Abstract
Purpose of Review
The challenging surgical field of endoscopic skull base surgery has undergone tremendous advancement in the past years. The aim of this review was to discuss the important factors that contributed to the evolution of this exciting field.
Recent Findings
Endoscopic skull base surgery started with pituitary surgeries and closure of cerebrospinal fluid (CSF) leaks. As the field progresses, it is now possible to operate on selected lesions located in areas ranging from the cribriform plate down to the second cervical vertebra and laterally to the infratemporal fossa and petrous apex. The key factors that contributed to the evolution of endoscopic intracranial surgeries include the development of modern endoscopy equipment, advancement of endoscopic anatomy knowledge, and improvement in skills to perform endoscopic surgical resection, reconstruction, and hemostasis.
Summary
Extended endonasal approaches can provide skull base surgeons with safe and effective access to the anterior, middle, and posterior cranial fossae in selected cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skull base is one of the most complex anatomical regions within the human body and forms the floor of the cranial cavity. Skull base surgeries include operations to the anterior, middle, and posterior cranial fossa via open, microscopic, or endoscopic approaches.
The surgical field of endoscopic skull base surgery started about 40 years ago. Advances in technology have led to the development of modern endoscopes, which enable surgeons to maintain adequate illumination and visualization of the surgical field when operating.
The key factors that contributed to the evolution of endoscopic intracranial surgeries include the development of modern endoscopy equipment, better understanding of the sinonasal pathophysiology, advancement of endoscopic anatomy knowledge, and improvement in skills to perform endoscopic surgical resection, reconstruction, and hemostasis.
The sinonasal tract acts as a natural corridor to a myriad of skull base lesions. The minimally invasive endoscopic transnasal approaches, coupled with sophisticated neuronavigation system, allow access to various midline skull base pathologies, where manipulation of neurovascular structures can be minimized and brain retraction (from the cribriform plate down to the second cervical vertebra) can be avoided. In addition to that, these approaches also enable access to lateral lesions up to the infratemporal fossa and petrous apex.
The aim of this review was to highlight the important factors that contributed to the evolution of endoscopic intracranial surgeries.
Advances in Technology—the “Modern” Endoscope
Advances in technology were fundamental in the development of endoscopic sinus and skull base surgery. Doglietto et al. recently summarized the key development of modern endoscopy [1] where the first endoscope was invented by Philipp Bozzini (1773–1809) more than 200 years ago [2]. However, the illumination for the endoscope was achieved with a candlelight reflected by mirrors inside a tube at the time, therefore limiting surgeons’ visualization [3,4,5]. The device was later improved by Max Nitze (1849–1906), where illumination of the scope was improved and magnification of images was made possible [3, 5]. Thomas Edison’s invention of the light bulb allowed further improvements to be made to the endoscope [6]. Max Nitze was the first to take endoscopic photographs inside the bladder [5, 7] while Hirschmann was the first to use an endoscope (a modified cystoscope) to inspect the maxillary sinus in 1901 [8].
The evolution of the “modern” endoscope being used today was driven by inventions of Harold H. Hopkins (1918–1994) and Karl Storz (1911–1996) [6, 9]. Hopkins invented the rod-lens system, which was an improvement from the previous Nitze system, by using neutral gas instead of air between a train of glass lenses. This results in a far better optic efficiency with greater light transmissions, wider views, and better image quality within a smaller system. Moreover, it was also made possible to document endoscopic findings using cameras and video system in the new endoscopy unit [6].
Basil Hirschowitz later on developed an endoscope with flexible glass-coated fibers, illuminated by a simple light bulb at the proximal end. This system was called fiberscope, and it was first introduced at the American Gastroscopic Society meeting in Colorado Spring on May 16, 1957 [10, 11]. Karl Storz, who was influenced by Hirschowitz, licensed the idea of the fiber optic external cold light transmission combined with the rod-lens system in 1965 [6]. The inventions of Hopkins’ rod-lens system coupled with Karl Storz’s external cold light transmission paved the way for modern endoscopic sinus and skull base surgery. Table 1 summarizes the milestones and key individuals involved in the development to the “modern endoscope.”
Understanding of Sinonasal Pathophysiology
Walter Messerklinger introduced the endoscope for diagnosis and surgical treatment of inflammatory sinus disease in 1970s [12]. The understanding of sinonasal pathophysiology and endoscopic anatomy led to a paradigm shift in the diagnosis and treatment of inflammatory sinus disease.
Messerklinger and Stammberger then advanced the endoscopic surgical techniques further in Europe [13] while David Kennedy introduced the endoscopic sinus surgery to the USA and introduced the term “functional endoscopic sinus surgery” (FESS) in 1985 [14]. Since then, rhinology and sinus surgery have undergone tremendous advancements. Before the turn of the last century, the use of endoscope has become part of the routine examination and has revolutionized the treatment of chronic rhinosinusitis. As the field advances, endoscopic sinus surgery has also pushed the boundaries of its application further due to improved visualization, better understanding of endoscopic anatomy, and advancements in image-guidance systems, therefore enabling its usage in the treatment of skull base pathologies.
Dawn of Endoscopic Skull Base Surgery—Pituitary Surgery
The use of endoscope in skull base surgeries started with trans-sphenoidal approach to the skull base, where Gerard Guiot was the first neurosurgeon to use an endoscope for pituitary surgery. However, routine use of this method was abandoned due to inadequate visualization of the surgical field at that time [1]. In the late 1970s, the endoscope was reintroduced as an adjunct to the microscope to look “around corners” [1, 15,16,17].
The partnership between neurosurgeons and otolaryngologist was a major step towards endoscopic skull base surgeries. This collaboration resulted in usage of endoscope as the only visualization tool for trans-sphenoidal pituitary surgery in the early 1990s. In 1992, Janokowski et al. reported the first three cases of a pure endoscopic endonasal approach to the pituitary [18], which was followed by more reports of such cases by Sethy and Pillay, Rodziewski et al., and Jho and Carrau [19,20,21]. Cappabianca et al. then introduced the term “functional endoscopic pituitary surgery” and made further improvements to the instrumentation and technique of this approach [22].
Since sphenoid sinus was the starting point to reach different regions of the skull base (especially in the medial plane), the pure endoscopic approach to the sphenoid sinus to treat sellar pathologies was regarded as the dawn of endoscopic skull base surgery.
The endoscopes provide monocular views compared to the binocular views of microscopes that most neurosurgeons are accustomed to. Therefore, compensatory maneuvers were necessary to overcome the lack of depth perceptions, for example using bimanual dissection with one hand constantly in the field, providing gentle palpation with a suction tip. Besides that, the surgeons can also move the endoscopes to provide a dynamic visualization of the surgical field [23, 24, 25••]. This highlights the importance of a true team approach between the neurosurgeons and otolaryngologist. As the field of endoscopic skull base surgery advances, the concept of extended endoscopic approaches was developed.
Evolution of Expanded Endonasal Approaches
The ideal approach to the skull base should provide adequate access to the target lesion, facilitate complete resection, and allow repair of any defects. Moreover, it should provide identification and protection of important neurovascular structures. Besides providing these surgical features in well-selected patients, expanded endonasal approaches (EEAs) also help patients avoid external incisions and preserve their neurological, visual, and masticatory functions [25••].
The main principle of EEA is to use pre-existing airspaces to gain access to the skull base. A wide surgical corridor has to be created to allow adequate visualization and instrumentation, and this can be achieved with customized removal of the bone. The collaboration between neurosurgeons and otorhinolaryngologists plays an important role for the success of surgery. By using the two-surgeons, bilateral nasal access and four-handed technique, adequate space for instrumentation can be achieved. Besides, the surgeon holding the endoscope can provide constant feedback to the surgeon who is performing tissue dissections, which is important for intraoperative decision-making and problem solving [25••]. The role of the co-surgeon can be reversed during the procedure. In our experience, the otorhinolaryngologist usually performs the role of removing bones, exposing the skull base and reconstructing the defects while the neurosurgeons perform the intradural resection part of the operation. However, it is worth noting that the concept of team surgery has its disadvantages as well because it is not always easy to find compatible surgical partners with similar personality, skills, and philosophy to ensure smooth proceedings of the surgery [25••].
The evolution of EEAs has produced a paradigm shift in skull base surgery, and the field continues to evolve as the surgeons’ experience and technology advances. Specialized instrumentation such as bipolar forceps, microdebrider, high-speed drills, computer-assisted surgical navigation, and Doppler ultrasound probes is some of the technological adjuncts that contributed to the evolution of EEAs.
Kassam et al. introduced anatomy-based modules in the sagittal and coronal plane that allow access to a myriad of skull base lesions [25••, 26, 27]. The sagittal plane provides surgical access to median structures from the frontal sinus to the second cervical vertebra (transfrontal, transcribiform, transplanum, transellar, transclival, transodontoid approach).
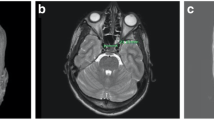
On the other hand, the coronal plane provides access to the paramedian skull base to reach the medial orbit including the orbital apex, Meckel’s cave, the petrous apex, middle cranial fossa, and the infratemporal fossa [26,27,28]. The transpterygoidal approach is common to access some of the lesions in the coronal plane. The main limitations of these approaches are the risks to important neurovascular structures such as the cranial nerves and internal carotid artery (ICA). When cranial nerves need to be sacrificed and major vessels need to be resected, alternative or combined approaches should be considered [25••]. The ICA is also an important anatomical landmark intraoperatively. Figure 1 illustrates the anatomical relation of the ICA.
This case illustrates the main principals of an expanded endonasal approach (EEA) in a patient with a petrous apex cholesteatoma. Imaging shows lateral extension and the close relation to the ICA (a, b). Pre-existing airspaces are used to gain access. The sphenoid keel and the posterior septum are removed. By using the two-surgeons, bilateral nasal access, and four-handed technique, adequate space for instrumentation can be achieved. Image guidance and a Doppler ultrasound probe can help to verify the location of the ICA. Images in c and d show the lesion before removal after the surgical corridor is established. Image in e shows the course of the ICA (petrous and paraclival segment) after tumor removal. Image in f illustrates the location of the ICA after tumor removal
A variety of different intracranial pathologies can be resected using the transnasal endoscopic approach. The most common pathologies in the sagittal plane are pituitary macroadenoma, CSF leak, meningocele or encephalocele, meningioma, craniopharyngeoma, Rathke cleft cyst, esthesioneuroblastoma, chondrosarcoma, chordoma, craniovertebral invagination, and inflammatory pannus [25••]. On the other hand, the common pathologies in the paramedian plane include inverted papilloma, sinonasal tumors, schwannoma, cholesterol granuloma, cholesteatoma, haemangioma, and extensive nasopharyngeal tumors [25••].
A detailed description of common pathologies, advantages, and limitations of EEAs to the skull base is beyond the scope of this article. This topic has been covered in an excellent review by Kasemsiri et al. [25••].
Hemostasis
Management of intraoperative hemorrhage during skull base surgery is essential. The tools used to control bleeding include diathermy or electrocautery, vessel clips, hemostatic agents (such as surgical gelfoam and floseal), bone wax, and hot saline irrigation. The principle of controlling intraoperative hemorrhage is the same regardless of whether an endoscope or a microscope is being used [29, 30]. The surgical corridor should allow enough space for instrumentation in order to deal with intraoperative bleeding. In diffuse “oozing” of mucosa or tumor, hot saline irrigation (40 °C) is sufficient to stop the bleeding; therefore, adequate space for instrumentation is not essential. However, in high flow venous or arterial bleeding, the surgical corridor has to allow adequate visualization and instrumentation to control hemostasis.
A potential catastrophic complication is the injury of the ICA. Therefore, a good understanding of the anatomical relationships of this structure is paramount to avoid causing trauma to the vessel. If ICA were to be injured during operation, it is important to obtain intraoperative control of the hemorrhage followed by angiography with stenting or sacrifice of the ICA. Recent published techniques for vascular control highlight the need of a team approach and the need of adequate visualization. Use of large suctions, lens cleansing devices, and application of crushed muscle patch can control intraoperative ICA bleeding [25••, 31, 32].
Reconstruction
The expansion of endoscopic endonasal approaches for resecting benign or malignant skull base tumors has led to improvements in the techniques available for reconstructing skull base defects.
Small and low flow dural defect can be repaired using free autologous tissue (fat, mucosa, fascia) or synthetic material in multilayer technique [33••]. On the other hand, vascularized flaps should be used in cases of high-flow intraoperative CSF leaks [33••]. Currently, reconstruction using vascularized pedicle flaps provides the most reliable outcomes, with postoperative CSF leak rate of less than 5% [34]. Commonly used skull base vascularized flaps include the Hadad–Bassagaisteguy nasoseptal flap [35, 36], nasoseptal rescue and modified rescue flaps [37], reverse rotation flap (Caicedo flap) [38, 39•], anterior pedicle lateral nasal wall flap (Hadad–Bassagaisteguy 2 flap) [40], posterior pedicle lateral nasal wall flap (Carrau–Hadad flap) [41], middle turbinate flap [42], transpterygoid temporoparietal fascia flap [43, 44], and transfrontal pericranial flap [45,46,47]. Temporoparietal fascia and pericranial flaps are regional or extranasal vascularized flaps, whereas all others are categorized as intranasal flaps. It should be noted that multiple flaps or a combination of vascularized flaps and free tissue grafts may be needed to complete skull base reconstruction.
A detailed description of skull base reconstruction is beyond the scope of this article. However, this topic was covered in an excellent review by Soudry et al. [33••] and Tang et al. [48••].
Limits
Several factors should be considered when deciding whether an endoscopic approach to a skull base pathology is suitable. Besides the pathology and vascularity of the lesion, it is also important to take into account the location of the lesion in relation to important neurovascular structures and tailor the surgical approach accordingly.
Therefore, endoscopic approach is contraindicated for lesions that extend laterally beyond the critical neurovascular structures, with the exception of cystic or soft lesions or if the goal of the operation was for debulking rather than complete excision of the tumor [25••]. The ICA is one of the main anatomical limitations for this surgery as injury to this vessel can result in major neurological sequelae. Even though ICA mobilization techniques have been described in the literature, they should only be reserved to very skilled and experienced surgeons [49, 50].
Cerebral involvement of tumor is also considered a contraindication for endoscopic approach for most authors [49, 51]. Orbital invasion via the infraorbital fissure or involvement of the periorbital region by malignancies requires surgical orbital exenteration [49]. Similarly, adequate resection of tumor involving the skin and maxillary and nasal bone cannot be achieved using the endoscopic approach. Posterior extension of the tumor to the inferior temporal fossa can be successfully treated endoscopically. However, it is not feasible to treat patients with osteolysis of the greater wing of the sphenoid using only the endoscopic method [49, 51].
Another contraindication for surgery is patients’ co-morbidities. Any active bacterial sinusitis should be adequately treated prior to undergoing an endoscopic skull base surgery [25••].
The surgical team’s experience and the department’s resources for endoscopic skull base surgery can also be limiting factors for endoscopic skull base operations. Proper training in endoscopic surgical technique is essential to achieve optimal surgical and oncological outcomes and to avoid complications [52]. There is a steep learning curve to be competent in this type of surgery [53, 54], and this can be achieved with a systematic training scheme that includes teachings on the complex anatomy, pathology, and technical skills required for the operation, as well as recognizing the potential risks of neural and vascular injury associated with the extent of intradural dissections [25••, 52, 55].
Essential resources for the operation include adequate specialized instrument and equipment, trained personnel, and an intensive neurosurgical care unit. Good functional working relationships between the otorhinolaryngology and the neurosurgical team, as well as with the anesthetists, radiologists, and pathologists of the skull base team are crucial for the operation to run smoothly [55].
A detailed description of the advantages and limitations of EEAs to the skull base is beyond the scope of this article. This topic has been covered in a review article by Kasemsiri et al. [25••].
Morbidity Related to Endoscopic Skull Base Surgery
Endoscopic skull base surgery uses pre-existing air spaces of the paranasal sinuses to gain access to the skull base pathology. Moreover, removal of bone and mucosa is necessary to allow access for a bi-nostril, 4-handed surgical approach, which is advantageous as described above.
However, this results in the disruption of the sinonasal tracts with loss of normal nasal epithelium ciliary function with potential loss of olfactory function. The loss of ciliary function can result in nasal crusting causing patient discomfort, malodor, and inflammation [25••]. Nasal crusting occurs in almost all patients (>95%) 1 month after surgery and can be treated with saline irrigations and regular endoscopic debridement. Fifty percent of these patients were found to be crust-free at 3 months postoperatively [56].
Other postoperative sinonasal complications include formation of nasal synechia, alar sill burn, hyposthesia in the areas innervated by the maxillary, palatal, or incisor nerve, taste disturbances, serous otitis media, and malodor [57]. Most patients regained their full nasal function 6 months after their operations with adequate postoperative care [56, 57].
Complications
The most common complication after endoscopic skull base surgery is CSF leak. The introduction of vascular pedicled flaps significantly decreases the rate of CSF leak to less than 5% [34].
In a study by Kassam et al. involving 800 patients (where the two most common pathologies were pituitary adenomas (39.1%) and meningionmas (11.8%)), they found that the most common complications after endoscopic endonasal skull base surgeries (beside CSF leaks) are transient neurologic deficits (2.5%), permanent neurologic deficits (1.8%), intracranial infections (1.6%), systemic complications (2.1%), and death (0.9%). In this series, seven patients died within 30 days of the perioperative period (six from systemic illness and one from infection) [58]. They concluded that with incremental acquisition of skills and experience, endoscopic endonasal approaches have an acceptable safety profile in selected patients presenting with various skull base pathologies.
Outcomes
There are several advantages to endoscopic approach such as lack of external incisions, avoidance of translocation of the maxillofacial skeleton, preservation of neurovascular structures, and avoidance of brain retraction [25••]. Therefore, the term “minimally invasive” is also being used to describe this approach occasionally. However, the word “minimal” can be misleading as it is only true when compared to open approaches for certain skull base lesions, but not for the extent of resections.
Endoscopic approaches to benign skull base lesions are well accepted [59, 60]. However, there are some controversies when it comes to management of malignancies. Many believed that endoscopic surgeries on malignancies are possible within the limitations of the approach as discussed above.
Studies have found that piecemeal resection by tumor disassembly have the same 5-year overall survival compared to traditional open approaches, with marked reduction in patients’ morbidity and mortality [59, 61, 62, 63•]. It is important to be radical in achieving negative resection margins and tumor debulking should not violate normal tissue planes [59, 61].
If negative margins cannot be obtained by an endoscopic approach, the surgeon must be able to convert the operation to an appropriate alternative (open) approach.
Endoscopic approach is also associated with less trauma to the normal soft tissue and facial skeleton, resulting in shorter recovery time as well as shorter delay between surgery and the start of adjuvant treatment compared to open approaches [58].
Several studies have compared the outcome of anterior craniofacial resections with transnasal endoscopic resections of anterior skull base malignancies [64•] where endoscopic resections were associated with excellent tumor visualization [65, 66], reduced hospital and intensive care unit stay, decreased blood loss and transfusion rate, faster recovery [65,66,67], and superior cosmetic outcome [65, 66]. No significant differences were found in terms of survival, recurrence, metastases, or complication rates between both approaches [65, 66].
Conclusions
Skull base pathologies are rare disorders and endoscopic approaches are the latest frontiers in rhinology surgeries. The advent of endoscopes, advancement in technology, better understanding of sinonasal anatomy, and pathophysiology have revolutionized this field. With competent surgical expertise and adequate resources, transnasal endoscopic approaches can provide safe and effective access to the anterior, middle, and posterior cranial fossae in selected cases. Even though most of the published studies came from retrospective case series with limited follow-up period, we believe that endoscopic skull base surgeries have tremendous potential and will continue to evolve as the field progresses.
References
Papers of Particular Interest, Published recently, Have Been Highlighted as: • Of Importance •• Of Major Importance
Doglietto F, Prevedello DM, Jane Jr JA, Han J, Laws Jr ER. Brief history of endoscopic transsphenoidal surgery-from Philipp Bozzini to the first world congress of endoscopic Skull Base surgery. Neurosurg Focus. 2005;19:E3.
Reuter M. The historical development of endophotography. World J Urol. 2000;18:299–302.
Jackson C. Bronchoscopy and Esophagoscopy: a manual of Peroral endoscopy and laryngeal surgery. Philadelphia: WB Saunders; 1922.
Modlin I, Kidd M, Lye KD. From the lumen to laparoscope. Arch Surg. 2004;139:1110–26.
Mouton WG, Bessell JR, Maddern GJ. Looking back to the advent of modern endoscopy: 150th birthday of Maximilian Nitze. World J Surg. 22:1256–8.
Linder TE, Simmen D, Stool SE. Revolutionary inventions in the twentieth century. The history of endoscopy. Arch Otolaryngol Head Neck Surg. 1997;123:1161–3.
Reuter HJ, Reuter MA. Philipp Bozzini and endoscopy in the nineteenth century. Stuttgart: Max Nitze Museum; 1988.
Hirschmann A. Über Endoskopie der Nase und deren Nebenhöhlen. Arch Laryngol Rhinol (Berl). 1993;14:194–202.
Jennings CR. Harold Hopkins. Arch Otolaryngol Head Neck Surg. 1998;124:1042.
Gow JG. Harold Hopkins and optical systems for urology - an appreciation. Urology. 1998;52:152–7.
Hirschowitz BI. Endoscopic examination of the stomach and duodenal cap with the fiberscope. Lancet. 1961;1:1074–8.
Messerklinger W. Diagnosis and endoscopic surgery of the nose and its adjoining structures. Acta Otorhinolaryngol Belg. 1980;34:170–6.
Stammberger H, Possawetz W. Functional endoscopic sinus surgery. Concepts, indications and results of the Messerklinger technique. Eur Arch Otorhinolarynol. 1990;247:63–76.
Kennedy DW. Functional endoscopic sinus surgery. Technique Arch Otolaryngol. 1985;111:643–9.
Liu JK, Das K, Weiss MH, Laws Jr ER, Couldwell WT. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083–96.
Apuzzo MLJ, Heifetz M, Weiss MH, Kurze T. Neurosurgical endoscopy using the side-viewing telescope. Technical note J Neurosurg. 1977;16:398–400.
Bushe KA, Halves E. Modifizierte Technik bei transnasaler Operation der Hypophysengeschwulste. Acta Neurochir. 1978;41:163–75.
Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;02:198–202.
Sethi DS, Pillay PK. Endoscopic management of lesions of the Sella turcica. J Laryngol Otol. 1995;109:956–62.
Rodziewicz GS, Kelley RT, Kellman RM, Smith MV. Transnasal endoscopic surgery of the pituitary gland: technical note. Neurosurgery. 1996;39:189–93.
Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44–51.
Cappabianca P, Aleri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the Sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66–73.
Fraser JF, Allen B, Anand VK, Schwartz TH. Three-dimensional neurostereoendoscopy: subjective and objective comparison to 2D. Minim Invasive Neurosurg. 2009;52:25–31.
Cappabianca P, Decq P, Schroeder HWS. Future of endoscopy in neurosurgery. Advantages and limitations of endoscopic endonasal approaches to the skull base. Surg Neurol. 2007;67:496–8.
•• Kasemsiri P, Carrau RL, Ditzel Filho LF, Prevedello DM, Otto BA, Old M, et al. Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg. 2014;82:12–21. Excellent review article
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I: crista galli to the sella turcica Neurosurg Focus. 2005;19:E3.
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II: posterior clinoids to the foramen magnum Neurosurg Focus. 2005;19:E4.
Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19:E6.
Paluzzi A, Gardner P, Fernandez-Miranda JC, Snyderman C. The expanding role of endoscopic skull base surgery. Br J Neurosurg. 2012;26:649–61.
Lund VJ, Stammberger H, Nicolai P, Castelnuovo P, Beal T, Beham A, et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. European rhinologic society advisory board on endoscopic techniques in the management of nose, paranasal sinus and skull base tumours. Rhinol Suppl. 2010;1:1–143.
Valentine R, Wormald PJ. Carotid artery injury after endonasal surgery. Otolaryngol Clin N Am. 2011;44:1059–79.
Valentine R, Wormald PJ. Controlling the surgical field during a large endoscopic vascular injury. Laryngoscope. 2011;121:562–6.
•• Soudry E, Turner JH, Nayak JV, Hwang PH. Endoscopic reconstruction of surgically created skull base defects: a systematic review. Otolaryngol Head Neck Surg. 2014;150:730–8. Excellent review about different skull base reconsruction options
Kassam AB, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approach. Neurosurg Focus. 2005;19:E8.
Hadad G, Bassagaisteguy L, Carrau RL, Mataza JC, Kassam A, Synderman CH, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–6.
Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63:44–52.
Rivero-Serrano CM, Snyderman CH, Gardner P, Prevedello D, Wheless S, Kassam AB, et al. Nasoseptal ‘rescue’ flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011;121:990–3.
Caicedo-Granados E, Carrau RL, Snyderman CH, Prevedello D, Fernandez-Miranda J, Gardner P, et al. Reverse rotation flap for reconstruction of donor site after vascular pedicled nasoseptal flap in skull base surgery. Laryngoscope. 2010;120:1550–2.
• Kasemsiri P, Carrau RL, Otto BA, Tang IP, Prevedello DM, Muto J, et al. Reconstruction of the pedicled nasoseptal flap donor site with a contralateral reverse rotation flap: technical modifications and outcomes. Laryngoscope. 2013;123:2601–4. Good article with an important modification in technique
Hadad G, Rivero-Serrano CM, Bassagaisteguy LH, Carrau RL, Fernandez-Miranda J, Prevedello DM, et al. Anterior pedicle lateral nasal wall flap: a novel technique for the reconstruction of anterior skull base defects. Laryngoscope. 2011;121:1606–10.
Rivera-Serrano CM, Bassagisteguy LH, Hadad G, Carrau RL, Kelly D, Prevedello DM, et al. Posterior pedicle lateral nasal wall flap: new reconstructive technique for large defects of the skull base. Am J Rhinol Allergy. 2011;25:212–6.
Prevedello DM, Barges-Coll J, Fernandez-Miranda JC, Morera V, Jacobson D, Madhok R, et al. Middle turbinate flap for skull base reconstruction: cadaveric feasibility study. Laryngoscope. 2009;119:2094–8.
David SK, Cheney SL. Ananatomy study of the temporoparietal fascial flap. Arch Otolaryngol Head Neck Surg. 1995;121:1153–6.
Fortes FS, Carrau RL, Snyderman CH, Kassam A, Prevedello D, Vescan A, et al. Transpterygoid transposition of a temporoparie- tal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117:970–6.
Yoshioka N, Rhoton Jr AL. Vascular anatomy of the anteriorly based pericranial flap. Neurosurgery. 2005;57:11–6.
Price JC, Loury M, Carson B, Johns ME. The pericranial flap for reconstruction of anterior skull base defects. Laryngoscope. 1988;98:1159–64.
Smith JE, Ducic Y. The versatile extended pericranial flap for closure of skull base defects. Otolaryngol Head Neck Surg. 2004;130:704–11.
•• Tang IP, Carrau RL, Otto BA, Prevedello DM, Kasemsiri P, Ditzel L, Muto J, et al. Technical nuances of commonly used vascularised flaps for skull base reconstruction. J Laryngol Otol. 2015;129:752–61. Excellent overview of vascularised flaps for skull base reconstruction
Verillaud B, Bresson D, Sauvaget E, Mandonnet E, Georges B, Kania R, et al. Endoscopic endonasal skull base surgery. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:190–6.
Zanation AM, Snyderman CH, Carrau RL, Gardner PA, Prevedello DM, Kassam AB. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119:19–25.
Dehdashti AR, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery. 2009;64:677–87.
Snyderman CH, Fernandez-Miranda J, Gardner PA. Training in neurorhinology: the impact of case volume on the learning curve. Otolaryngol Clin N Am. 2011;44:1223–8.
Smith SJ, Eralil G, Woon K, Sama A, Dow G, Robertson I. Light at the end of the tunnel: the learning curve associated with endoscopic transsphenoidal skull base surgery. Skull Base. 2010;20:69–74.
Snyderman C, Kassam A, Carrau R, Mintz A, Garnder P, Prevedello DM. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117:699–705.
Snyderman CH, Pant H, Carrau RL, Prevedello D, Gardner P, Kassam AB. What are the limits of endoscopic sinus surgery? The expanded endonasal approach to the skull base. Keio J Med. 2009;58:152–60.
O’Malley Jr BW, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25:E10.
de Almeida JR, Zanation AM, Snyderman CH, Carrau RL, Prevedello DM, Gardner PA, et al. Defining the nasopalatine line: the limit for endonasal surgery of the spine. Laryngoscope. 2009;119:239–44.
Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2011;114:1544–68.
Castelnuovo P, Dallan I, Battaglia P, Bignami M. Endoscopic endonasal skull base surgery: past, present and future. Eur Arch Otorhinolaryngol. 2010;267:649–63.
Pasquini E, Sciaretta V, Frank G, Cantaroni C, Modugno GC, Mazzatenta D, et al. Endoscopic treatment of benign tumors of the nose and paranasal sinuses. Otolaryngol Head Neck Surg. 2004;131:180–6.
Snyderman CH, Carrau RL, Kassam AB, Zanation A, Prevedello D, Gardner P, et al. Endoscopic skull base surgery: principles of endonasal oncological surgery. J Surg Oncol. 2008;97:658–64.
Nicolai P, Battaglia P, Bignami M, Bolzoni Villaret A, Delù G, Khrais T, et al. Endoscopic surgery for malignant tumors of the sinonasal tract and adiacent skull base: a 10-year experience. Am J Rhinol. 2008;222:308–16.
• Farag A, Rosen M, Evans J. Surgical Techniques for Sinonasal Malignancies. Neurosurg Clin N Am. 2015;26:403–12. Article focusing on surgical techniques
• Husain Q, Patel SK, Soni RS, Patel AA, Liu JK, Eloy JA. Celebrating the golden anniversary of anterior skull base surgery: reflections on the past 50 years and its historical evolution. Laryngoscope. 2013;123:64–72. Review with a historical overview and summary of the evolution of the field
Eloy JA, Vivero RJ, Hoang K, Civantos FJ, Weed DT, Morcos JJ, et al. Comparison of transnasal endoscopic and open craniofacial resection for malignant tumors of the anterior skull base. Laryngoscope. 2009;119:834–40.
Wood JW, Eloy JA, Vivero RJ, Sargi Z, Civantos FJ, Weed DT, et al. Efficacy of transnasal endoscopic resection for malignant anterior skull-base tumors. Int Forum Allergy Rhinol. 2012;2:487–95.
Cohen MA, Liang J, Cohen IJ, Grady MS, O’Malley Jr BW, Newman JG. Endoscopic resection of advanced anterior skull base lesions: oncologically safe? ORL J Otorhinolaryngol Relat Spec. 2009;71:123–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Y Brand, Dr. IP Tang, Dr. V Waran, Dr. E Wong, and Dr. N Prepageran declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Rhinology: Advances in Endoscopic Sinus Surgery
Rights and permissions
About this article
Cite this article
Brand, Y., Tang, I., Waran, V. et al. The Evolution of Endoscopic Intracranial Surgeries. Curr Otorhinolaryngol Rep 5, 16–23 (2017). https://doi.org/10.1007/s40136-017-0141-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-017-0141-9