Abstract
Purpose of Review
In this article, we review the pathogenesis, clinical features, imaging modalities, and latest management options for optic pit maculopathy (OPM).
Recent Findings
The pathogenesis of OPM remains to be unclear, but imaging tools such as optical coherence tomography (OCT) and OCT angiography are enhancing our knowledge. Observation continues to be the best management strategy for patients with good visual acuity, and many cases have demonstrated spontaneous resolution. For more advanced, progressive vision loss, treatment options involving vitrectomy can be considered and discussed with the patient. Supplementary techniques to vitrectomy have been reported in small studies with relative success such as glial tissue peeling, inverted internal limiting membrane flap, optic pit plugging, and retinal fenestration.
Summary
While there are multiple treatment options available for OPM, there is no consensus on the technique and surgical timing. Individual patient factors and the risks-benefits of treatment must be taken into account in guiding management. Larger clinical trials will further assist in decision-making for treating OPM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optic pits (OP) are oval-shaped depressions that are commonly found in the temporal region of the optic disc. OP were first described in 1882 by Weithe as small, dark depressions in the optic discs that he observed in both eyes in a 62-year-old woman [1]. Halbertsma in 1927 first described the association of OP with maculopathy [2], and later Kranenburg described central vision loss due to optic pit maculopathy (OPM) in 65% of patients with OP [3]. Petersen also described OP associate with serous retinal detachment of the macula [4]. OP are usually asymptomatic but can have visual field defect such as enlarged blind spots and paracentral scotoma. Vision loss is commonly due to maculopathy associated with OP. OPM can exist with subretinal fluid, retinoschisis, and lamellar or full-thickness macular holes leading to outer retinal atrophy.
Epidemiology
Affecting men and women equally, optic pits are rare with an incidence of 1 in 11,000 people [3, 5]. OP occur unilaterally in 85% of individuals and are usually sporadic [5, 6]. OP are usually singular in nature, but have less commonly been described as multiple pits [7]. While there has been no genetic link with OP, there have been isolated cases describing possible autosomal inheritance in pedigrees of multiple affected family members [8, 9]. OPM can occur in 25–75% of these patents especially when the OP is temporally located [3, 10, 11]. OPM often presents during the third to fourth decades, although patients may become symptomatic even earlier [12].
Pathogenesis
Improper Closure of Embryonic Tissue
Congenital OP results from an improper closure of the superior edge of the embryonic fissure [13, 14]. Specifically, it is proposed that the anomalous differentiation of the neuroectodermal folds of the primitive papilla causes the OP [13, 14]. This improper closure results in a temporal pit that may allow access to the retina. Brockhurst, Bonnet, and Postel reported the presence of a small hole overlying the pit in two cases that they believed was a passage for macular fluid [15,16,17]. Lincoff in 1988 proposed that the fluid from OP forms a schisis-like cavity initially in the nerve fiber layer of the retina and then progresses to form retinal holes and outer retinal pathology eventually leading to subretinal tracking [18]. However, subsequent optical coherence tomography (OCT) studies have demonstrated that subretinal fluid can occur without intraretinal schisis associated with OP [19, 20]. Moon et al. hypothesized that accumulation of fluid in the retina depends upon the level where the fluid enters from the optic pit [19]. Histologically, OP are a herniation of dysplastic retina through a defective lamina cribosa excavation into the subarachnoid space [21]. This connection creates a communication between the intraocular and subarachnoid space [21].
Source of Fluid
The source of macular fluid in OPM is controversial [12, 15]. OPM onset is commonly seen in patients around the third or fourth decade, which corresponds to the start of vitreous liquefaction [12, 15]. Tractional vitreous strands and posterior vitreous detachment (PVD) are theorized to be associated with vitreous entrance into the pit. Brown et al. demonstrated that India ink dye injected into the vitreous cavity was found in the subretinal fluid of collie dogs with optic pits [22]. This was the first evidence that vitreous may be the source of fluid in optic pits; however, there were no glycosaminoglycans, a component of the vitreous, present in the subretinal fluid [22]. Histopathologic studies of two eyes with OPM however did demonstrate mucopolysaccharides, another component of the vitreous [21]. Postel et al. proposed that there may be a pocket of liquefied vitreous over the abnormally developed optic nerve and that tractional forces allow for liquefied vitreous to enter into the retina [17]. Several studies have reported in patients with OPM the migration of silicone oil or gas into the subretinal space that was in the vitreous cavity [23,24,25]. In conjunction with theories of the vitreous entering the retina, it has been postulated that pressure gradient differences between the intraocular pressure and intracranial pressure allows for the vitreous to enter into the OP leading to maculopathy [26, 27]. However, while large studies have demonstrated that majority of OPM occur in patients with PVD, few cases especially in children have also reported the presence of OP without posterior vitreous detachments or liquification [10, 26, 28, 29]. OCT studies have also demonstrated cases with OPM without vitreous traction [20, 29, 30]. Imaging and histopathologic studies have failed to demonstrate a clear connection between the subretinal space and the vitreous via the OP [22].
Another postulated source of retinal fluid is cerebrospinal fluid. An OCT-based study by Krivoy et al. demonstrated a direct communication between the optic pit cavity and the subarachnoid space [31]. Studies involving pars plana vitrectomy and gas infusion have demonstrated gas bubbles penetrating the optic nerve sheath, further supporting a connection between optic pits and the subarachnoid space [32]. Kuhn et al. reported a patient with OPM previously treated with vitrectomy and silicone oil, and later developed intracranial migration of silicone oil from the OP [33]. However, in another study where a patient was injected with intrathecal fluorescein did not demonstrate a spread to the subretinal space via the OP [34].
Leakage of vessels near the OP is a third theory for the source of OMP. The association between OPM and cilioretinal arteries had been described previously. Fluorescein angiography with late hyperfluorescence at the OP was the first clue regarding this theory of leaking vessels, although this phenomenon does not occur with all patients. Theodosssiadis et al. reported cilioretinal arteries accompanying 64% of cases with OP, as opposed to 30% in eyes without OP [35]. Adams et al. recently described leaking, anomalous cilioretinal vessels in and around the optic cavity using swept-source optical coherence tomography angiography (SS-OCT) and fluorescein angiography [36•].
Other Possible Associations
An OP was described in one eye of a patient with incontinentia pigmenti [37]. OP has also been associated with basal encephaloceles along with other optic nerve anomalies [38]. OP have also been reported in cases of midline neurological developmental malformations [39]. Aicardi syndrome and Alagille syndrome have also been associated with OP [40,41,42,43]. Alagille syndrome is caused by an autosomal dominant mutation in the JAG1 gene, a critical signaling pathway for development [41]. Bilateral renal hypoplasia has also been associated with OP [43]. OPM has also been described in the literature in the setting of trauma [44,45,46]. One report has proposed laser in situ keratomileusis (LASIK) associated with OPM [47].
Clinical Features
Symptoms and Clinical Presentation
OP has been reported in all ages ranging from 3 to 82 years old, with mean age being 35 years [48]. The size of OP can range from 1/10th or 7/10ths of the disc size [42]. Posterior vitreous detachment can exist in less than 10% of patients with OP [48]. While OP themselves may be asymptomatic, visual disturbances usually occur due to macular involvement. Visual acuity changes can be gradual. There have been reports of visual changes that occur with positional changes such as bending. Visual field loss and color changes have also been described in these patients [15, 49, 50]. Maculopathy affected 84% of patients with OP, with 20% of those affected being asymptomatic [48]. Visual acuity can range from log MAR − 0.04 to 2, mean being 0.54 (estimated conversion to Snellen range being 20/20 to counting fingers, average being 20/60) [48]. Spherical equivalent refractive error was between − 7 D and + 8 D, mean being − 0.10 D [48].
Imaging
Multimodal imaging can be used to guide management of OPM. Established imaging techniques include fundus photography, autofluorescence, fluorescein angiography, and visual fields as well as optical coherence tomography (OCT) and OCT angiography (OCTA). Fundus autofluorescence (FAF) is an adjunct tool in imaging patient with OPM. Serous detachments of the macula from OP may develop subretinal flecks with outer retinal changes, and FAF can help detect the fluorophore levels in the subretinal space and RPE [51]. Visual fields deficits include an arcuate scotoma from displacement of nerve fibers by the OP, and central scotomas from serous detachment from OPM [3, 10]. Lincoff et al. demonstrated that mild scotomas may occur when inner retinal separation occurs, but dense, large central scotomas may occur when the outer layer is detached [52]. Fluorescein angiography (FA) techniques demonstrate OP hypofluorescence in the early phases and staining in the late phases [53]. Theodossiadis et al. reported that there was increased hyperfluorescence in the late phases of patients with OPM attributed to leakage of the dye into the retinal schisis cavity and subretinal fluid [54].
Multiple studies of OP have used OCT to capture the structural changes and the excavations [55,56,57]. The use of OCT has enhanced our knowledge on the optic nerve head and theories on OPM. Theodossiadis et al. demonstrated vitreous abnormalities including vitreomacular traction, vitreous strands, and partial or complete PVD in patients with OP using OCT [58]. They concluded that the development of maculopathy was likely due to the role of the vitreous tractional forces [58]. More recently, Lorusso et al. using SD-OCT demonstrated spontaneous resolution of OPM over 3 months after the development of PVD [59]. On the other hand, enhanced depth imaging OCT by Gowdar et al. demonstrated connectivity between the macular schisis and lamina cribrosa in OP supporting the theory that fluid comes from the cerebrospinal fluid [60]. Imamura et al. characterized retinal manifestations of OPM using high-resolution OCT in 16 patients, concluding that the fluid from OP can do directly below the internal limiting membrane into the inner retinal, outer retinal, or subretinal space [20]. They reported the outer nuclear layer to be the most commonly affected area of the retina, and did not find holes in the OP to be frequent [20]. Roy et al. also confirmed that all eyes in their study had fluid in the outer nuclear layer, and about half of the eyes had concurrent subretinal fluid along with outer retinoschsis [61]. Michalewski et al. demonstrated in 20 eyes using 3-dimensional spectral-domain OCT that the subretinal and intraretinal fluid in OPM may have both a vitreous and cerebrospinal origin [62]. Intraoperative OCT can also play a major role during surgery with assisting in guiding and managing membranes during vitrectomy [63].
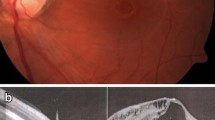
OCTA is a useful clinical tool to understand the structural and vascular mechanisms in OPM. Recent studies by Bach et al. and others demonstrated capillary dropout in a pediatric patient with OP using OCTA [64]. Recently, Michalewska et al. used SS-OCTA to predict outcomes of patients with OPM undergoing vitrectomy with stuffing of the OP with ILM, focusing on the superficial foveal avascular zones before and after surgery [65••]. Adams et al. demonstrated anomalous vessels in and around the optic pit in patients with OPM using SS-OCTA [36•]. The current review demonstrates a patient with OPM imaged using en face OCTA and corresponding b-scans to demonstrate OPM (Fig. 1).

source optical coherence tomography (PLEX Elite 9000, Carl Zeiss Meditec, Dublin, CA), 12 × 12 en face structural image demonstrates macular striae corresponding to macular schisis. Color thickness map (bottom, left) demonstrates macular thickening. Corresponding B-scan demonstrates retinal schisis that tracks from the optic pit; there is subretinal fluid with retinal break. This example demonstrates that utility of widefield swept-source optical coherence tomography. Six-month follow-up demonstrated stable visual acuity and imaging findings
Outer retinal atrophy in optic pit maculopathy. Optic pit maculopathy (OPM) of the right eye in a 24-year-old man. He presented with a visual acuity of 20/100. Fundus color photography of the right eye demonstrates a temporal optic pit. Using swept
Management Options
Observation
Spontaneous resolution of OPM has been described in multiple case reports and series [66,67,68]. The rate of spontaneous regression has been estimated to be 25% of cases [66,67,68]. Sugar in 1967 reported 8 eyes with spontaneous resolution over a 2-month to 10-year follow-up period; of those 8 eyes, 5 eyes regained a visual acuity of 20/30 or better [66]. Yuen et al. reported an 8-year old girl presenting with a visual acuity of count fingers in the left eye with OPM [67]. After 1 year of observation, serous maculopathy resolved with residual retinal pigment epithelial changes and a visual acuity of 20/30 [67]. Parikakis et al. in 2014 reported a 63-year-old female with a 6-month history of blurry vision in her left eye, with a visual acuity of 6/24 and serous macular detachment associated with OPM in the left eye [68]. At 3-year follow-up, patient presented with complete resolution of macular fluid and a visual acuity of 6/12. The authors concluded that spontaneous resolution after long-standing macular detachment can result in favorable visual acuities depending on the integrity of the IS/OS layer, which should be used as a prognostic factor for visual acuity and necessity of surgical management [68]. Spontaneous regression of OPM with visual recovery over a 6-month period in pediatric populations has also been reported. Tzu et al. described multiple cases of OPM with schisis and subretinal fluid with stability in anatomy and visual acuity over time [69]. One particular case of OPM had been followed with stability for over 6 years, and while the maculopathy progressed, visual acuity remained stable ranging from 20/20 to 20/30 [69]. The favorable prognosis was likely because the schisis-cavity did not affect the foveal center [69]. The authors continued to follow this patient for more than 20 years (Fig. 2).
A Observational management of optic pit maculopathy (OPM) of the left eye in a 39-year-old woman. Optical coherence tomography (OCT) for over 15 years with shifting of the macular fluid without intervention. The subretinal fluid and intraretinal fluid, and visual acuity continued to remain stable at 20/30 despite the changes in maculopathy. B Vitrectomy, PVD induction, ILM peeling and flap, endolaser, and gas versus observation for bilateral optic pit maculopathy optic pit maculopathy (OPM) of both eyes in a 10-year-old girl. Fundus photography of the right eye demonstrated a small pit superotemporally; pre-operative OCT revealed subretinal fluid, intraretinal fluid, and an outer retinal defect. Three years later, the right eye OCT demonstrated a flat retina with central atrophy and trace intraretinal cysts, with a visual acuity of 20/800 after scleral buckle, pars plana vitrectomy with posterior vitreous detachment, glial tissue removal, internal limiting membrane (ILM) peel, endolaser to the region of the pit, and C3F8 gas was exchanged. Fundus photography of the left eye on presentation demonstrates a small optic pit, and OCT revealed intraretinal cystic fluid with an outer retinal break. Over 3 years, the visual acuity improved to 20/40 in the left eye, and OCT demonstrated resolution of the maculopathy with observation only. C Vitrectomy, PVD induction, endolaser, and gas for optic pit maculopathy. OPM of the right eye in a 5-year-old girl with superotemporal optic pit, macular atrophy temporal to the disc, and OCT demonstrated subretinal fluid tracking from the optic pit as well as intraretina schisis. Two years after patient underwent pars plana vitrectomy, posterior hyaloid detachment, endolaser around the pit region, and C3F8 gas, vision was 20/60. Areas of pigment changes where the laser was applied and OCT demonstrated persistent subretinal and intraretinal fluid
Laser Photocoagulation
Anatomical and visual outcomes of laser treatment vary significantly. In previous decades, laser photocoagulation was applied to the temporal edge of the optic disc and the laser scars theoretically create a barrier between the OP and subretinal space, preventing the entrance of fluid into the macula. Gass used xenon photocoagulation along the temporal disc margin to minimize fluid going into the macula but this treatment was not successful [70]. Mustonen and Brockhurst reported the use of argon laser photocoagulation with some success anatomically, but with variable visual acuity improvements [15, 71]. Few studies have shown resolution of maculopathy in patients after krypton laser therapy [72, 73]. Lincoff et al. reported that laser application showed either no response to treatment or slight improvement [74]. Although some success, laser treatment option has had overall low success rates with significant visual field defects reported and visual acuity not recovered [75]. This is likely due to the fact that laser absorption by the retinal pigment epithelium and choroid, possibly not affecting the macular schisis [72].
Acetazolamide
Acetazolamide use was first reported in a pediatric patient with incontinetia pigmenti and OPM [37]. Over 1 year, the use of oral acetazolamide resulted in complete resolution of subretinal fluid and improvement in visual acuity [37]. By inhibiting carbonic anhydrase enzyme, the mechanism is speculated to be due to a decrease in cerebrospinal fluid production, and would be effective if the course of fluid is due to communication of the OP with the subarachnoid space. Since then, other case reports and series have advocated for oral acetazolamide as primary or adjunct treatment [76•, 77, 78]. Osigian et al. more recently described successful treatment of adjuvant oral acetazolamide along with pars plana vitrectomy, posterior hyaloid peel, internal limiting membrane peel, fluid-air exchange, endolaser, and gas tamponade [76•]. This pediatric patient improved from 20/400 to 20/60 with this approach [76•]. Although the response may be rapid and sustained with low-dose acetazolamide, there can be unwanted systemic adverse events in higher dosages.
Gas Tamponade vs Gas Tamponade With or Without Laser Photocoagulation
Lincoff et al. described the use of gas displacement for OPM in 3 patients [79]. Using OCT, the authors reported temporary anatomic success with gas tamponade, which was not sustained because of persistent flow of fluid from the pit [79]. Akiyama et al. described a small group of patients receiving gas tamponade, with complete retinal reattachment occurring in half of those patients and requiring more than 1 injection in certain cases [80]. After an average follow-up period of 94 months, there were no recurrences after complete reattachment by gas tamponade [80]. Lei et al. reported treatment of 9 eyes with combination of intravitreal C3F8 gas and temporal disc laser photocoagulation; they described this technique as an effective and minimally invasive solution to OPM, reporting reduction or resolution of fluid in all eyes with good visual outcomes [81].
Macular Buckling
Theodossiadis et al. first used macular buckling technique using a scleral sponge fixed at the posterior pole corresponding to the area of macular schisis from OP [82, 83]. Macular buckling has reported a success rate of 85% regarding fluid absorption after a follow-up period of 13 years [83]. Macular buckling may be a beneficial technique independent of the source of fluid of OPM. Macular buckling results in an inward force of the macula, relieving vitreous traction on the macula. Long-term follow-up has demonstrated success over 10 years, with low rates of complications and stable vision [82]. Theodossiadis also used OCT to show restoration of the foveal outer retinal layers and photoreceptor layers using macular buckling over time [84]. This approach has not been widely utilized.
Pars Plana Vitrectomy (PPV)
PPV is a common option for treatment of OPM [85]. Steel et al. in a 2-year nationwide prospective population-based study reported surgery to be anatomically successful in 75% of cases [48]. OCT studies have reported multilayer intraretinal and subretinal fluid outcomes after surgery [86]. Rayat et al. used triamcinolone in some cases to aide posterior hyaloid removal [87]. There are many combinations of vitrectomy with adjunct techniques that are described below.
-
a.
PPV with posterior hyaloid removal
The induction of a PVD by PPV may release the vitreous traction from the vitreous, and assist in resolution of macular fluid from OPM. PPV with PVD induction has been described by multiple studies [30, 88,89,90,91]. Hirakata et al. described 8 patients who underwent PPV with PVD induction without gas tamponade and laser photocoagulation; 7 of the 8 patients achieved anatomic success and visual improvement after PPV alone [90]. Teke and Citirik in 2015 reported 10 eyes underwent 23-g PPV, endolaser, and gas tamponade versus 7 eyes that underwent 23-g PPV [92]. They reported no difference in postoperative visual acuity and SRF in the central foveal region [92].
-
b.
PPV with ILM peeling
Talli et al. described 8 patients who underwent PPV, ILM peeling, and tamponade with SF6 without endolaser for OPM; 7 of the 8 patients had complete resolution of subretinal fluid, mean visual acuity improved from 20/83 to 20/40, and mean foveal thickness improved from 973 microns to 363.5 microns [91].
-
c.
PPV with ILM inverted flap
Mohammed and Pai first described the inverted autologous ILM flap procedure for OPM in 2013, in which they peeled the ILM adjacent to the superotemporal edge of the optic disc and inverted over the optic disc [93]. Hara in 2017 described their refined ILM inverted flap technique by creating the ILM flap temporal to the optic disc edge around 1.5-disc diameters excluding the fovea and covering the optic disc and OP [94]. Sborgia et al. recently described a case report using ILM-flap for OPM, and found OCT to be a tool in assisting surgical maneuvers [95]; while visual acuity and multifocal electroretinography results improved after performing PPV with inverted ILM-flap technique, microperimetry analysis reduced in this patient during follow-up [95]. The ILM flap works as an effective plug to reduce fluid in OPM. This technique is promising but evidence is mainly with case reports.
-
d.
Glial tissue peeling
Inoue et al. presented a case of OPM treated with PPV, PVD induction, and glial tissue removal; the patient’s visual acuity improved to 20/20 from 20/200, retinoschisis resolved, there were no visual field defects, and results sustained for 10 years without recurrence [96]. Yannuzzi et al. reported a 31-year-old woman with OPM who underwent PPV with ILM peeling, laser photocoagulation, and removal of OP plug [97]. The histopathologic examination of the glial plug showed fibrovascular neural tissue and spindle-shaped cells [97]. The fibroglial tissue may contribute to continuous vitreous traction on the pit, and removing it may make it possible to relieve vitreous traction and to seal the connection at the OP via wound healing.
-
e.
OP plugging
In 2013, Travassos et al. described their novel surgical technique of scleral plugging of OP [98]. After a failed attempt at removing fibroglial tissue and closing the OP communication with autologous blood, the blood clot reabsorbed and a scleral plug was chosen to close the pit communication [98]. The scleral plug remained in the correct position after at least 1 year of follow-up and visual improvement was seen in the three cases [98]. Rosenthal et al. reported one case of a patient with OPM who underwent PPV with autologous platelets injected into the optic pit with successful anatomic and functional results [99]. Ozdek et al. described the use of autologous fibrin injected over the optic pit to seal it in two cases with anatomic success as well [100]. Recently, Rizzo et al. used a human amniotic membrane patch implanted inside the optic pit in three patients; subretinal fluid gradually resolved over 6 months, and visual acuity improved after surgery without recurrence during a 6-month follow-up period [101].
-
f.
PPV with endolaser vs with endolaser and gas/oil tamponade
Rizzo et al. in 2011 reported outcomes of 10 patients with OPM undergoing small-gauge PPV, gas tamponade, and laser photocoagulation [102]. Complete retina reattachment was observed in 5 of the 10 patients and 7 of the 10 patients gained 2 lines of vision [102]. Rayat et al. in 2015 reported a retrospective chart review of 32 eyes with OPM, all undergoing PPV with PVD induction; 8 eyes also underwent ILM peeling, 7 eyes underwent endolaser, and 31 eyes underwent gas tamponade [87]. Mean visual acuity improved by 5 lines, and foveal attachment was achieved in 81% of eyes [87]. Adjunct techniques such as ILM peel and temporal endolaser did not improve outcomes. Increased central retinal thickness was found to be a poor surgical prognostic sign. Avci et al. reported 13 eyes with OPM undergoing PPV, PVD induction, endolaser to temporal margin of the optic disc, and gas tamponade without ILM peeling; anatomic success was achieved in all 12 eyes and 2 lines or more improvement in 11 eyes [103]. Gosh et al. described one case of OPM treated with PPV, posterior hyaloid peeling, endolaser, and silicone oil with good anatomic reattachment. Kuhn et al. described a case report of a patient with proliferative diabetic retinopathy treated with silicone oil and OPM, demonstrate migration of silicone oil into the subarachnoid space [104].
-
g.
PPV with retinal fenestration
Moon et al. observed that juxtapapillary thickening with fluid concurrently entering the retinal stroma and subretinal space [19]. Ooto et al. used the concept of juxtapapillary thickening to report the first large study describing inner retinal fenestration for the treatment of OPM [105]. Inner retinal fenestration involves making partial thickness retinal holes radial to OP that resulted in redirection of the fluid flow, allowing egress of fluid into the vitreous. They performed this novel technique in 18 eyes following the core vitrectomy [105]. No eyes had ILM peeling or peripapillary laser, and only a few patients had posterior hyaloid removal. Ninety-four percent of patients had complete resolution of the fluid without the need for additional treatment. Best corrected visual acuity improved post-operatively to a mean 20/48. Thus, this procedure had anatomic and functional improvement without need additional treatments [105].
Discussion
The rarity of this entity along with the lack of prospective controlled clinical trials comparing different techniques and unclear pathogenic mechanisms makes OPM management challenging despite multiple treatment options available. There is no consensus regarding surgical intervention and the ideal timing for surgery. Since there are no guidelines, the risks and benefits of each strategy must be considered along with the patient’s history, age, vision, and foveal integrity. In patients with good visual acuity and minimal symptoms, the best management strategy may be observation. In patients with moderate visual loss, observation can also be considered because the clinical course can be variable with possible spontaneous recovery. For more advanced vision loss especially when progressive, treatment options should be considered. Laser photocoagulation at the border of the disc has often been felt to be inadequate. Acetazolamide may have some benefit in reducing maculopathy, but the effects are temporary. Macular buckling was described prior to modern vitreoretinal surgical procedures and is no longer a clinically utilized technique. Vitrectomy with removal of the posterior hyaloid and vitreous traction is an often utilized technique which can be supplemented with ILM peel, fenestration, gas, laser, and other strategies. Silicone oil has the risk of severe complication including migration of oil droplets into the subarachnoid space. Surgery is probably contraindicated in eyes with advanced RPE atrophy and outer retinal loss.
Conclusion
In this overview of management options for OPM, the pros and cons of each approach have been discussed. Caution should be exercised in surgery when the eye with OPM is the only functional eye. Individualized patient care can be based on specific patient clinic findings, potential risks, probable benefits, and cost of each option. The physicians should consider the patient’s visual needs, visual disability, and general health when deciding between observation and treatment. Analysis from evidence-based outcomes and ongoing clinical trials and future results will add to our ability to make the best decisions for our patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wiethe T. Ein Fall von angeborener Deformitat der Sehnervenpapille. Arch Augenheilkd. 1882;11:14–9.
Halbertsma KT. Crater-like hole and coloboma of the disc associated with changes at the macula. Br J Ophthalmol. 1927;11(1):11–7.
Kranenburg EW. Crater-like holes in the optic disc and central serous retinopathy. Arch Ophthalmol. 1960;64:912–24.
Petersen HP. Pits or crater-like holes in the optic disc. Acta Ophthalmol (Copenh). 1958;36(3):435–43.
Gass JDM. Serous detachment of the macula: secondary to congenital pit of the optic nervehead. Am J Ophthalmol. 1969;67(6):821–41.
Theodossiadis G, Panopoulos M, Kollia A, Georgopoulos G. Long-term study of patients with congenital pit of the optic nerve and persistent macular detachment. Acta Ophthalmol. 1992;70(4):495–505.
Babu N, Baliga G, Kohli P, Ramasamy K. Management of double optic disc pit complicated by maculopathy. Indian J Ophthalmol. 2020;68(4):663–5.
Stefko ST, Campochiaro P, Wang P, Li Y, Zhu D, Traboulsi EI. Dominant inheritance of optic pits. Am J Ophthalmol. 1997;124(1):112–3.
Slusher MM, Weaver RG Jr, Greven CM, Mundorf TK, Cashwell LF. The spectrum of cavitary optic disc anomalies in a family. Ophthalmology. 1989;96(3):342–7.
Brown GC, Shields JA, Goldberg RE. Congenital pits of the optic nerve head: II. Clinical studies in human. Ophthalmology. 1980;87(1):51–65.
Bonnet M. Serous macular detachment associated with optic nerve pits. Graefe’s Arch Clin Exp Ophthalmol. 1991;229(6):526–32.
Brodsky MC. Congenital optic disk anomalies. Surv Ophthalmol. 1994;39(2):89–112.
Giuffrè G. Optic pit syndrome. Documenta Ophthalmol Adv Ophthalmol. 1986;64(2):187–99.
Apple DJ. New aspects of colobomas and optic nerve anomalies. Int Ophthalmol Clin. 1984;24(1):109–21.
Brockhurst RJ. Optic pits and posterior retinal detachment. Trans Am Ophthalmol Soc. 1975;73:264–91.
Bonnet M. Serous macular detachment associated with optic nerve pits. Graefe’s Arch Clin Exp Ophthalmol. 1991;229(6):526–32.
Postel EA, Pulido JS, McNamara JA, Johnson MW. The etiology and treatment of macular detachment associated with optic nerve pits and related anomalies. Trans Am Ophthalmol Soc. 1998;96:73–88; discussion 88–93.
Lincoff H, Lopez R, Kreissig I, Yannuzzi L, Cox M, Burton T. Retinoschisis associated with optic nerve pits. Arch Ophthalmol. 1988;106(1):61–7.
Moon SJ, Kim JE, Spaide RF. Optic pit maculopathy without inner retinal schisis cavity. Retina (Philadelphia, Pa). 2006;26(1):113–6.
Imamura Y, Zweifel SA, Fujiwara T, Freund KB, Spaide RF. High-resolution optical coherence tomography findings in optic pit maculopathy. Retina (Philadelphia, Pa). 2010;30(7):1104–12.
Ferry AP. Macular detachment associated with congenital pit of the optic nerve head. Pathologic findings in two cases simulating malignant melanoma of the choroid. Arch Ophthalmol. 1963;70:346–357.
Brown GC, Shields JA, Patty BE, Goldberg RE. Congenital pits of the optic nerve head. I. Experimental studies in collie dogs. Arch Ophthalmol. 1979;97(7):1341–1344.
Dithmar S, Schuett F, Voelcker HE, Holz FG. Delayed sequential occurrence of perfluorodecalin and silicone oil in the subretinal space following retinal detachment surgery in the presence of an optic disc pit. Arch Ophthalmol. 2004;122(3):409–411.
Salam A, Khan-Lim D, Luff AJ. Superior retinal detachment in an oil-filled eye with a colobomatous optic disc. Retinal Cases Brief Rep. 2008;2(2):124–5.
Coll GE, Chang S, Flynn TE, Brown GC. Communication between the subretinal space and the vitreous cavity in the morning glory syndrome. Graefe’s Arch Clin Exp Ophthalmol. 1995;233(7):441–443.
Jain N, Johnson MW. Pathogenesis and treatment of maculopathy associated with cavitary optic disc anomalies. Am J Ophthalmol. 2014;158(3):423–35.
Johnson TM, Johnson MW. Pathogenic implications of subretinal gas migration through pits and atypical colobomas of the optic nerve. Arch Ophthalmology (Chicago, Ill : 1960). 2004;122(12):1793–1800.
Rii T, Hirakata A, Inoue M. Comparative findings in childhood-onset versus adult-onset optic disc pit maculopathy. Acta Ophthalmol. 2013;91(5):429–33.
Hirakata A, Okada AA, Hida T. Long-term results of vitrectomy without laser treatment for macular detachment associated with an optic disc pit. Ophthalmology. 2005;112(8):1430–5.
Theodossiadis PG, Grigoropoulos VG, Emfietzoglou J, Theodossiadis GP. Vitreous findings in optic disc pit maculopathy based on optical coherence tomography Graefe’s Arch Clin Exp Ophthalmol. 2007;245(9):1311–1318.
Krivoy D, Gentile R, Liebmann JM, et al. Imaging congenital optic disc pits and associated maculopathy using optical coherence tomography. Arch Ophthalmol. 1996;114(2):165–170.
Irvine AR, Crawford JB, Sullivan JH. The pathogenesis of retinal detachment with morning glory disc and optic pit. Trans Am Ophthalmol Soc. 1986;84:280–92.
Kuhn F, Kover F, Szabo I, Mester V. Intracranial migration of silicone oil from an eye with optic pit. Graefe’s Arch Clin Exp Ophthalmol. 2006;244(10):1360–1362.
Kalina RE, Conrad WC. Letter: Intrathecal fluorescein for serous macular detachment. Arch Ophthalmol. 1976;94(8):1421.
Theodossiadis GP, Kollia AK, Theodossiadis PG. Cilioretinal arteries in conjunction with a pit of the optic disc. Ophthalmologica Journal international d’ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 1992;204(3):115–21.
• Adams MK, Cohen SY, Souied EH, et al. Multimodal imaging of choroidal and optic disk vessels near optic disk pits. Retinal Cases Brief Rep. 2020;14(4):289–296. This case series demonstrated well anomalous vessels near the optic pit using multimodal imaging.
Prasad PS, Shah SP, Reddy S, Hubschman JP. Resolution of serous maculopathy associated with optic disk pits and incontinentia pigmenti using oral acetazolamide. Retinal Cases Brief Rep. 2011;5(3):267–9.
Caprioli J, Lesser RL. Basal encephalocele and morning glory syndrome. Br J Ophthalmol. 1983;67(6):349–51.
Corbett JJ, Savino PJ, Schatz NJ, Orr LS. Cavitary developmental defects of the optic disc. Visual loss associated with optic pits and colobomas. Arch Neurol. 1980;37(4):210–213.
Fea A, Grosso A, Rabbione M, Grignolo F. Alagille syndrome and optic pit. Graefe’s Arch Clin Exp Ophthalmol. 2007;245(2):315–317.
Kim BJ, Fulton AB. The genetics and ocular findings of Alagille syndrome. Semin Ophthalmol. 2007;22(4):205–10.
Reed ODDD. Congenital pits of the optic nerve. Clin Eye Vision Care. 1999;11(2):75–80.
Asensio Sánchez V, Corral Azor A, Bartolomé Aragón A, Paz García Md. Síndrome renal-coloboma. Arch Soc Esp Oftalmol. 2002;77(11):635–637.
Meyer CH, Rodrigues EB. Optic disc pit maculopathy after blunt ocular trauma. Eur J Ophthalmol. 2004;14(1):71–3.
Colyer MH, Weichel ED, Ward TP. Blast injury-associated optic disc pit maculopathy. Br J Ophthalmol. 2007;91(4):558.
Hirakata A, Hida T, Wakabayashi T, Fukuda M. Unusual posterior hyaloid strand in a young child with optic disc pit maculopathy: intraoperative and histopathological findings. Jpn J Ophthalmol. 2005;49(3):264–6.
Rodriguez-Coleman H, Schiff WM, Hwang JC, Speaker MG. Optic pit maculopathy after laser-assisted in situ keratomileusis. Can J Ophthalmol J Can D’ophtalmol. 2007;42(1):123–4.
Steel DHW, Suleman J, Murphy DC, Song A, Dodds S, Rees J. Optic disc pit maculopathy: a two-year nationwide prospective population-based study. Ophthalmology. 2018;125(11):1757–64.
Theodossiadis GP, Panopoulos M, Kollia AK, Georgopoulos G. Long-term study of patients with congenital pit of the optic nerve and persistent macular detachment. Acta Ophthalmol (Copenh). 1992;70(4):495–505.
Gordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. Br J Ophthalmol. 1969;53(7):481–9.
Laud K, Visaetsilpanonta S, Yannuzzi LA, Spaide RF. Autofluorescence imaging of optic pit maculopathy. Retina (Philadelphia, Pa). 2007;27(1):116–9.
Lincoff H, Lopez R, Kreissig I, Yannuzzi L, Cox M, Burton T. Retinoschisis associated with optic nerve pits. Arch Ophthalmol. 1988;106(1):61–67.
Kalogeropoulos D, Ch’ng SW, Lee R, et al. Optic disc pit maculopathy: a review. Asia-Pac J Ophthalmol. 2019;8(3):247–255.
Theodossiadis GP, Ladas ID, Panagiotidis DN, Kollia AC, Voudouri AN, Theodossiadis PG. Fluorescein and indocyanine green angiographic findings in congenital optic disk pit associated with macular detachment. Retina (Philadelphia, Pa). 1999;19(1):6–11.
Zaidi AA, Brucker AJ, Johnson MW. Diagnostic and therapeutic challenges. Retina (Philadelphia, Pa). 2011;31(10):2125–8.
Hedels C, Krohn J. Enhanced depth imaging optical coherence tomography of optic disc maculopathy without a visible optic pit. Clin Exp Ophthalmol. 2013;41(9):894–6.
Spaide RF, Costa DL, Huang SJ. Macular schisis in a patient without an optic disk pit optical coherence tomographic findings. Retina (Philadelphia, Pa). 2003;23(2):238–40.
Theodossiadis PG, Grigoropoulos VG, Emfietzoglou J, Nikolaidis P, Theodossiadis GP. Optical coherence tomography study of vitreoretinal interface in full thickness macular hole associated with optic disk pit maculopathy. Eur J Ophthalmol. 2007;17(2):272–6.
Lorusso M, Zito R, Micelli Ferrari L, et al. Spontaneous resolution of optic pit maculopathy: an OCT report. Ther Adv Ophthalmol. 2020;12:2515841420950843.
Gowdar JP, Rajesh B, Giridhar A, Gopalakrishnan M, Hussain R, Thachil T. An insight into the pathogenesis of optic disc pit–associated maculopathy with enhanced depth imaging. JAMA Ophthalmol. 2015;133(4):466–9.
Roy R, Waanbah AD, Mathur G, Raman R, Sharma T. Optical coherence tomography characteristics in eyes with optic pit maculopathy. Retina (Philadelphia, Pa). 2013;33(4):771–5.
Michalewski J, Michalewska Z, Nawrocki J. Spectral domain optical coherence tomography morphology in optic disc pit associated maculopathy. Indian J Ophthalmol. 2014;62(7):777–81.
Ehlers JP, Kernstine K, Farsiu S, Sarin N, Maldonado R, Toth CA. Analysis of pars plana vitrectomy for optic pit–related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011;129(11):1483–6.
Bach A, Cavuoto KM, Capo H. Visualization of capillary dropout emanating from an optic disc pit using optical coherence tomography angiography. JAMA Ophthalmol. 2018;136(8):e182327–e182327.
•• Michalewska Z, Nawrocka Z, Nawrocki J. Swept-source OCT and swept-source OCT angiography before and after vitrectomy with stuffing of the optic pit. Ophthalmol Retina. 2020;4(9):927-937. Using swept-source OCT and OCTA, the authors were able to demonstrate improvements in anatomy and visual acuity when performing vitrectomy with optic pit stuffing using internal limiting membrane.
Sugar HS. Congenital pits in the optic disc and their equivalents (congenital colobomas and colobomalike excavations) associated with submacular fluid. Am J Ophthalmol. 1967;63(2):298–307.
Yuen CH, Kaye SB. Spontaneous resolution of serous maculopathy associated with optic disc pit in a child: a case report. J AAPOS. 2002;6(5):330–1.
Parikakis EA, Chatziralli IP, Peponis VG, et al. Spontaneous resolution of long-standing macular detachment due to optic disc pit with significant visual improvement. Case Rep Ophthalmol. 2014;5(1):104–10.
Tzu JH, Flynn HW Jr, Berrocal AM, Smiddy WE, Murray TG, Fisher YL. Clinical manifestations of optic pit maculopathy as demonstrated by spectral domain optical coherence tomography. Clin Ophthalmol (Auckland, NZ). 2013;7:167–72.
Gass JD. Serous detachment of the macula. Secondary to congenital pit of the optic nervehead. Am J Ophthalmol. 1969;67(6):821–841.
Mustonen E, Varonen T. Congenital pit of the optic nerve head associated with serous detachment of the macula. Acta Ophthalmol. 1972;50(5):689–98.
Theodossiadis G. Treatment of retinal detachment with congenital optic pit by krypton laser photocoagulation. Graefe’s Arch Clin Exp Ophthalmol. 1988;226(3):299–299.
Annesley W, Brown G, Bolling J, Goldberg R, Fischer D. Treatment of retinal detachment with congenital optic pit by krypton laser photocoagulation. Graefe’s Arch Clin Exp Ophthalmol. 1987;225(5):311–4.
Lincoff H, Yannuzzi L, Singerman L, Kreissig I, Fisher Y. Improvement in visual function after displacement of the retinal elevations emanating from optic pits. Arch Ophthalmol. 1993;111(8):1071–9.
Monin C, Le Guen Y, Morel C, Haut J. Treatment of coloboma pitS of the optic nerve complicated by serous detachment of the neuroepithelium. J Francais D’ophtalmol. 1994;17(10):574–9.
• Osigian CJ, Gologorsky D, Cavuoto KM, Berrocal A, Villegas V. Oral acetazolamide as a medical adjuvant to retinal surgery in optic disc pit maculopathy in a pediatric patient. Am J Ophthalmol Case Rep. 2020;17:100599. Description of a a combining surgical management with oral acetazolamide to treat optic nerve pit maculopathy suggesting a role for systemic carbonic anhydrase inhibitor decreasin fluid associated with optic pit.
Martins D, Bacalhau C, Santos M. Etiology and treatment of serous macular detachment associated with optic pit–clinical case. 2012.
Mehta RA, Mehta NR, Mehta S, Mehta A. Acetazolamide assisted rapid resolution of optic disc pit maculopathy in a 15 year old child. Indian J Clin Exp Ophthalmol. 2020;6(4):650–3.
Lincoff H, Kreissig I. Optical coherence tomography of pneumatic displacement of optic disc pit maculopathy. Br J Ophthalmol. 1998;82(4):367–72.
Akiyama H, Shimoda Y, Fukuchi M, et al. Intravitreal gas injection without vitrectomy for macular detachment associated with an optic disk pit. Retina (Philadelphia, Pa). 2014;34(2):222–7.
Lei L, Li T, Ding X, et al. Gas tamponade combined with laser photocoagulation therapy for congenital optic disc pit maculopathy. Eye (Lond). 2015;29(1):106–14.
Theodossiadis GP, Theodossiadis PG. The macular buckling technique in the treatment of optic disk pit maculopathy. Semin Ophthalmol. 2000;15(2):108–15.
Theodossiadis GP, Chatziralli IP, Theodossiadis PG. Macular buckling in optic disc pit maculopathy in association with the origin of macular elevation: 13-year mean postoperative results. Eur J Ophthalmol. 2015;25(3):241–8.
Theodossiadis GP, Grigoropoulos VG, Liarakos VS, Rouvas A, Emfietzoglou I, Theodossiadis PG. Restoration of the photoreceptor layer and improvement of visual acuity in successfully treated optic disc pit maculopathy: a long follow-up study by optical coherence tomography. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(7):971–9.
Cox MS, Witherspoon CD, Morris RE, Flynn HW. Evolving techniques in the treatment of macular detachment caused by optic nerve pits. Ophthalmology. 1988;95(7):889–96.
Steel DH, Williamson TH, Laidlaw DA, et al. Extent and location of intraretinal and subretinal fluid as prognostic factors for the outcome of patients with optic disk pit maculopathy. Retina (Philadelphia, Pa). 2016;36(1):110–8.
Rayat JS, Rudnisky CJ, Waite C, et al. Long-term outcomes for optic disk pit maculopathy after vitrectomy. Retina (Philadelphia, Pa). 2015;35(10):2011–7.
Bakri SJ, Beer PM. Vitreoretinal surgery for optic pit associated serous macular detachment: a discussion of two cases. Int Ophthalmol. 2004;25(3):143–6.
Georgalas I, Petrou P, Koutsandrea C, Papaconstadinou D, Ladas I, Gotzaridis E. Optic disc pit maculopathy treated with vitrectomy, internal limiting membrane peeling, and gas tamponade: a report of two cases. Eur J Ophthalmol. 2009;19(2):324–6.
Hirakata A, Inoue M, Hiraoka T, McCuen BW 2nd. Vitrectomy without laser treatment or gas tamponade for macular detachment associated with an optic disc pit. Ophthalmology. 2012;119(4):810–8.
Talli PM, Fantaguzzi PM, Bendo E, Pazzaglia A. Vitrectomy without laser treatment for macular serous detachment associated with optic disc pit: long-term outcomes. Eur J Ophthalmol. 2016;26(2):182–7.
Teke MY, Citirik M. 23 gauge vitrectomy, endolaser, and gas tamponade versus vitrectomy alone for serous macular detachment associated with optic disc pit. Am J Ophthalmol. 2015;160(4):779-785.e772.
Mohammed OA, Pai A. Inverted autologous internal limiting membrane for management of optic disc pit with macular detachment. Middle East Afr J Ophthalmol. 2013;20(4):357–9.
Hara R, Tsukahara Y, Simoyama T, Mori S. Refined internal limiting membrane inverted flap technique for intractable macular detachment with optic disc pit. Case Rep Ophthalmol. 2017;8(1):208–13.
Sborgia G, Recchimurzo N, Sborgia L, et al. Inverted internal limiting membrane-flap technique for optic disk pit maculopathy: morphologic and functional analysis. Retinal Cases Brief Rep. 2021;15(1):31–7.
Inoue M, Shinoda K, Ishida S. Vitrectomy combined with glial tissue removal at the optic pit in a patient with optic disc pit maculopathy: a case report. J Med Case Reports. 2008;2(1):1–4.
Yannuzzi NA, Zhou XY, Monsalve P, Dubovy SR, Smiddy WE. Clinicopathologic correlation of the optic pit glial plug in optic disc pit maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):e287–91.
Travassos AS, Regadas I, Alfaiate M, Silva ED, Proença R, Travassos A. Optic pit: novel surgical management of complicated cases. Retina (Philadelphia, Pa). 2013;33(8):1708–14.
Rosenthal G, Bartz-Schmidt KU, Walter P, Heimann K. Autologous platelet treatment for optic disc pit associated with persistent macular detachment. Graefe’s Arch Clin Exp Ophthalmol. 1998;236(2):151–153.
Ozdek S, Ozdemir HB. A new technique with autologous fibrin for the treatment of persistent optic pit maculopathy. Retinal Cases Brief Rep. 2017;11(1):75–8.
Rizzo S, Caporossi T, Pacini B, De Angelis L, De Vitto ML, Gainsanti F. Management of optic disk pit-associated macular detachment with human amniotic membrane patch. Retina (Philadelphia, Pa). 2020.
Rizzo S, Belting C, Genovesi-Ebert F, et al. Optic disc pit maculopathy: the value of small-gauge vitrectomy, peeling, laser treatment, and gas tamponade. Eur J Ophthalmol. 2012;22(4):620–5.
Avci R, Yilmaz S, Inan UU, et al. Long-term outcomes of pars plana vitrectomy without internal limiting membrane peeling for optic disc pit maculopathy. Eye (Lond). 2013;27(12):1359–67.
Kuhn F, Kover F, Szabo I, Mester V. Intracranial migration of silicone oil from an eye with optic pit. Graefe’s Arch Clin Exp Ophthalmol. 2006;244(10):1360–2.
Ooto S, Mittra RA, Ridley ME, Spaide RF. Vitrectomy with inner retinal fenestration for optic disc pit maculopathy. Ophthalmology. 2014;121(9):1727–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PGI, HWF, KCF, RG: None.
AMB: Alcon, Allergan, Bayer, DORC, Novartis, Phoenix Clinical, Visunex Medical Systems, Zeiss.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Retina
Rights and permissions
About this article
Cite this article
Iyer, P.G., Flynn, H.W., Fan, K.C. et al. Optic Pit Maculopathy: Clinical Features and Management Options. Curr Ophthalmol Rep 9, 158–167 (2021). https://doi.org/10.1007/s40135-021-00274-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40135-021-00274-0