. The first group (n = 39) consisted of young children (1.7 (SD 1.8) years of age) who had congenital (n = 35), very early progressive unilateral hearing loss (n = 3), or early sudden unilateral hearing loss from bacterial meningitis infection (n = 1). The second group (n = 10) was older (10.3 (SD 4.2) years of age) and primarily included children with unilateral deafness, which was acquired suddenly either through trauma (n = 6) or an idiopathic mechanism (n = 2), and two children with what was suspected to be late discovered congenital hearing loss where a prolonged period of deprivation (> 4 years) disqualified them from further participating in the study.
Audiometric Assessment
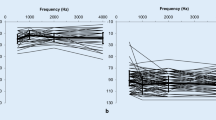
Ear-specific pure tone audiometric thresholds for all children are shown in Fig. 1. Most of the children (47/49, 96%) had unilateral sensorineural hearing loss with no evidence of middle ear involvement, and most of the children (46/49, 94%) had unilateral profound sensorineural deafness. The mean pure tone average at 500, 1000, and 2000 Hz (PTA) was 14 (SD 12) dB HL for the normal hearing ear and 95 (SD 12) dB HL for the deaf ear. One child had severe to profound sensorineural hearing loss; two had better hearing at 250 Hz (mild to moderate hearing loss), which sloped to profound at higher frequencies. Two children had hearing thresholds outside the normal limits at high frequencies in their better hearing ear; one child had mild sensorineural hearing loss at frequencies ≥ 4 kHz and the other child had mild to moderate sensorineural hearing loss ≥ 3 kHz. None had a conductive component, nor did any have abnormalities of the pinnae or external auditory canal.
Etiologic Assessment
Etiologic workup included imaging, assessment for congenital exposure to congenital CMV where possible, and genetic evaluation when desired by the family. Etiology of the unilateral sensorineural hearing loss was determined in 37/49 children and remained unknown in 12/49. A summary of etiologies is found in Table 1. The findings of the specific investigations relevant to etiology within our cohort are reviewed in detail below.
Imaging
Magnetic Resonance Imaging
The majority (37/49; 76%) of children underwent radiologic assessment of the brain and internal auditory canal using MRI. The families of 10 children opted to not complete MRI, as they were not interested in the surgical aspect of the study (i.e., cochlear implantation). An additional two children did not complete MRI due to concerns about extended duration of deafness disqualifying them from candidacy. Of the 37 children who underwent MR imaging, over a third (12/37; 32%) demonstrated aplasia/hypoplasia of the cochlear nerve (Fig. 1), 8/37 (22%) children showed evidence of non-specific white matter changes associated with congenital CMV) [34], and 6/37 of whom (16%) also had temporal lobe cysts (Fig. 2). Four children (4/37; 11%) demonstrated cochleovestibular anomalies: these were two children with EVA, one child with incomplete partition type 1 (IP-1) (Fig. 3), and one child with mild modiolar deficiency. Four of the children, where the predominant cause of deafness was identified to be an absent cochlear nerve on MRI, also demonstrated labyrinthine abnormalities, which included three children with mild modiolar deficiency and one child with a cystic cochlea and remnant superior semicircular canal. One child had intra-labyrinthine hemorrhage due to a severe arteriovenous malformation involving the right temporal lobe, thalamus, and basal ganglia (Fig. 4). Vestibular abnormalities were observed in two of the 10 children whose unilateral deafness was acquired after trauma to the head. In both cases, loss of signal within the labyrinth suggesting post-traumatic labyrinthitis ossificans was noted, most prominently in the lateral semicircular canal (Fig. 5). The remaining 10 children demonstrated no abnormalities on MR imaging.
Cochlear nerve dysplasia. Axial T2W (a) demonstrates a normal right cochlea and modiolus. Axial T2W (b) reveals aplasia of the CNC (arrow). Direct parasagittal T2W (c) image demonstrates four nerves in the IAC, (d) while the cochlear and vestibular nerves are not identified on the contralateral side (short arrow)
Incomplete partition type 1. Axial T2W image (a) through the basal turns of the cochlea demonstrate asymmetry. The left (arrow) is bulbous. Contiguous T2W image (b) demonstrates a normal right cochlea, vestibule, and horizontal semicircular canal. On the left, the cochlea (short arrow) is amorphous, bulbous, and lacking a modiolus. The vestibule is enlarged (long arrow), and the central bone island of the horizontal semicircular canal is smaller than the contralateral side. Findings are typical for IP1 malformation
Arteriovenous malformation. Axial enhanced CT (a) demonstrates a massive arteriovenous malformation (arrow). Coronal T2W image (b) again shows the massive AVM (arrow) in addition to temporal lobe atrophy. The cochlea on the right (arrow) is normal in signal and symmetrical with the contralateral side
Computed Tomography
Twenty percent (10/49) of children underwent non-enhanced high-resolution computed tomography (CT) of the temporal bones. The indication for CT imaging was a post-traumatic hearing loss in 5/10 and post-meningitic hearing loss in 1/10 (Fig. 6). The remaining 4/10 children had had CT imaging performed by their referring physician as part of their etiologic workup, prior to presentation to our clinic. Of the six children presenting with a history of traumatic injury to the temporal bone or inner ear leading to SNHL, 5 children underwent CT. Of these, ossification of the horizontal canal was observed in one child (Fig. 5), temporal bone fracture with otic capsule violation in another child, and evidence of stapes suprastructure lying within the vestibule in a third child. The remaining two CT scans were normal, and the one child who received only MRI also had a normal scan.
Post-traumatic ossifying labyrinth. Axial CT (a) reveals a linear fracture (short arrow) entering the vestibule. There is increased density within the horizontal semicircular canal (long arrow) consistent with labyrinthine ossification. T2W axial image (b) in the same child reveals loss of signal in the semicircular canal (arrow). Coronal T2W image (c) confirms loss of signal in the left superior and horizontal semicircular canals (long arrow) when compared with the contralateral side
Genetic Evaluation
All families were offered a comprehensive 80-gene NGS panel which included genes associated with both syndromic and non-syndromic forms of hearing loss. None were completed pre-operatively although 15/49 children had samples taken at the time of cochlear implantation. In total, three results were still pending at the time of writing this review, four children demonstrated no pathogenic variants in GJB2/GJB6 while the remainder of the NGS panel is pending. Of the remaining eight children who had completion of the NGS panel, none demonstrated pathogenic variants; however, all but one had variants of uncertain significance.
Congenital Cytomegalovirus
The majority of children (38/49; 78%) included in the present study had a dried blood spot taken at birth available for polymerase chain reaction (PCR) testing for CMV DNA. Eleven children did not have the dried blood spot available for assessment, either because they were born elsewhere or their specimens could not be located or had been discarded. In these instances, the possibility of congenital CMV as the underlying etiology could only be assessed by the presence or absence of the characteristic central nervous system findings seen on MRI brain as outlined above [34], if it had been performed. As a result, most (46/49, 94%) children could be assessed for congenital CMV by dried blood spot (DBS) testing either by PCR or by MR Imaging findings. The PCR test for congenital CMV DNA on their dried blood spot was positive in nine children. In another two children, the diagnosis of congenital CMV was based on MRI findings as outlined above, one of which had a negative DBS for CMV and one whose DBS was not available, as he was born in another province. Neither of these children went on to implantation. Six of the children whose dried blood spot showed evidence of CMV DNA were interested in cochlear implantation and thus underwent MRI; all six (100%) showed brain changes associated with congenital CMV, thus further confirming the diagnosis. In summary, a total of 11/49 children were considered to have an etiology of cCMV in our cohort. Of the 11 children, 7 underwent unilateral cochlear implantation, and the remaining 4 families opted against implantation.
Decision to Pursue Cochlear Implantation
Of the 49 children that presented with single-sided deafness to our clinic, 15 were not considered candidates for cochlear implantation by the cochlear implant team using the Graded Profile Analysis [33]. Of note, this workup includes an assessment of realistic expectations of the implant by the family and child, where appropriate [33]. Twelve of these 15 children had aplasia/hypoplasia of the cochlear nerve, 2 children had longer durations of deafness (> 4 years) than was initially appreciated at presentation, and 1 child’s hearing thresholds in the poor ear suggested sufficient residual hearing to make use of a hearing aid in that ear. Of the remaining 34 children, 17 families elected not to pursue cochlear implantation. This decision to not be included in the surgical arm of the study was made by families at various time points throughout the candidacy assessment. Of note, 10 families made this decision before imaging was performed, citing concerns, which included risks of the general anesthesia needed to obtain the imaging. It is expected that some of these 10 children would have had cochlear nerve aplasia/hypoplasia and have been excluded on those grounds. Of the children who went on to have imaging, where cochlear nerves were present, seven additional families decided not to pursue implantation once the entire candidacy process was known. In the remaining 17 children who were considered candidates for cochlear implantation of the poorer ear, 16 have been implanted to date and 1 is scheduled for surgery. The etiologies of deafness in these 17 children (Table 1), from most common to least, were congenital CMV (7/17, 41%), traumatic injury (4/17, 24%), noise induced trauma (1/17, 6%), meningitis (1/17, 6%), enlarged vestibular aqueduct (1/17, 6%), idiopathic hearing loss (1/17, 6%), and unknown etiology (2/17, 12%).
Discussion
Data from the present study confirms a high prevalence (32%) of cochlear nerve dysplasia in children presenting with unilateral severe to profound sensorineural hearing loss. While still a predominant cause of single-sided deafness, the prevalence of cochlear nerve dysplasia in our study was less than the nearly 50% previously reported in a similar cohort [30]. Children presenting with unilateral cochlear nerve dysplasia are not candidates for cochlear implantation. While the pathophysiology responsible for cochlear nerve dysplasias remains unknown, it is hypothesized to represent a defect in embryogenesis. What remains equally unclear is the pathophysiology underlining the predilection for it to be a unilateral deficit in the great majority of cases. The prevalence of bilateral cochlear nerve dysplasias was much lower. Specifically, bilateral cochlear nerve dysplasias are reported to be in the order of 5% [35] with the degree of stenosis related to hearing deficit [36] and ultimately performance with cochlear implants where indicated [37].
While unilateral cochlear nerve dysplasias are well known to represent the most common cause of unilateral sensorineural hearing loss in children, the current study highlights also the high prevalence of congenital CMV (24%) in our cohort. This prevalence is in keeping with that reported by several prevalence studies of congenital CMV in children presenting with any form of sensorineural hearing loss [38,39,40,41,42].
If we then exclude the children with cochlear nerve dysplasia (n = 12), as they are not candidates for implantation, as well as other non-candidates (n = 2), children with congenital CMV-related unilateral sensorineural hearing loss make up the largest proportion of children (11/34, 32% in the present cohort) who are candidates for unilateral cochlear implantation. In addition to being the most common cause, the characteristics and behaviors of sensorineural hearing loss due to congenital CMV also make these children particularly relevant candidates for early single-sided implantation. Specifically, it is known that sensorineural hearing loss from congenital CMV carries a high risk of progression, in both the ear with hearing loss and the normal hearing ear. In their cohort of children with asymptomatic congenital CMV, Lanzieri et al. demonstrated that 65% of children experienced progression in their poorer hearing ear and, of particular relevance to the current study, 45% experienced progression in the better hearing ear [32]. This group also demonstrated that those children presenting with either congenital or early progressive profound unilaterally sensorineural hearing loss developed bilateral hearing loss in 75% of cases. Considering the risk of progressive deterioration in the better hearing ear [32], the role of early unilateral implantation in the presence of normal contralateral hearing may be viewed as the first stage in preserving consistent bilateral auditory access over a lifetime. While our cohort of children with cCMV were infants, our historical experience, which is supported by the Lanzieri et al. study, demonstrates that progressive loss in the contralateral normal hearing ear can occur over a highly variable and, at times protracted, time frame. Lanzieri et al. demonstrated that the median time to bilateral hearing loss in children presenting with congenital or early progressive profound loss, similar to those children in our study, was 4 years, ranging from 4 months to 18 years [32]. If progression to bilateral loss occurs early, any aural preference created as a result of asymmetric auditory input should be resolvable following bilateral implantation. If however one ear experiences a long period of deprivation before the other ear develops hearing loss within the range of implant candidacy, the induced cortical asymmetries are expected to persist following bilateral implantation, which in turn would yield limits on functional performance. As a result, a strategy that leads to implanting the poorer ear early will protect against the development of abnormal aural preference in children with contralateral normal hearing. In the absence of such a strategy, and in the presence of long durations of asymmetric auditory development, the cortex will reorganize to prefer the better ear while waiting to implant, and these changes will not be resolved after, on average, 2 years of bimodal use [43]. In addition, our data suggests that children who experience progressive hearing loss will do well with an implant once hearing deteriorates to the point of meeting candidacy for implantation. Specifically, we have shown that children who were implanted later/had longer periods of hearing aid use did better on some music perception tests. Their duration of hearing aid use related to their degree of hearing at 250 Hz so we suggested that the better music perception was due to the period of residual low-frequency hearing pre-implantation [44, 45]. The risk of progressive deterioration in the only hearing ear could be a major factor involved in families’ decision to choose unilateral implantation for their children with unilateral deafness after congenital CMV. Specifically, the majority (7/11, 64%) of children with congenital CMV went on to agree to implantation.
In addition to cochlear nerve dysplasias and congenital CMV, the prevalence of unilateral sudden deafness was also very high in our cohort (7/49, 14%) with the most common cause being secondary to temporal bone trauma (5/49, 10%). If we again exclude the children with cochlear nerve dysplasia, as well as other non-candidates for implantation, children with sudden unilateral sensorineural hearing loss make up 20% (7/34) of children who are potential candidates for unilateral cochlear implantation, with traumatic injury being the underlying etiology in the majority, making up 15% (5/34) of our potentially “implantable cohort.” Similar to our cohort of children with congenital CMV, the acceptability of implantation to families of older children with sudden onset loss was remarkably high, with all but one child who presented with sudden single-sided deafness due to temporal bone trauma, choosing to proceed with cochlear implantation. This is in contrast to the large proportion of infants (16/27, 59%) who presented with congenital or early unilateral hearing loss who elected not to proceed with intervention a number of which did not even wish to complete candidacy assessment. There are likely several factors that contribute to this difference. First, this may speak to the impact of the sudden onset of the deficit in a child who has had access to binaural/bilateral hearing and experiences a sudden change. Essentially, these children are acutely aware of what they are missing. This is in keeping with adult data, which suggests a significant and prolonged impairment in quality of life following the onset of sudden unilateral deafness. Improvements in quality of life appear to occur in proportion with the intensity of the auditory rehabilitation in these individuals as well as the prevalence of associated tinnitus and vertigo [46]. Likewise, there also appears to be an acute adjustment phase, which is mirrored by cortical reorganization, with much of this change occurring in the first 12 to 18 months following the loss [47, 48]. Secondly, children with sudden unilateral sensorineural hearing loss were older and could actively relay their perceived deficits and, as a result, share the burden of the decision making with their caregivers. This is in contradistinction to infants with unilateral sensorineural hearing loss where the burden of decision making fell exclusively to the caregivers.
Following from this, what has become apparent to our implant team through the social work interviews conducted with these families is the distinct challenges these families face in deciding whether or not to proceed with unilateral implantation under our experimental protocol. While families presenting for implantation with a child with bilateral hearing impairment face their own challenges, the non-acceptance rate for implantation in this cohort is exceedingly low. In comparison, half of potential candidates for our study did not proceed to implant, with many not even wishing to complete some parts of the candidacy assessment. We have eluded above to some of the factors that we think contribute to a family’s decision to proceed with implantation, including risk of hearing loss in the contralateral ear as well as sudden onset of hearing loss and will no doubt learn more about the characteristics of these children and their families as the study continues. There is no doubt that the “optional” nature of participation, the invasive nature of the intervention, and the unknown long-term benefits of unilateral implantation act as significant stressors for these families and should not be underestimated. The burden of decision making for these families needs to be actively managed by the implant team, and in our center, this is done through universal evaluation and individual counseling by social work.
The long-term impact of early unilateral implantation in our cohort of children with single-sided deafness and limited duration of auditory deprivation will be seen over the next decade. However, early findings in our initial cohort are promising. Simply put, children who underwent implantation wear their devices. Specifically, datalog information available from the implant speech processor in the first seven implanted children indicates that they wear their devices consistently from the date of activation for on average 7.4 (SD 1.7) h/day [49]. Broken down by age, two teenagers with sudden sensorineural hearing wore their devices for approximately 10 h/day, preschoolers approximately 7 to 9 h/day, and toddlers approximately 4 to 5 h/day, which is typical for this age group given their daytime sleeping pattern and similar to that of children with bilateral cochlear implants. Beyond duration of use, these children are wearing their devices in a variety of situations including noisy environments [49]. Finally, the children in our cohort wore their implant more than children who have lesser degrees of unilateral hearing loss and use a hearing aid in the worse ear [49].
Beyond usage, a number of studies report early findings suggesting benefits to spatial hearing in children who undergo implantation in treatment of unilateral hearing loss [2, 4]. Our own early data demonstrates that while the cortical responses to the deaf ear stimulated with a cochlear implant in young children with limited duration single-sided deafness are unexpectedly abnormal, these responses rapidly resolve and normalize within 6 months in the majority of children (4/5) [50].
In summary, even though implantation as a treatment for single-sided deafness is currently not considered standard of practice and the decision to proceed with implantation is a challenging one, half of those families whose children are candidates chose intervention. If willingness to proceed is a marker of true or perceived disability from single-sided deafness and our early usage and cortical data are an indication of benefit following implantation in this population, the rehabilitation strategies for short duration single-sided deafness in children may routinely include implantation in the future.
Conclusions
Our cohort of children who have limited durations of unilateral deprivation reveals both expected and novel findings about their etiologies of deafness. While the high rate of cochlear nerve hypoplasia/aplasia would have been predicted, there was a high proportion of children whose unilateral deafness was associated with congenital CMV or acquired after injury. The congenital CMV group provides an ideal opportunity to prospectively follow potential deterioration of hearing in their better ear to determine whether early implantation of the deaf ear protects lifelong bilateral hearing.
References
Gordon K, Henkin Y, Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 2015;136(1):141–53. https://doi.org/10.1542/peds.2014-3520.
Thomas JP, Neumann K, Dazert S, Voelter C. Cochlear implantation in children with congenital single-sided deafness. Otol Neurotol. 2017;38(4):496–503. https://doi.org/10.1097/MAO.0000000000001343.
Arndt S, Laszig R, Aschendorff A, Hassepass F, Beck R, Wesarg T. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO. 2017; https://doi.org/10.1007/s00106-016-0297-5.
Arndt S, Prosse S, Laszig R, Wesarg T, Aschendorff A, Hassepass F. Cochlear implantation in children with single-sided deafness: does aetiology and duration of deafness matter? Audiol Neurootol. 2015;20(Suppl 1):21–30. https://doi.org/10.1159/000380744.
Keating P, King AJ. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front Syst Neurosci. 2013;7:123. https://doi.org/10.3389/fnsys.2013.00123.
Agterberg MJ, Hol MK, Van Wanrooij MM, Van Opstal AJ, Snik AF. Single-sided deafness and directional hearing: contribution of spectral cues and high-frequency hearing loss in the hearing ear. Front Neurosci. 2014;8:188. https://doi.org/10.3389/fnins.2014.00188.
Lieu JE. Speech-language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg. 2004;130(5):524–30. https://doi.org/10.1001/archotol.130.5.524.
Lieu JE, Tye-Murray N, Fu Q. Longitudinal study of children with unilateral hearing loss. Laryngoscope. 2012;122(9):2088–95. https://doi.org/10.1002/lary.23454.
Morita S, Suzuki M, Iizuka K. Non-organic hearing loss in childhood. Int J Pediatr Otorhinolaryngol. 2010;74(5):441–6. https://doi.org/10.1016/j.ijporl.2010.01.003.
Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1–2):134–43. https://doi.org/10.1016/j.heares.2004.12.010.
SLaden DP, Rothpletz A, Bess FH, Newton V. Paediatric audiological medicine. In: Series in Human Communication Science. 2nd ed. Hoboken: Wiley; 2009.
Dancer J, Burl NT, Waters S. Effects of unilateral hearing loss on teacher responses to the SIFTER. Screening Instrument for Targeting Educational Risk. Am Ann Deaf. 1995;140(3):291–4.
Wolter NE, Cushing SL, Vilchez-Madrigal LD, James AL, Campos J, Papsin BC, et al. Unilateral hearing loss is associated with impaired balance in children: a pilot study. Otol Neurotol. 2016;37(10):1589–95. https://doi.org/10.1097/MAO.0000000000001218.
Haffey T, Fowler N, Anne S. Evaluation of unilateral sensorineural hearing loss in the pediatric patient. Int J Pediatr Otorhinolaryngol. 2013;77(6):955–8. https://doi.org/10.1016/j.ijporl.2013.03.015.
Rachakonda T, Shimony JS, Coalson RS, Lieu JE. Diffusion tensor imaging in children with unilateral hearing loss: a pilot study. Front Syst Neurosci. 2014;8:87. https://doi.org/10.3389/fnsys.2014.00087.
Schmithorst VJ, Holland SK, Ret J, Duggins A, Arjmand E, Greinwald J. Cortical reorganization in children with unilateral sensorineural hearing loss. Neuroreport. 2005;16(5):463–7.
Wang X, Fan Y, Zhao F, Wang Z, Ge J, Zhang K, et al. Altered regional and circuit resting-state activity associated with unilateral hearing loss. PLoS One. 2014;9(5):e96126. https://doi.org/10.1371/journal.pone.0096126.
Yang M, Chen HJ, Liu B, Huang ZC, Feng Y, Li J, et al. Brain structural and functional alterations in patients with unilateral hearing loss. Hear Res. 2014;316:37–43. https://doi.org/10.1016/j.heares.2014.07.006.
Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117(9):645–52. https://doi.org/10.1177/000348940811700903.
Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol. 2009;14(3):163–71. https://doi.org/10.1159/000171478.
Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32(1):39–47. https://doi.org/10.1097/MAO.0b013e3181fcf271.
Buechner A, Brendel M, Saalfeld H, Litvak L, Frohne-Buechner C, Lenarz T. Results of a pilot study with a signal enhancement algorithm for HiRes 120 cochlear implant users. Otol Neurotol. 2010;31(9):1386–90. https://doi.org/10.1097/MAO.0b013e3181f1cdc6.
Firszt JB, Holden LK, Reeder RM, Cowdrey L, King S. Cochlear implantation in adults with asymmetric hearing loss. Ear Hear. 2012;33(4):521–33. https://doi.org/10.1097/AUD.0b013e31824b9dfc.
Stelzig Y, Jacob R, Mueller J. Preliminary speech recognition results after cochlear implantation in patients with unilateral hearing loss: a case series. J Med Case Rep. 2011;5:343. https://doi.org/10.1186/1752-1947-5-343.
Tavora-Vieira D, De Ceulaer G, Govaerts PJ, Rajan GP. Cochlear implantation improves localization ability in patients with unilateral deafness. Ear Hear. 2015;36(3):e93–8. https://doi.org/10.1097/AUD.0000000000000130.
Tavora-Vieira D, Marino R, Acharya A, Rajan GP. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol Neurotol. 2015;36(3):430–6. https://doi.org/10.1097/MAO.0000000000000707.
Gordon KA, Wong DD, Papsin BC. Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain. 2013;136(Pt 5):1609–25. https://doi.org/10.1093/brain/awt052.
Hassepass F, Aschendorff A, Wesarg T, Kroger S, Laszig R, Beck RL, et al. Unilateral deafness in children: audiologic and subjective assessment of hearing ability after cochlear implantation. Otol Neurotol. 2013;34(1):53–60. https://doi.org/10.1097/MAO.0b013e31827850f0.
Tavora-Vieira D, Rajan GP. Cochlear implantation in children with congenital and noncongenital unilateral deafness. Otol Neurotol. 2015;36(8):1457–8. https://doi.org/10.1097/MAO.0000000000000806.
Clemmens CS, Guidi J, Caroff A, Cohn SJ, Brant JA, Laury AM, et al. Unilateral cochlear nerve deficiency in children. Otolaryngol Head Neck Surg. 2013;149(2):318–25. https://doi.org/10.1177/0194599813487681.
Laury AM, Casey S, McKay S, Germiller JA. Etiology of unilateral neural hearing loss in children. Int J Pediatr Otorhinolaryngol. 2009;73(3):417–27. https://doi.org/10.1016/j.ijporl.2008.11.012.
Lanzieri TM, Chung W, Flores M, Blum P, Caviness AC, Bialek SR et al. Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics. 2017;139(3). doi:https://doi.org/10.1542/peds.2016-2610.
Daya H, Figueirido JC, Gordon KA, Twitchell K, Gysin C, Papsin BC. The role of a graded profile analysis in determining candidacy and outcome for cochlear implantation in children. Int J Pediatr Otorhinolaryngol. 1999;49(2):135–42.
van der Knaap MS, Vermeulen G, Barkhof F, Hart AA, Loeber JG, Weel JF. Pattern of white matter abnormalities at MR imaging: use of polymerase chain reaction testing of Guthrie cards to link pattern with congenital cytomegalovirus infection. Radiology. 2004;230(2):529–36. https://doi.org/10.1148/radiol.2302021459.
Nakano A, Arimoto Y, Matsunaga T. Cochlear nerve deficiency and associated clinical features in patients with bilateral and unilateral hearing loss. Otol Neurotol. 2013;34(3):554–8. https://doi.org/10.1097/MAO.0b013e3182804b31.
Wilkins A, Prabhu SP, Huang L, Ogando PB, Kenna MA. Frequent association of cochlear nerve canal stenosis with pediatric sensorineural hearing loss. Arch otolaryngol Head Neck Surg. 2012;138(4):383–8. https://doi.org/10.1001/archoto.2012.237.
Valero J, Blaser S, Papsin BC, James AL, Gordon KA. Electrophysiologic and behavioral outcomes of cochlear implantation in children with auditory nerve hypoplasia. Ear Hear. 2012;33(1):3–18. https://doi.org/10.1097/AUD.0b013e3182263460.
Stehel EK, Shoup AG, Owen KE, Jackson GL, Sendelbach DM, Boney LF, et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics. 2008;121(5):970–5. https://doi.org/10.1542/peds.2006-3441.
Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2008;41(2):57–62. https://doi.org/10.1016/j.jcv.2007.09.004.
Barbi M, Binda S, Caroppo S, Ambrosetti U, Corbetta C, Sergi P. A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J. 2003;22(1):39–42. https://doi.org/10.1097/01.inf.0000047673.38917.e4.
Park AH, Duval M, McVicar S, Bale JF, Hohler N, Carey JC. A diagnostic paradigm including cytomegalovirus testing for idiopathic pediatric sensorineural hearing loss. Laryngoscope. 2014;124(11):2624–9. https://doi.org/10.1002/lary.24752.
Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–90.
Polonenko MJ, Papsin BC, Gordon KA, editors. Developmental protection of aural preference in children with asymmetric hearing loss through bimodal hearing. Conference on Implantable Auditory Prostheses; 2015; Lake Tahoe, CA.
Polonenko MJ, Giannantonio S, Papsin BC, Marsella P, Gordon KA. Music perception improves in children with bilateral cochlear implants or bimodal devices. J Acoust Soc Am. 2017;141(6):4494. https://doi.org/10.1121/1.4985123.
Hopyan T, Peretz I, Chan LP, Papsin BC, Gordon KA. Children using cochlear implants capitalize on acoustical hearing for music perception. Front Psychol. 2012;3:425. https://doi.org/10.3389/fpsyg.2012.00425.
Carlsson PI, Hall M, Lind KJ, Danermark B. Quality of life, psychosocial consequences, and audiological rehabilitation after sudden sensorineural hearing loss. Int J Audiol. 2011;50(2):139–44. https://doi.org/10.3109/14992027.2010.533705.
Noguchi Y, Takahashi M, Ito T, Fujikawa T, Kawashima Y, Kitamura K. Delayed restoration of maximum speech discrimination scores in patients with idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx. 2016;43(5):495–500. https://doi.org/10.1016/j.anl.2015.12.003.
Li LP, Shiao AS, Chen KC, Lee PL, Niddam DM, Chang SY, et al. Neuromagnetic index of hemispheric asymmetry prognosticating the outcome of sudden hearing loss. PLoS One. 2012;7(4):e35055. https://doi.org/10.1371/journal.pone.0035055.
Polonenko MJ, Papsin BC, Gordon KA. Children with single-sided deafness use their cochlear implant. Ear Hear. 2017; https://doi.org/10.1097/aud.0000000000000452.
Polonenko MJ, Papsin BC, Gordon KA, editors. Cortical activity in children with single-sided deafness pre- and post-implantation. Conference of the Association for Research in Otolaryngology; 2017; Baltimore, MD.
Funding Information
Canadian Institutes of Health Research Grant #MOP-130420 for Dr. Karen Gordon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Sokolov has received a Fellowship from the Canadian Institutes of Health Research.
Dr. Cushing reports other from Cochlear Corporation, other from Oticon, non-financial support and other from Interacoustics, outside the submitted work. In addition, Dr. Cushing has a patent Systems and Methods for Balance Stabilization. Issued. Patent no.: 7041-0. Joint Holder issued.
Dr. Polonenko has received a Fellowship from the Canadian Institutes of Health Research.
Dr. Blaser has nothing to disclose.
Dr. Papsin reports personal fees from Cochlear Americas Corporation, outside the submitted work. In addition, Dr. Papsin has a patent Systems and Methods for Balance Stabilization. Applied. Patent no.: 7041-0. Joint Holder issued.
Dr. Gordon reports grants from Canadian Institute of Health Research, during the conduct of the study, and is also a member of the Speaker’s Bureau for Cochlear Corporation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Otology
Rights and permissions
About this article
Cite this article
Sokolov, M., Cushing, S.L., Polonenko, M. et al. Clinical Characteristics of Children With Single-Sided Deafness Presenting for Candidacy Assessment for Unilateral Cochlear Implantation. Curr Otorhinolaryngol Rep 5, 275–285 (2017). https://doi.org/10.1007/s40136-017-0173-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-017-0173-1