Abstract
Purpose
The purpose of this study is to highlight the changing pattern of retinopathy of prematurity (ROP) incidence with improvement in economy and health care in Africa, pointing out the challenges and recommendations for sustainable, cost-effective screening and management.
Recent Findings
ROP was initially thought to be rare in some parts of Africa. Recent findings have shown this not to be true. Studies done over 2011–2016 reported the presence of any ROP stage in 12–52% of screened babies with the prevalence of treatable ROP at 2.9–9.8%. ROP-trained ophthalmologists available to screen with binocular indirect ophthalmoscope and manage babies are few. Awareness of this blinding disease, disease screening, adequate follow-up, treatment issues, and physician competing duties are the major factors militating against effective ROP programs.
Summary
Creating awareness and collaboration among stakeholders is urgently needed in most parts of Africa. Cost-effective, regional ROP screening program across several contiguous states using a telemedicine approach with widefield retinal imaging by middle-level personnel is strongly advocated to best address the growing problem of ROP in many parts of Africa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Africa is the world’s second largest and second most populous continent after Asia; it has a population of about 1.216 billion (2016) people. Africa has 54 fully recognized sovereign countries. It is geographically broadly divided into North Africa (Algeria, Libya, Mauritania, Morocco, Tunisia, and Egypt) which lies north of the Sahara and runs along the Mediterranean coast and the sub-Saharan regions of East, West, Central, and Southern African. Nigeria, Egypt, and South Africa have the most developed economies [1, 2].
Vision 2020—the right to sight initiative of the World Health Organization (WHO)—recognized retinopathy of prematurity (ROP) as a major cause of blindness in babies. The disease is however neglected in most blindness prevention programs in Africa. It is said to be rare in Sub-Sahara Africa by previous workers [3, 4]. The reason for the assumption of rare ROP was non-survival of preterm babies due to inadequate facilities for neonatal care. However, with improving economy and health care, more babies are surviving in Sub-Sahara Africa.
Retinopathy of prematurity is known to increase with improvement in health indices and technology that allows more babies born early to survive. There are limited available publications from Africa on the magnitude of the ROP problem. Some of the known risk factors include prematurity, low birth weight, use of supplemental oxygen, sepsis, anemia, respiratory distress, poor weight gain, and multiple births have been well documented across the world. Recent studies have shown that ROP is indeed present in Africa (Table 1). It was also previously suggested that there may be a genetic influence moderating the presentation and incidence of ROP with one study reporting an increased risk of severe ROP [3] and other studies describing lower incidence among babies of African descent [5,6,7].
Risk Factors for Retinopathy of Prematurity in Africa
High Prevalence of Babies Born Prematurely
Nigeria has the world’s third largest number of premature infants in 2010 after India and China, and the USA is the sixth [8]. A significant 52% of the global live births occur in South Asia and Sub-Saharan Africa, where more than 60% of preterm babies were found to be born [9].
Multiple Pregnancy
The rate of twin births in West Africa is about four times higher than in the rest of the world, especially among Nigeria’s Yoruba community which is largely concentrated in the southwestern part of the country and in particular at Igbo-Ora-tagged “The Land of Twins” with about 40.2 per 1000 deliveries [10]. Multiple pregnancy is often associated with being born early and having low birth weight.
In a study of 119 low birth weight (LBW) twin infants to determine the incidence and document some of the characteristics, 74 (69.2%) were preterm and 33 (30.8%) of twins born at term were small-for-gestational age (SGA) infants. Twenty-six (35.1%) of the 74 twins born preterm were less than 32 weeks gestation [11]. Another study showed that 52 (14%) of 372 twin babies had birth weight less than 1500 g [12].
Fertility Treatments
The increase in assisted reproductive technique is not in itself found to increase the incidence of ROP; however, there is an increased association with low birth weight and prematurity [13, 14].
Improved Health Care
Improvement in maternal and neonatal medical technology and care can affect premature birth. A study identified many maternal socio-demographic and antenatal variables, including previous preterm delivery, antepartum hemorrhage, rupture of membrane, urinary tract infection, pregnancy-induced hypertension, type of labor, and booking status, as determinants of preterm delivery [15].
Education
Awareness of ROP among pediatricians is also increasing. Forty-six (95.8%) pediatricians during a continuous medical education program were aware of some risk factors for ROP while 14 (29.2%) of them knew the ROP screening guidelines. Knowledge on treatment options was low with laser being the only treatment modality for ROP known by 18 (37.5%) [16]. The need to improve awareness among all health professionals and the public has been emphasized [17].
Improved Neonatal Care
In a 2008 study in Ilorin, North Central Nigeria, at a neonatal intensive care unit (NICU) which provides level II care to both inborn and outborn (out of base hospital referrals ) neonates; it admits between 1000 and 1200 patients annually out of which about 35% are babies born preterm. Among 2489 inborn deliveries over a 9-month period, 293 (11.8%) were preterm and 97 (3.9%) were early preterm (<34 weeks) giving a preterm delivery rate (<37 weeks) of 120 per 1000 deliveries and early preterm delivery rate (<34 weeks) of 40 per 1000 deliveries [15].
In another study from Southeast Nigeria from 2009 to 2013, among 592 babies born, the preterm birth rate increased from 9.8% in 2009 to 17.1% in 2013, 188 (31.7%) of 592 preterm were born earlier than 32 weeks, and 147 (23.8%) of 592 of preterm babies had birth weight less than 1500 g out of which 47 (7.9%) were less than 1000 g. The prevalence of early neonatal death was about 29.7% especially among the extremely preterm babies (86% mortality) born less than 28 weeks compared to very preterm (50.5%) born between 28 and <32 weeks) [18].
An unpublished work from 2009 to 2013 from the University of Ilorin Teaching Hospital, Nigeria, showed babies with birth weight ranging from 500 to 750 g surviving due to improved neonatal care. Among 6539 admissions into the NICU, 113 (1.7%) babies were born extremely preterm less than 28 weeks gestational age, survival was overall about 28%, a trend which improved gradually from 15% in year 2009 to 22% in 2011 and 46% in year 2012 and was 661 (74%) among 891 28–34 gestational age. Survival among those whose birth weights are below 1000 g was about 30% overall, improving from 24% in 2009 to 42 and 55% in years 2011 and 2012. This improvement is most probably related to a special federal government of Nigeria intervention commissioned in 2011 to revamp the tertiary hospital equipment including providing incubators and a few neonatal oxygen monitors in about 12 teaching hospitals.
An unpublished study by the author1 from 10 of the 12 federal government hospitals in Nigeria revealed that in each of the hospitals, an average of 11 (5–30) babies less than 1500 g birth weight are admitted monthly per hospital. The survival rate of the babies under 1500 g was 60% (42/70). On the average, there are about 8 (1-15) functional incubators and 2 (0–10) neonatal oxygen monitors. Some incubators have oxygen delivered by pipes, while others use oxygen concentrators to deliver oxygen. Pulse oximeters to measure oxygen saturation are irregularly used as noted in another study from South Africa [17]. The survey also showed that only a third of about 45 pediatric ophthalmologists and retinal specialists in Nigeria were specifically trained in ROP care.
ROP Screening Criteria, Prevalence and Challenges
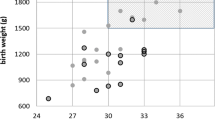
In Nigeria, there is no national or regional ROP program. A previous study done in 2007 on 58 eyes of babies born at gestational age (GA) of ≤35 weeks, birth weight of ≤1.9 kg performed within 30 days of birth, showed that at the first examination, retina avascularity in about 52 (90%) eyes, mostly located in zone 2, among the very low birth weight <1500 g babies and in GA <32 weeks, there was no treatable ROP [19•]. It also identified many challenges to screening including difficulties with follow-up visits because there was no dedicated personnel to coordinate screening; overwhelming competing clinical and administrative responsibilities for the few pediatric and retinal specialists with ROP training and lack of facilities for treatment of ROP at the time (LASER treatment facilities only became available in 2011 in four tertiary hospitals) [19•]. This study therefore recommended for ROP screening criteria in Nigeria to include only babies with birth weight of 1500 g and gestational age of 32 weeks; this is similar to the conclusion in a South African study [20•]. In South Africa, there was also a report of a seeming low prevalence of 1.6–2.9% of treatable ROP. Competing demands and limited resources in the public sector precluded prioritizing the diagnosis and treatment of ROP [21].
Studies from Nigeria [22•, 23, 24•], South Africa [21, 25•, 26•], and Egypt [27, 28•] reported ROP (Table 1).
A study from Nigeria in 2012 based on the criteria previously set reported any stage of ROP in 25 (47.2%) of 53 children born at 32 weeks or earlier with birth weight 1500 or less where survival was 87% among all 100 babies born. ROP was reported among 6 (75%) of 8 babies weighing <1300 g compared to 19 (42%) 1300 or more and among 18 (58%) of 31 delivered before 30-week gestation. Zone II stage 3 disease was the most common in 19 (76%), and all cases of ROP regressed spontaneously except in one baby who had zone 1 stage 3 disease but unfortunately died before treatment. The significant association of ROP with prematurity and receiving supplemental oxygen (chi (2) = 6.17; P = 0.01), presence of sepsis (chi (2) = 7.47; P = 0.006), multiple blood transfusions (chi (2) = 5.11; P = 0.02), and delivery by caesarian section (chi (2) = 4.22; P = 0.04) was also reported [23].
Another study from 2011 to 2014 found that 12 (15%) of 80 babies had any stage of ROP and 6 (7.5%) needed treatment [24•].
Even though the need to screen for ROP has became emphasized in Nigeria, the challenges remain daunting. These challenges include the following:
-
1.
The limited number of trained personnel who are also encumbered by other duties
-
2.
The time required to do the ROP screening using bedside indirect ophthalmoscopy
-
3.
The absence of a widefield retinal camera that might be used by middle-level personnel
-
4.
The lack of an ROP coordinator to ensure routine follow-up screening [19•]
Given these challenges, a mobile smartphone application with a 20D lens considered to be less expensive was introduced for ROP screening. The method is limited by the learning curve similar to that of the indirect ophthalmoscopy. Some concerns about safety of the neonate to exposure of unregulated smartphone phone light, which is believed to be addressed by the ability of the App (Filmic Pro) to regulate the intensity of the light, has been voiced. This has become a popular tool worldwide [22•] (Fig. 1).
An ongoing (2016) study in Ibadan, Nigeria, on incidence of ROP has so far found 7 (24.6%) cases of ROP among 29 babies. With treatable cases in 2 (8.9%) (personal communication with authors).
Cases of missed ROP probably abound in our communities. Routine screening is negligible leading to a high possibility of missed ROP. A well-structured screening program in South Africa decreased the rate of missed cases from 33/33 (100%) in year 1, before the interventions, to 23/292 in the final year (7.9%) [25•].
In Nigeria, this is exemplified by a recent referral (October 2016) of a 6-month-old infant who presented to an ophthalmologist with leukocoria in the left eye noticed 3 months after birth. There was a history of prematurity with non-judicious oxygen use. There was no ROP screening at the time in the hospital. Comprehensive examination revealed in the right eye severe macular heterotopia and straightened retinal vessel with market peripheral fibrosis. The refraction was a minus -12D sphere -2Cyl and the left eye had no view with indirect ophthalmoscopy. Ocular ultrasound revealed a retrolental mass with complete retinal detachment (Fig. 2). This is suggestive of an undiagnosed case of ROP leading to lack of treatment and a very poor outcome.
Conclusion
Retinopathy of prematurity is taking an important position in the prevention of childhood blindness in Africa. This is as a result of improvement in neonatal care for the very low birth weight babies.
There is no national ROP program in Nigeria, the most populous country in Africa. The creation of awareness among obstetricians and neonatologists about the presence of ROP, the need to adhere to judicious use of oxygen in babies born prematurely, and ROP screening guidelines is imperative. Collaboration between neonatologists and ophthalmologists needs to be strengthened. The general public needs to be educated about the possible eye complications of prematurity.
The effect of genetics on ROP may also be explored in the indigenous African environment to confirm or refute the previous reports that black children have a lesser risk of developing ROP.
Due to the relatively small number of trained ophthalmologists (with competing other duties) available to screen and manage babies for ROP, it is strongly recommended that a regional screening program should be put in place in African countries. This will involve screening across several contiguous states within the region using a telemedicine approach. Infant retinal photos can be taken by middle-level trained personnel. They will coordinate, take fundus pictures, screen, and send images for real-time highly trained specialist review. The initial cost may be high; however, the long-term benefit may well be worth it in the prevention of ROP-induced blindness.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Africa- https://en.wikipedia.org/wiki/Africa Accessed 16th Oct.
GDP Ranking Table. http://dataworldbank.org/data-catalog/GDP-ranking-table Accesses 16th Oct. 2016.
Aralikatti AK, Mitra A, Denniston AK, Haque MS, Ewer AK, Butler L. Is ethnicity a risk factor for severe retinopathy of prematurity? Arch Dis Child Fetal Neonatal Ed. 95(3):F174–6.
Baiyeroju-Agbeja AMOF. Screening for retinopathyof prematurity. Nigerian J Ophthalmol. 1998;6:23–5.
Csak K, Szabo V, Szabo A, Vannay A. Pathogenesis and genetic basis for retinopathy of prematurity. Front Biosci. 2006;11:908–20.
Delport SD, Swanepoel JC, Odendaal PJ, Roux P. Incidence of retinopathy of prematurity in very-low- birth-weight infants born at Kalafong Hospital, Pretoria. S Afr Med J. 2002;92(12):986–90.
Good WV, Hardy RJ, Wallace DK, Bremer D, Rogers DL, Siatkowski RM, et al. Beta-blocking and racial variation in the severity of retinopathy of prematurity. Arch Ophthalmol. 2012;130(1):117–8.
World Health Organization Preterm Birth Fact sheet No 363. Available from: http://www.whoint/mediacentre/factsheets/fs363/en/ Accessed September 10, 2014.
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72.
Akinboro A, Azeez MA, Bakare AA. Frequency of twinning in Southwest Nigeria. Indian J Hum Genet. 2008;14(2):41–7.
Onyiriuka AN. Incidence of delivery of low birthweight infants in twin gestations. Niger J Clin Pract. 2010;13(4):365–70.
Kullima AA, Audu BM, Geidam AD. Outcome of twin deliveries at the University of Maiduguri Teaching Hospital: a 5-year review. Niger J Clin Pract. 2011;14(3):345–8.
Orhue AA, Aziken ME, Osemwenkha AP, Ibadin KO, Odoma G. In vitro fertilization at a public hospital in Nigeria. Int J Gynaecol Obstet. 2012;118(1):56–60.
Ezechi OC, Ndububa VI, Loto OM, Ezeobi PM, Kalu BK, Njokanma OF, et al. Pregnancy, obstetric and neonatal outcome after assisted reproduction in Nigerians. J Matern Fetal Neonatal Med. 2008;21(4):261–6.
Mokuolu OASB, Adesiyun O, Adeniyi A. Prevalence and determinants of pre-term deliveries in the University of Ilorin Teaching Hospital, Ilorin, Nigeria. Pediatric Reports. 2010;2(1):4.
Uhumwangho OI-AY. Awareness and screening for retinopathy of prematurity among paediatricians in Nigeria. Journal of the West African College of Surgeons. 2013;3(3):13.
Visser L, Singh R, Young M, Lewis H, McKerrow N. Guideline for the prevention, screening and treatment of retinopathy of prematurity (ROP). S Afr Med J. 2012;103(2):116–25.
Iyoke CALO, Ezugwu EC, et al. Prevalence and perinatal mortality associated with preterm births in a tertiary medical center in South East Nigeria. International Journal of Women’s Health. 2014;6:8.
• Ademola-Popoola D, Adesiyun O, Obasa TO. Screening programme for retinopathy of prematurity in Ilorin, Nigeria: a pilot study. West Afr J Med. 2013;32(4):281–5. Recommended screening criteria, identified and provided solutions to the many challenges to ROP screening and difficulties with follow-up visits in Africa.
• Visser Kift E, Freeman N, Cook C, Myer L. Retinopathy of prematurity screening criteria and workload implications at Tygerberg Children’s Hospital, South Africa: a cross-sectional study. S Afr Med J. 2016;106(6). Identified that 41 babies need to be screen to detect a neonate to treat and while confirming previous screening criteria that will pick most neonates with ROP even in an African setting.
Varughese S, Gilbert C, Pieper C, Cook C. Retinopathy of prematurity in South Africa: an assessment of needs, resources and requirements for screening programmes. Br J Ophthalmol. 2008;92(7):879–82.
• Oluleye TS, Rotimi-Samuel A, Adenekan A. Mobile phones for retinopathy of prematurity screening in Lagos, Nigeria, Sub-Saharan Africa. Eur J Ophthalmol. 2015;26(1):92–4. Described the use of smartphone in ROP screening in the absence of wide field cameras, making caregiver’s education and documentation possible
Adio AO, Ugwu RO, Nwokocha CG, Eneh AU. Retinopathy of prematurity in Port Harcourt, Nigeria. ISRN Ophthalmol.2014:481527.
• Fajolu IB, Rotimi-Samuel A, Aribaba OT, Musa KO, Akinsola FB, Ezeaka VC, et al. Retinopathy of prematurity and associated factors in Lagos, Nigeria. Paediatr Int Child Health. 2015;35(4):324–8. Reported a relatively high number of African babies with ROP(15%) contrary to previous assumptions.
• Jacoby MR, Du Toit L. Screening for retinopathy of prematurity in a provincial hospital in Port Elizabeth, South Africa. S Afr Med J. 2016;106(6). Provided key strategies to improve number of babies Screened for ROP and followed up thereby eliminating missed ROP cases.
Mayet I, Cockinos C. Retinopathy of prematurity in South Africans at a tertiary hospital: a prospective study. Eye (Lond). 2006;20(1):29–31.
Hakeem AH, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19(3):289–94.
• Hadi AM, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol. 2013;7:831–7. Reported high incidence (34.4%)of ROP identified when a wide field camera is used in an African country with reasonable economy and improving neonatal care services while also confirming main risk factors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dupe Ademola-Popoola and Tunji Oluleye declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Ophthalmology
Rights and permissions
About this article
Cite this article
Ademola-Popoola, D.S., Oluleye, T.S. Retinopathy of Prematurity (ROP) in a Developing Economy with Improving Health Care. Curr Ophthalmol Rep 5, 114–118 (2017). https://doi.org/10.1007/s40135-017-0129-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40135-017-0129-0